Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Effects of Chinese Herbal Medicine on Acute Exacerbations of COPD: A Randomized, Placebo-Controlled Study
Authors Li J , Zhang H, Ruan H , Si Y, Sun Z, Liu H, Feng J, Wang Y, Li L, Bai L, Sun H
Received 10 August 2020
Accepted for publication 19 October 2020
Published 12 November 2020 Volume 2020:15 Pages 2901—2912
DOI https://doi.org/10.2147/COPD.S276082
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Jiansheng Li,1,2,* Hailong Zhang,1,3,* Huanrong Ruan,3 Yimei Si,3 Zikai Sun,4 Hong Liu,5 Jihong Feng,6 Yanqing Wang,7 Lihua Li,8 Li Bai,9 Hui Sun10
1Co-Construction Collaborative Innovation Center for Chinese Medicine and Respiratory Diseases by Henan & Education Ministry of P.R. China, Henan University of Chinese Medicine, Zhengzhou, People’s Republic of China; 2Henan Key Laboratory of Chinese Medicine for Respiratory Disease, Henan University of Chinese Medicine, Zhengzhou, People’s Republic of China; 3The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, People’s Republic of China; 4Jiangsu Province Hospital of Traditional Chinese Medicine, Nanjing, People’s Republic of China; 5The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 6The Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 7Haici Hospital of Qingdao, Qingdao, People’s Republic of China; 8Zhengzhou People’s Hospital, Zhengzhou, People’s Republic of China; 9Shanxi Hospital of Integrated Traditional and Western Medicine, Taiyuan, People’s Republic of China; 10Nanyang City Center Hospital, Nanyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiansheng Li No. 156 Jinshui East Road, Zhengdong New District, Zhengzhou, Henan 450046, People’s Republic of China
Tel +86 371 65676568
Fax +86 371 65944307
Email [email protected]
Purpose: Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is an essential occurrence in COPD management and is the leading cause of morbidity and mortality. Chinese herbal medicine is widely used in the treatment of AECOPD, but high quality randomized controlled trials are limited. This study aimed to evaluate the efficacy and safety of Chinese herbal medicine as adjuvant therapy for patients with AECOPD.
Methods: This was a randomized, double-blind, placebo-controlled study of 378 participants from eight centers in China. Participants were randomly assigned to receive 10 g of Chinese herbal medicine (according to the type of Traditional Chinese medicine syndrome: Sanhanhuayin, Qingrehuatan, or Zaoshihuatan granules) or placebo, two times per day, for 14 days, in addition to conventional medicine. Participants were followed up for 84 days after the treatment. The primary end point was the COPD assessment test (CAT) score. Secondary end points included the Modified British Medical Research Council (mMRC) questionnaire and the COPD patient-reported outcome scale (COPD-PRO). We also assessed treatment failure and treatment success rate, length of hospitalization, number of patients with acute exacerbations, number of patients readmitted due to AECOPD, and number of deaths and intubation.
Results: The between-group difference in the change from baseline for CAT on day 14 (end of treatment) was − 2.11 (95% confidence interval [CI], − 3.198 to − 1.050; P< 0.001), exceeding the minimal clinically important difference. The mMRC and COPD-PRO scores were lower in the intervention group compared to the control group (between-group difference in the change from baseline, − 0.28; 95% CI, − 0.48 to − 0.08; P=0.007 and − 2.51; 95% CI, − 4.087 to − 0.929; P=0.002, respectively) on day 14. The intervention group had a significantly shorter duration of hospital stay than the control group (mean difference, − 1.21days; 95% CI, − 2.041 to − 0.419; P=0.003), significantly lower of number of exacerbations (risk ratio [RR], 0.60; 95% CI, 0.409 to 0.892; P=0.010), and significantly lower number of readmissions due to AECOPD (RR, 0.41; 95% CI, 0.193 to 0.865; P=0.015). Significant differences in the number of treatment failures or successes, deaths, and intubation were not observed. The difference in safety variables and adverse events between the two groups was not observed.
Conclusion: Chinese herbal medicine appears to be safe and beneficial for AECOPD and can be considered a complementary treatment for patients with AECOPD.
