Back to Journals » Cancer Management and Research » Volume 11
Effects of anesthetic technique and surgery on myeloid-derived suppressor cells and prognosis in women who underwent breast cancer surgery: a prospective study
Authors Yan T , Zhang GH, Cheng YZ, Wu LX, Liu XY, Sun YL, Zheng H, Sun L
Received 12 August 2018
Accepted for publication 14 March 2019
Published 18 June 2019 Volume 2019:11 Pages 5513—5522
DOI https://doi.org/10.2147/CMAR.S183519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Beicheng Sun
Tao Yan,1* Guo-Hua Zhang,1* Yao-Zhong Cheng,1* Lin-Xin Wu,1 Xiao-Yan Liu,1 Yu-Lin Sun,2 Hui Zheng*,1 Li Sun3
1Department of Anesthesiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China; 2State Key Laboratory of Molecular Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China; 3Department of Anesthesiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen 518116, People’s Republic of China
*These authors contributed equally to this work
Background: Surgery and anesthesia-induced immunosuppression may play a critical role in tumor progression and metastasis. Myeloid-derived suppressor cells (MDSCs) are highly immunosuppressive myeloid cells, closely linked with tumor staging, clinical therapeutic efficacy and prognosis. This study aims to investigate the effect of anesthetic technique and surgery on the expression of MDSCs and prognosis in women who received breast cancer surgery.
Methods: From March 2016 to January 2017, a total of 80 patients with breast cancer were prospectively enrolled and randomized into two anesthetic groups: sevoflurane-based anesthetic group (SEV; n=38) and propofol-based total intravenous anesthetic group (TIVA; n=42). The expression of MDSCs and prognosis between different anesthetic techniques and stresses of surgical methods were compared. The primary endpoint is the postoperative expression of MDSCs and prognosis between SEV and TIVA groups. The secondary endpoint is the VAS scores at 24 hr post-operation between SEV and TIVA groups.
Results: There was no significant difference in postoperative expression of MDSCs (P=0.202) and prognosis (P=0.138) between SEV and TIVA groups. Compared to breast-conserving surgery (BCS), patients who underwent breast mastectomy had significantly fewer MDSCs (P=0.040) and lower VAS score at 24 hr post-operation (P=0.044), while no significant difference in prognosis was found (P=0.953). When MDSCs were classified as subtypes of granulocytic/polymorphonuclear (PMN)-MDSCs and monocytic (Mo)-MDSCs, it showed higher ratio of Mo-MDSCs (P=0.018) or lower ratio of (PMN)-MDSCs (P=0.022) correlates to later tumor stage.
Conclusion: Sevoflurane and propofol-based anesthesia do not show significant difference in MDSCs expression and prognosis after breast cancer surgery. Compared to BCS, although mastectomy with high extent of surgical stress exhibits lower levels of MDSCs, there is no significant difference in prognosis. The ratio of MDSCs subtype correlates to tumor stage.
Keywords: breast cancer, anesthesia, surgery, propofol, sevoflurane, MDSCs
Introduction
Each year over 1.5 million women are diagnosed with breast cancer globally. Breast cancer is the first leading cause of cancer-related deaths among women.1,2 In China, it was estimated that 278,800 new cases in 2013 and the mortality rate of 69.5%.3,4 Surgery and anesthesia-induced immunosuppression may play a critical role in tumor progression and metastasis.5,6 Myeloid-derived suppressor cells (MDSCs) are critical in immunosuppression for tumor immune escape, elevation of which represents late tumor staging, aggravated progression and poor prognosis.7–9 Nowadays, general anesthesia contains two main classes of drugs: the most widely used volatile inhalation and the alternative propofol. Although many basic science studies suggest the potential benefits of propofol over volatile agents on cell-mediated immunity,5 there is still debate on if propofol was superior to sevoflurane during anesthesia for breast cancer surgery in a clinical environment.10,11 Hitherto, few previous literature focused on the influence of anesthetic techniques on MDSCs clinically. In this study, we compare the effect of anesthetic technique and surgery on the expression of MDSCs in women received breast cancer surgery. The primary endpoint is the postoperative expression of MDSCs and prognosis between sevoflurane-based anesthetic (SEV) and total intravenous anesthetic (TIVA) groups. The secondary endpoint is the VAS scores at 24 hr post-operation between SEV and TIVA groups.
Methods
Study population
Adult female patients scheduled to receive breast cancer resection were recruited between January 2016 and January 2017 at National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Patients were randomly assigned to two general anesthetic groups using a sealed envelope technique: SEV group (sevoflurane anesthesia and remifentanil; n=38) and TIVA group (total intravenous anesthesia with propofol and remifentanil; n=42). Written informed consent was obtained from each patient, and the protocol was approved by the ethical committee/institutional review board of National Cancer Center (NCC EC/IRB) (approval number: NCC2013YZ-06). This study was conducted in accordance with the Declaration of Helsinki.