Keywords: acute exacerbations, chronic obstructive pulmonary disease, randomized controlled trial, Chinese herbal medicine
Introduction
Chronic obstructive pulmonary disease (COPD) is a common and frequently- occurring disease that seriously endangers human health.1–3 Its mortality rate had risen to third place among non-communicable diseases in the world and China in 2013.4–6 Acute exacerbations of COPD (AECOPD) are important occurrences when managing COPD because they negatively impact health status, rates of hospitalization and readmission, and disease progression.7 AECOPD constitutes a significant part of the medical expenses for patients with COPD. It is a common cause of death. Therefore, active treatment of exacerbations is essential in reducing the risk of death and reducing the economic burden, particularly in patients with moderate to severe AECOPD.
AECOPD is commonly treated with three classes of medications, such as bronchodilators, corticosteroids, antibiotics, and in more severe cases, requires oxygen therapy and ventilatory support. However, these therapies can lead to significant side effects such as headaches, insomnia, nausea, and pneumonia.1 Therefore, there is a need to improve the management of AECOPD. Traditional Chinese medicine (TCM), especially herbal medicine, plays a vital role in improving respiratory symptoms of COPD and reducing exacerbations. It is widely used in the treatment of AECOPD.8–10 However, due to unreasonable trial design and low-quality clinical research, the efficacy of Chinese herbal medicine in the treatment of AECOPD has not been widely recognized.11 Therefore, we performed a multi-center, randomized, double- blind, placebo-controlled clinical trial to assess the effectiveness and safety of Chinese herbal medicine in treating patients with AECOPD.
Patients and Methods
Study Design
This study was a randomized, double-blind, placebo-controlled, multi-center clinical trial. Three hundred seventy-eight participants were randomized in a 1:1 ratio to Chinese herbal medicine or matching placebo, using a computer-generated code. Stratified and block randomization were used to generate a randomization list containing numerical codes (001–378), with a block size of 6. Participants, trial participants, care providers, and outcome assessors, except for the data analysts, were blind to treatment allocation. Jiangsu Famous Medical Technology Co., Ltd. in Nanjing, China, as a third-party contract research organization, was responsible for data management and analysis. The study was registered (ClinicalTrials.gov, registration number: NCT03428412), and performed according to the published protocol.12
Participants
Participants were eligible if they were diagnosed with moderate to severe AECOPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 criteria, and were aged 40–80 years. They had to meet the Chinese medicine syndrome classification of external cold and internal fluid, phlegm-heat congesting lung, or phlegm-damp amassing in the lung, not participated in other interventional trials in the past month, and signed the written informed consent form.
The diagnosis of AECOPD is completely depended on the clinical manifestations, the sudden change of respiratory symptoms that exceeded the normal range of variation and result in additional therapy. At same time, it must be differentiated clinically from other diseases that could explain sudden changes in these symptoms. The key symptom of AECOPD is increased dyspnea, and other symptoms include increased sputum purulence and volume, together with increased cough and wheeze. The TCM diagnostic criteria referred to the Diagnostic criteria of TCM syndromes of chronic obstructive pulmonary disease (update 2011).13 In order to ensure the accuracy of the diagnosis of TCM syndromes, the researchers were trained before the start of the study to make them master the diagnostic criteria of TCM syndromes. In the process of the study, each subject was diagnosed by two researchers respectively, and the person in charge of the research unit was finally determined if there was any inconsistency.
Patients were excluded if they were pregnant or lactating, unable to communicate, had heart failure (NYHA Class IV), severe cardiac arrhythmias, unstable hemodynamics, non-COPD respiratory disorders (eg, asthma, bronchiectasis, active tuberculosis, pneumothorax, pleural effusion, pulmonary thromboembolic, or neuromuscular diseases affecting respiratory movement function), had a history of tumor, had been treated outside the hospital for more than seven days, required invasive mechanical ventilation, had severe concomitant liver, or kidney disease, required long-term bed rest, or had known hypersensitivity to the study medication or some of its ingredients.
Interventions
Eligible participants were randomly assigned to the intervention group or the control group. Based on health education and conventional medicine, the intervention group will be was treated with Chinese herbal medicine granules, and the control group was given a placebo according to TCM syndromes. Sanhanhuayin granule for a syndrome of external cold and internal fluid; Qingrehuatan granule for a syndrome of phlegm-heat congesting lung and Zaoshihuatan granule for a syndrome of phlegm-damp amassing in the lung. The Chinese herbal medicine granules and placebo were supplied by Jiangyin Tian Jiang Pharmaceutical Co., Ltd. Jiangsu, People’s Republic of China. The Chinese herbal medicine granules were manufactured strictly by the standards of the Chinese Pharmacopoeia (2010). Each type of granule was dissolved in warm water and taken orally, 10 g, two times daily for 14 days. The components of Chinese herbal medicine granules are shown in Table 1.
 |
Table 1 The Components of Chinese Herbal Medicine Granules |
The conventional medicine for AECOPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 criteria and the Expert consensus on Management of Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD) in China (update 2017).14 The treatment of AECOPD includes Pharmacological treatment and respiratory support. The three classes of medications commonly used for AECOPD are bronchodilators, corticosteroids, and antibiotics. Respiratory support mainly includes oxygen therapy and noninvasive mechanical ventilation.
Outcome Measures
The COPD assessment test (CAT) score, the primary end point, was measured at baseline, 14 days (end of treatment), 42 days (after a follow-up of 4 weeks), and 98 days (after a follow-up of 12 weeks). Secondary end points included the following: treatment failure and success rates over 14 days, length of hospitalization, number of patients of with acute exacerbations, and number of patients readmitted due to AECOPD in the 12-week follow-up period, rate of mortality and intubation over 98 days, dyspnea accessed by the Modified British Medical Research Council (mMRC) questionnaire, and COPD patient-reported outcome scale (COPD-PRO).
The safety points included adverse events, serious adverse events, and laboratory test results. Adverse events or serious adverse events were documented throughout the study. Blood routine, liver and kidney function were measured before and after treatment.
Sample Size and Statistical Analysis
Based on previous studies, we estimated that 189 participants in the intervention group and 189 participants in the control group would be necessary to detect a difference of two points (minimal clinically important difference) in the CAT score between the intervention and control groups at the end of the treatment with 10% drop-out rate, 90% power, using a significance level of 0.05.
An independent statistician analyzed data using SPSS version 23.0 software (SPSS Inc., Chicago, IL, USA). The intention-to-treat population data was used for baseline data and efficacy, and the missing data of withdrawn participants were replaced with the last-observation-carried-forward method. The continuous or categorical variables of baseline characteristics between the two groups were compared using t-test or chi-squared test. For between-group comparisons of changes from baseline to 14days, 42 days, and 98 days in continuous variables were conducted using an analysis-of-variance model including the baseline values as covariates (ANCOVA). Results with treatment failure, treatment success, COPD exacerbations, readmission due to AECOPD and deaths are expressed as numbers, percentages, Risk Ratio (RR) and the related 95% confidence interval (CI). Groups were compared using the Crosstabs test. Two-sided tests were used throughout and with the statistical significance level set at 0.05.
Results
The study was conducted between August 2018 and January 2020. A total of 378 participants, 189 per group, were enrolled from eight study sites. Of the 378 participants, 18 dropped out by day 98 (11 in the intervention group and 7 in the control group). The flow chart of the study is presented in Figure 1. Eventually, 378 patients were included in the full analysis set population. Participants’ demographics and clinical characteristics were not significantly different between the two groups at baseline (Table 2).
 |
Table 2 Baseline Demographics and Clinical Characteristics |
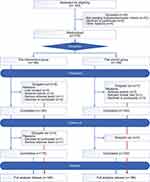 |
Figure 1 Patient enrollment distribution diagram. |
Primary End Point
CAT Scores
The average change in the total CAT score on day 14 (end of treatment) was −7.69±5.39 in the intervention group and −5.58±5.21 in the control group. The between-group difference value was −2.11 (95% CI, −3.198 to −1.050), with a statistically significant difference between the groups (P<0.001). On day 42 (after a follow-up of 4 weeks), the mean change was −8.05±5.59 in the intervention group and −6.15±6.00 in the control group (mean difference, −1.90; 95% CI, −3.072 to −0.727; P=0.002). There was no difference between groups in the mean change of the total CAT score on day 98 (after a follow-up of 12 weeks) (mean difference, −1.30; 95% CI, −2.615 to 0.022; P=0.054), the mean change was −7.53±6.36 in the intervention group and −6.24±6.67 in the control group (Table 3).
 |
Table 3 Effects of Intervention on Primary and Secondary End Points in the Intention-to-Treat Population |
Secondary End Points
Treatment Failure on Day 14
On day 14, clinical failure was observed in 7 patients (3.7%) from the intervention group and 10 patients (5.3%) from the control group (risk ratio [RR], 0.70; 95% CI, 0.272 to 1.800; P=0.457) (Table 3).