Female patients between 18 and 70 years of age were eligible for the study if they had histologically confirmed breast cancer with the American Society of Anesthesiologists (ASA) grades I–III. Including sentinel lymph node biopsy or axillary lymph node dissection, mastectomy and breast-conserving surgery (BCS) were performed by the same group of surgeons. Female patients with a previous history of surgery, radiotherapy, or chemotherapy; recent infection or on immunosuppressive drugs; liver/kidney dysfunction; or body mass index <19 or >31 kg per square meter (kg/m2) were excluded from the study. Contraindication history for drug use in other medical research in the past month was another factor for exclusion. Appearance of malignant events during the operation, such as intraoperative blood loss of >300 mL and cardiac arrest, or withdrawal from the study, were the reasons for termination from the trial. Classification of malignant tumors staging (TNM 8th) was performed for the patient using National Comprehensive Cancer Network (NCCN) guideline (2018, Version 4.0).12
Anesthetic procedure
Before endotracheal intubation, all patients were induced intravenously with 1–2 mg per kilogram (mg/kg) of propofol (AstraZeneca, Italy), 2–3 µg per kilogram (μg/kg) of fentanyl (Renfu, China), and 0.6 mg/kg of rocuronium (Organon, Holland). After insertion of a laryngeal mark airway, the patients were mechanically ventilated to maintain the end-tidal carbon dioxide concentration at 35–45 mmHg with a fresh gas flow of 2 L/min oxygen. Anesthesia was maintained in sevoflurane (SEV) or propofol (TIVA). For the SEV group, anesthesia was maintained with sevoflurane (1.5–2%) and rocuronium (0.15 mg/kg). For the TIVA group, anesthesia was maintained with continuous infusion of propofol (3–6 mg/kg/hr), rocuronium (0.15 mg/kg) and remifentanil (0.1–0.2 µg/kg/min) until the end of surgery. To ensure similar anesthetic depth during surgery, bispectral index (BIS) value was maintained between 40 and 60 for both groups. The dose of sevoflurane and propofol were adjusted according to monitoring parameters such as noninvasive blood pressure, heart rate, electrocardiography, pulse oximetry and BIS. Intraoperative fentanyl (1 µg/kg) was added as required. At the end of surgery, NSAIDs of 50 mg flurbiprofen axeil were used in all patients. Postoperative analgesia contained flurbiprofen axeil (50 mg) for all patients and fentanyl (1 µg/kg) if necessary in post-anesthesia care unit and ward. Patient-controlled analgesia was not used for promoting early ambulation after surgery.
Flow cytometry analyses of MDSC
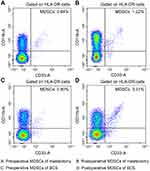
The samples were obtained before induction of anesthesia and 24 hrs after the surgery. Blood cell samples centrifuged from peripheral blood were stored at −80°C and then thawed at 39°C in water bath for analysis. Peripheral blood mononuclear cells were suspended at a concentration of 1×107 mL in PBS buffer (NaCl 137 mmol/L, KCl 2.7 mmol/L, Na2HPO4 4.3 mmol/L, KH2PO4 1.4 mmol/L, pH 7.4). Cells were incubated with fluorescently labeled antibodies at 4°C. The antibodies included CD14 (eBioscience), human leukocyte antigen-D related (HLA-DR) PERCP-Cy5.5-A (eBioscience), CD33PE-A (eBioscience), and CD11b fluorescein isothiocyanate (FITC-A) (eBioscience). Cells were washed with PBS, fixed with 1% formalin, and stored at 4°C until analysis. All analyses were performed using a BD LSR II flow cytometry (BD Biosciences, San Jose, CA, USA).
Follow-up
Adverse reactions and analgesia effect were assessed at 2 and 24 hrs post operation. VAS was used to evaluate analgesic effect. VAS was self-report measure and consisted of a 10 cm line scale (0 signified no pain; 1–3 – mild pain; 4–6 – moderate pain; 7–9 – severe pain; 10 – worst pain the patient had ever experienced).
Overall survival (OS) time of patients was measured from the date of surgery to the date of last follow-up or death. Whether and when a patient had died was obtained from inpatient and outpatient records, patients’ families, or local Public Security Census Register Office through follow-up telephone calls. The last date of follow-up was June 1, 2018 and no patients were lost to follow-up. Patients alive on the last follow-up date were considered censored.