Treatment Success on Day 14
Clinical success was observed in 174 patients (92.1%) from the intervention group and 172 patients (91.0%) from the control group (RR, 1.01; 95% CI, 0.951 to 1.076; P=0.712) (Table 3).
Length of Hospitalization
The duration of hospital stay was significantly shorter in the intervention group (11.23±3.59) than that in the control group (12.44±4.24) (mean difference, −1.21days; 95% CI, −2.041 to −0.419; P=0.003) (Table 3).
COPD Exacerbations
After a follow-up of 12 weeks, 85 participants (32 in the intervention group and 53 in the control group) experienced at least one COPD exacerbation. The rate of exacerbations in the intervention group (16.9%) was significantly lower than that in the control group (28.0%) (RR, 0.60; 95% CI, 0.409 to 0.892; P=0.010) (Table 3).
Readmission Due to AECOPD
After a follow-up of 12 weeks, 31 participants (9 in the intervention group and 22 in the control group) experienced at least one hospitalization for COPD exacerbation. The rate of readmission due to AECOPD in the intervention group (4.8%) was significantly lower than the control group (11.6%) (RR, 0.41; 95% CI, 0.193 to 0.865; P=0.015) (Table 3).
Intubation
There was no difference between groups in terms of intubation rate measured as the number of intubation over 98 days (RR, 1.01; 95% CI, 0.995 to 1.016; P=0.500, intervention group n=0 and control group n=1).
Deaths
There was no difference between groups in terms of mortality rate measured as the number of deaths over 98 days (RR, 1.00; 95% CI, 0.984 to 1.005; P=0.500, intervention group n = 1 and control group n = 0).
mMRC Score
The mean change in mMRC on day 14 was −1.10±1.02 in the intervention group and −0.81±0.98 in the control group (mean difference, −0.28; 95% CI, −0.48 to −0.08; P=0.007). On day 42, the mean change was −1.11±1.01 in the intervention group and −0.88±0.98 in the control group (mean difference, −0.23; 95% CI, −0.43 to −0.03; P=0.027). There was no difference between groups in the mean change of the mMRC score on day 98 (mean difference, −0.20; 95% CI, −0.41 to 0.005; P=0.055); the mean changes were −1.11±1.00 in the intervention group and −0.90±1.03 in the control group (Table 3).
Health-Related Quality of Life
There was a statistically significant difference between the intervention and control group in the mean change of the total COPD-PRO score on day 14 (mean difference, −2.51; 95% CI, −4.087 to −0.929; P=0.002), and the mean changes were −12.08±8.51 and −9.57±7.04, respectively. On day 42, the mean change was −13.35±8.53 in the intervention group and −10.83±7.81 in the control group (mean difference, −2.53; 95% CI, −4.183 to −0.875; P=0.003). There was no difference between groups in the mean change on day 98 (mean difference, −1.62; 95% CI, −3.265 to 0.016; P=0.052), the mean change was −12.36±8.03 in the intervention group and −10.74±8.19 in the control group (Table 3). Clinical symptoms and effectiveness satisfaction were the same as the change of the total score, but not health satisfaction (Table 3).
Safety
Sixty-five participants (26 from the intervention group, and 39 from the control group) reported 68 adverse events. The most frequent event was the common cold (Table 4).
 |
Table 4 Adverse Events in the Intervention and Control Groups |
There were two serious adverse events in the intervention group. One participant died of respiratory failure. Another participant was diagnosed with lung cancer during the follow-up period, and he withdrew from the study. The serious adverse events were not considered to be related to the intervention.
The participants’ full blood count and blood biochemistry tests were not clinically significant before or after treatment.
Discussion
According to the previous literature and clinical investigation results, our team have developed the Diagnostic criteria of TCM syndromes of chronic obstructive pulmonary disease (update 2011)13 and the Guidelines for TCM Diagnosis and Treatment of Chronic Obstructive Pulmonary Disease (2011 edition).14 In this study, we selected the three most common syndrome types of AECOPD. According to the Guidelines for TCM Diagnosis and Treatment of Chronic Obstructive Pulmonary Disease, and combined with the pathogenesis of AECOPD, we have formulated the syndrome differentiation treatment plan. This syndrome differentiation treatment plan has been used for a long time and have obtained a good clinical effect in the clinic. In order to study the efficacy and safety of Chinese herbal medicine with syndrome differentiation therapy for patients of AECOPD, the first randomized, double-blind, placebo-controlled, multi-center clinical trial has been carried out.
With health education and conventional drug treatment, Chinese herbal medicine was superior to placebo in terms of the total CAT score and mMRC score on day 14 (end of treatment) and day 42 (after a follow-up of 4 weeks) as well as the total COPD-PRO score. Besides, cases of COPD exacerbations and readmission due to AECOPD occurred, and the duration of hospital stay was significantly shorter in patients assigned to Chinese herbal medicine. We did not see a difference in the rate of treatment failure and success, and the cases of intubation and deaths between the two groups. Overall, we concluded that Chinese herbal medicine was more beneficial than a placebo for treating AECOPD.
The goal of the treatment for COPD exacerbations is to minimize the negative impact of the current exacerbation, reduce clinical symptoms, and reduce the risk of death.15 CAT is a simple, easy-to-use test consisting of eight questions to assess the impact of the disease on social life.16 It proved to be a comprehensive questionnaire, with excellent repeatability for evaluating the clinical symptoms of COPD.17 CAT is a potentially useful instrument to assess the efficacy of treatments following COPD exacerbations.18 Therefore, CAT was selected as the primary outcome measure in our study. The minimum clinically important difference (MCID) for CAT is conventionally considered to be 2 points.19 A statistically significant between-group difference emerged for CAT with a magnitude (2.11 points) that exceeded the MCID, indicating that Chinese herbal medicine was better than the placebo at the end of treatment. The current study further showed that the Chinese herbal medicine as an adjunct to regular treatment could alleviate the clinical symptoms and improve the symptom score of patients with AECOPD.
In TCM theory, AECOPD is mainly an excess syndrome with phlegm (phlegm-heat, phlegm-damp), blood stasis, or mutual resistance. The treatment should follow the principle of “treating the symptoms for acute onset,” The methods should be clearing, resolving, ventilating, and descending. In this study, the three most common syndromes of AECOPD were selected. Different treatment methods and prescriptions, genuinely embodying the pattern identification as the basis for determining the treatment of TCM, were selected according to the different syndrome types. In addition to the significant reduction of CAT score, there were significant differences in the length of hospital stay, the number of exacerbations of COPD, and readmission due to AECOPD between the two groups after treatment or during the follow-up period. The current study further showed that the use of Qingrehuatan granule and Zaoshihuatan granule as an adjunct to regular treatment could reduce the high-level expression of tumor necrosis factor, intercellular adhesion molecule-1, interleukin-8, and endothelin-1, and increase the expression level of calcitonin gene-related peptide of patients with AECOPD.20,21 This may be one of the therapeutic mechanisms of Chinese herbal medicine in the treatment of AECOPD.
Our study group developed and validated the COPD-PRO scale according to the highest standards and procedures of international scales.22,23 It can provide patients’ responses to the treatments and then evaluate the effect of treatment in a standardized way. The published MCIDs of mMRC score and COPD-PRO scores were not be awarded. However, a statistically significant between-group difference emerged for mMRC score and COPD-PRO total scores of participants after treatment. Therefore, we speculate that this may reduce clinical symptoms and promote disease recovery by Chinese herbal medicine.
We did not find a significant treatment effect at the number of treatment failure and treatment success on day 14, and the number of intubation and deaths on day 98. Several factors could explain this observation: first, routine medications, according to guidelines, have proven to be highly beneficial in patients with AECOPD. Second, we excluded patients with respiratory failure requiring invasive mechanical ventilation. Third, the follow-up time was short, only three months.