Statistical analysis plan
The primary endpoint was defined a priori as the change in preoperative versus postoperative MDSC. The sample size was calculated based on the preliminary study, we assumed that TIVA group would increase their preoperative MDSC by 200% and that SEV group would demonstrate an attenuated response of 100%, which would nonetheless represent a remarkable numerical change between the groups. Statistical result showed 37 patients should be recruited to each group with a Type I error of 0.05 and a power of 80%. Student t-test or Mann–Whitney rank test was used based on whether the data follow the normal distribution. Categorized variables were analyzed by Chi-square or Fisher exact test. Kaplan-Meier survival was plotted and log-rank tests were used to assess P-values. All statistical analyses were performed using SPSS 25.0 for mac (SPSS, Inc., Chicago, IL, USA). Differences with P-values <0.05 were considered to be statistically significant.
Results
Patient characteristics
A total of 92 patients were eligible for the study. Twelve patients were excluded for following reasons: three cases of contraindicated to NSAIDs, three cases of surgery cancellation, five cases of surgical type change, one case of patient’s withdrawal. Therefore, remnant 80 patients were included in the final analysis (Figure 1), 15 cases for mastectomy and 23 for BCS in the SEV group, 16 cases for mastectomy and 26 for BCS in the TIVA group. Patient characteristics, including age, height, weight, ASA staging, duration of anesthesia, tumor size, carcinoma cell embolus, nerve invasion, estrogen receptor, progesterone receptor, surgical approach, pathology grade as well as TNM staging, showed no significant difference between the SEV group and TIVA group (P>0.05; Table 1).
 | Table 1 Comparison of clinical characteristics of the SEV and TIVA groups |
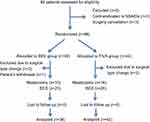 | Figure 1 CONSORT flow diagram.Abbreviations: BCS, breast-conserving surgery; SEV, sevoflurane-based anesthetic; TIVA, total intravenous anesthetic. |
Comparison of perioperative factors in different anesthetic groups
For serological level of MDSCs, analgesia medication, VAS score and adverse reaction, there was no difference for the two anesthetic groups (P>0.05, Table 2, Figure 2).
 | Table 2 Analgesia and adverse reactions and in the SEV and TIVA groups |
Comparison of perioperative factors in different surgical methods
Surgical methods were divided into mastectomy group and BCS group in our study. Our result showed patients who underwent breast mastectomy had significantly fewer MDSCs (P=0.040, Figure 3) and lower VAS score at 24 hr post-operation (P=0.044). For anesthetic method and perioperative analgesia medication, there was no difference in the two groups of surgical methods (Table 3).
 | Table 3 Comparison of perioperative factors in different surgical methods |
Serological MDSCs and tumor stage
There was no significant difference in the correlation of MDSCs and tumor stage (P=0.605). By different expressions of CD14, MDSCs are classified as two main subtypes, monocytic (Mo)-MDSCs (CD11b+CD33+HLA-DR−CD14+) and granulocytic/polymorphonuclear (PMN)-MDSCs (CD11b+CD33+HLA-DR−CD14−). Our study showed higher ratio of Mo-MDSCs (P=0.018) or lower ratio of (PMN)-MDSCs (P=0.022) in MDSCs correlates to later tumor stage (Figure 4).
Survival statistic of the patients
The follow-up time ranged from 6 to 28 months. By the last date of follow-up, recurrence and metastasis occurred in 5 (6.3%) patients, including 4 cases for SEV group and 1 case for TIVA group. SEV group has 2 cases of death but TIVA group has none. For all 80 cases analyzed by Kaplan-Meier analysis, the 1- and 2-year disease-free survival (DFS) rate is 95.0% and 93.8%. The 1- and 2-year OS rate is 100% and 96.2%. The 1-year DFS rate of SEV and TIVA patients were 92.1% and 97.6%, and the 2-year DFS rate of SEV and TIVA patients were 89.5% and 97.6% (P=0.138, Figure 5). The 1-year OS rate of SEV and TIVA patients were 100.0%, respectively, and the 2-year OS rate of SEV and TIVA patients were 92.8% and 100%, respectively (P=0.182, Figure 5). No difference of DFS (P=0.953) and OS (P=0.281) was found in the two surgical methods groups.