The clinical studies on the treatment of AECOPD with Chinese herbal medicine have been reported.10,24–27 However, these studies choose one prescription10,24,25 or Chinese patent medicine26,27 for one syndrome type, and they do not reflect syndrome differentiation treatment. Besides, these studies had certain design shortcomings, such as the lack of randomized or double-blind design,25 lack of follow-up,10,25 or the absence of a multi-center design that could lead to population and geographical bias.25 The current multi-center study was conducted strictly according to the protocol and requirements of good clinical practice. However, it also had some limitations. First, the type or dosage of conventional western medicine drugs was not specified; we only provided a scheme. Second, the changes in related inflammation indicators were not evaluated during the treatment. Third, the follow-up time was short. Finally, clinical trials with larger sample sizes are needed to confirm the results of our study.
Conclusion
This study showed that the use of Chinese herbal medicine for 14 days, in conjunction with conventional treatment, significantly improved the symptom and quality of life. Additional effects of Chinese herbal medicine include shorter hospital stay, significantly reduced the number of patients with acute exacerbations, and a reduced number of patients readmitted due to AECOPD. Based on Chinese herbal medicine’s efficacy and safety, it should be considered for clinical application for the complementary treatment of patients with AECOPD.
Data Sharing Statement
Individual participant data collected during the trial are not publicly available owing to data privacy, but the anonymised dataset can be obtained on reasonable request from the corresponding author following publication and ending 5 years after publication.
Ethics Approval
This study was approved by the Ethical Committee of the First Affiliated Hospital of the Henan University of Chinese Medicine (approval No 2017HL-069-01) as the coordinating center. The written informed consents signed by all participants included in the study.
Patient Consent for Publication
Not required.
Acknowledgments
We would like to thank the participants who enrolled in this study, and the study investigators and their study team for essential contributions. The authors particularly thank Jiangsu Famous Medical Technology Co., Ltd. in Nanjing, China, to manage the data and statistical analysis. Jiangyin Tian Jiang Pharmaceutical Co., Ltd. provided the Chinese medicine granules and placebo used in this study. We would like to thank Editage (www.editage.cn) for English language editing.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81973781, 81603556) and the construction project of the characteristic discipline of Chinese medicine in Henan province, China (Grant No. STS-ZYX- 2017006).
Disclosure
The authors report that there are no conflicts of interest in this work.
References
1. Gold Executive Committee. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of COPD 2020. Available from: http://www.goldcopd.org.
2. National Institute for Health and Clinical Excellence. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. London: National Clinical Guideline Centre; 2010. Available from: http://guidance.nice.org.ukCG101/Guidance/pdf/English.
3. Niewoehner DE. Outpatient management of severe COPD. N Engl J Med. 2010;362:1407–1416. doi:10.1056/NEJMcp0912556
4. Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–272. doi:10.1016/S0140-6736(15)00551-6
5. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England). 2015;385(9963):117–171.
6. Mannino DM, Higuchi K, Yu TC, et al. Economic burden of COPD in the presence of comorbidities. Chest. 2015;148(1):138–150. doi:10.1378/chest.14-2434
7. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi:10.1016/S0140-6736(07)61382-8
8. Liu S, Shergis J, Chen X, et al. Chinese Herbal Medicine (Weijing Decoction) combined with pharmacotherapy for the treatment of acute exacerbations of chronic obstructive pulmonary disease. Evid Based Complement Alternat Med. 2014;2014:257012. doi:10.1155/2014/257012
9. Gao Z, Jing J, Liu Y. Xiaoqinglong decoction (a traditional Chinese medicine) combined conventional treatment for acute exacerbation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Medicine. 2020;99(14):e19571.
10. Liu M, Zhong X, Yuhang L, et al. Xuan Bai Cheng Qi formula as an adjuvant treatment of acute exacerbation of chronic obstructive pulmonary disease of the syndrome type phlegm-heat obstructing the lungs: a multicenter, randomized, double-blind, placebo-controlled clinical trial. BMC Complement Altern Med. 2014;14:239.
11. Zhang H, Wang M, L F, et al. Systematic review of therapeutic effect assessment index of randomized controlled trials on acute exacerbation of chronic obstructive pulmonary disease by Chinese medicine syndrome differentiation and treatment. China J Chin Med. 2013;28(6):797–804.
12. Zhang H, Li J, Yu X, et al. Effects of Chinese medicine on patients with acute exacerbations of COPD: study protocol for a randomized controlled trial. Trials. 2019;20(1):735. doi:10.1186/s13063-019-3772-y
13. Internal Medicine Branch of Chinese Society of Chinese Medicine. Pulmonary Disease Branch of Chinese Society of Chinese Medicine. Diagnostic criteria of TCM syndromes of chronic obstructive pulmonary disease (update 2011). J Trad Chin Med. 2012;53(02):177–178.
14. Boqiang CAI, Rongchang CHEN. Expert consensus on management of Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD) in China (update 2017). Int J Respir. 2017;37(14):1041–1057.
15. Martinez FJ, Han MK, Flaherty K, et al. Role of infection and antimicrobial therapy in acute exacerbations of chronic obstructive pulmonary disease. Expert Rev Anti Infect Ther. 2006;4(1):101–124. doi:10.1586/14787210.4.1.101
16. Kelly JL, Bamsey O, Smith C, et al. Health status assessment in routine clinical practice: the chronic obstructive pulmonary disease assessment test score in outpatients. Respiration. 2012;84:193–199. doi:10.1159/000336549
17. Xiong W, Zhang Q-S, Zhao W, et al. A 12-month follow-up study on the preventive effect of oral lansoprazole on acute exacerbation of chronic obstructive pulmonary disease. Int J Exp Path. 2016;97:107–113. doi:10.1111/iep.12173
18. You-Hui T, Zhang Y, Fei G-H. Utility of the CAT in the therapy assessment of COPD exacerbations in China. BMC Pulm Med. 2014;14:42. doi:10.1186/1471-2466-14-42
19. Polkey MI, William D, Man C. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2:195–203. doi:10.1016/S2213-2600(14)70001-3
20. Bin L, Zhengkun H, Jiansheng L, et al. Huatan Qingre prescription treatment of chronic obstructive pulmonary disease with acute exacerbation and syndrome of heat-phlegm stagnating the lung the effects of cytokines. Liaoning J Trad Chin Med. 2010;37(03):403–405.
21. Suyun L, Ping C, Jiansheng L, et al. Dampness and phlegm prescriptions treatment of chronic obstructive pulmonary disease with acute exacerbation and syndrome of phlegm obstructing lung the effects of cytokines. Acta Chin Med. 2010;25(01):122–124.
22. Wang MH, Yu XQ, Li SY, et al. Study and preliminary assessment of chronic obstructive pulmonary disease patients report circulating ending scale. Acta Chin Med. 2011;26(03):270–274.
23. Li JS, Wang MH, Yu XQ, et al. Development and validation of a patient reported outcome instrument for chronic obstructive pulmonary diseases. Chin J Integr Med. 2015;21(9):667–675. doi:10.1007/s11655-014-1982-4
24. Gao Z, Jing J, Liu Y, et al. Xiaoqinglong decoction (a traditional Chinese medicine) combined conventional treatment for acute exacerbation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Medicine (Baltimore). 2020;99(14):e19571. doi:10.1097/MD.0000000000019571
25. Jin J, Zhang H, Demin L, et al. Effectiveness of Xin Jia Xuan Bai Cheng Qi Decoction in treating acute exacerbation of chronic obstructive pulmonary disease: study protocol for a multicentre, randomised, controlled trial. BMJ Open. 2019;9(11):e030249. doi:10.1136/bmjopen-2019-030249
26. Tingqian L, Gang W, Jing C, et al. Randomized controlled trial of tanreqing injection in treatment of acute exacerbation of chronic obstructive pulmonary Disease (Syndrome of Retention of Phlegm-Heat in the Lung). Chin J Evid Based Med. 2004;5:
27. Xia R-Y, Xiao-yang H, Fei Y-T, et al. Shufeng Jiedu capsules for treating acute exacerbations of chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Complement Med Ther. 2020;20(1):151. doi:10.1186/s12906-020-02924-5
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