Discussion
Immunity plays a critical role in promoting cancer metastasis. MDSCs, produced in the bone marrow from hematopoietic stem cells, are a class of immune suppressor cells inhibiting immune responses by T cells and natural killer cells.13,14 MDSCs are generally defined as being positive for CD33/CD11b, but express low levels of HLA-DR in humans. They account for <1% of circulating tumor cells in normal individuals. Elevated MDSCs are proven to be associated with the tumor patient’s immunosuppression, late stage, poor prognosis and resistance to therapy.15–18 In our study, there was no significant difference in the correlation of tumor stage and MDSCs (P=0.605). By different expressions of CD14, MDSCs are classified as two main subtypes, Monocytic (Mo)-MDSCs (CD11b+CD33+HLA-DR−CD14+) and granulocytic/polymorphonuclear (PMN)-MDSCs (CD11b+CD33+HLA-DR−CD14−).7,9 The two subtypes inhibit immune responses via different mechanisms,19 Mo-MDSCs are related to more severe disease, more severe stage and metastases, while (PMN)-MDSCs correlate with poor OS in breast cancer.7 Our result is similar to the conclusion of previous literature, higher ratio of Mo-MDSCs or lower ratio of (PMN)-MDSCs correlates to later tumor stage (Figure 4). In Verma et al.’s study, Women with locally advanced breast cancers have abnormal blood regulatory cell levels (Tregs and MDSCs) and cytokine profiles (Th1, Th2, Th17). Neoadjuvant chemotherapy followed by surgery failed to abolish the abnormal Treg and Th profiles. There was a significant correlation between the circulatory levels of Tregs and the pathological response of the breast cancers to neoadjuvant chemotherapy.20
There have long been some debates on effects of immunity and tumor progression for volatile anesthesia and intravenous anesthesia (TIVA). Many studies confirmed that volatile anesthesia suppresses cell-mediated immunity and promotes angiogenesis and cancer cell proliferation, whereas propofol, the most commonly used agent for TIVA, does not suppress cell-mediated immunity5,20,21 and has better prognosis.22 While the other works showed no significant differences between these two techniques in immunity, tumor progression and prognosis.10,11,23 In our study, it revealed propofol- and sevoflurane-based anesthesia during breast cancer surgery did not affect postoperative MDSCs (P=0.202, Figure 2) and prognosis (P=0.138 for DFS, P=0.182 for OS, Figure 5–6).
Surgery-induced stress responses play an important role in stimulating MDSCs growth and attenuating cell-mediated immunity by metabolic and neuroendocrine interruptions.5,9,24 As various surgical extents induce different stress responses,24 surgical methods are divided to mastectomy group and BCS group in our study. We hypothesized that breast mastectomy with high extent of surgical stress might exhibit higher levels of MDSCs. Of note, similar to previous literature result,24 our result showed patients who underwent breast mastectomy had significantly fewer MDSCs (P=0.040). Opioids and pain have well been confirmed to be associated with immune depression and tumor progression,25,26 both maybe the cause for our result. The factor of opioids was excluded because of the same dosage of perioperative opioids were applied in the two surgical method group. For the factor of pain, previous literatures demonstrated greater the pain cause fewer the MDSC levels.24 In our study, the patients receiving breast mastectomy had less VAS value than the alternative group at 24 hr post-operation (P=0.044). Compared to BCS, eradication of the remaining nerves in mastectomy maybe the reason for lower VAS score and lower MDSCs elevation. Although postoperative MDSCs are different between the two surgical groups, no prognostic difference was found for different surgical methods. This is in accordance to our clinic principle of treatment, namely, patients receiving mastectomy or radiotherapy assisted BCS have the same prognosis.
There are certain limitations in our study. Firstly, fentanyl’s immune influence and propofol’s induction interference in inhalation group all make it difficult to differentiate the properties of sevoflurane and propofol on immune response, respectively. Of note, the aim of our study is to provide clinical reference in certain anesthetic technique comparison rather than single drug comparison. Furthermore, as analgesia affects immunity and sevoflurane itself has analgesic effect, it is difficult to precisely achieve and control the same analgesic effect for the two anesthetic groups. Lastly, long-term survival follow-up and multi-centered studies need to be performed.
Our present study has some limitations. Firstly, the most important part of this study is whether the difference between sevoflurane and propofol-based anesthetic technique is associated with breast cancer recurrence. Despite the short follow-up period, the relapse rate of the TIVA group appears to be lower than that of the SEVO group. However, the follow-up period is 6–28 months, which seems somewhat insufficient to evaluate the effects of the anesthesia method on the recurrence and prognosis of breast cancer in patients undergoing a breast surgery. We are still constantly following the patient’s conditions. When the follow-up period is longer enough, we will provide updated and more accurate reports in a timely manner. Secondly, we only observed and analyzed the ratio of MDSCs in the SEVO group and the TIVA group, and found the proportion of MDSCs was related to the advanced tumor stage. With respect to cell-mediated immunity affected by anesthetic technique, changes in NK, T cell subsets, and immunosuppressive cytokines need to be assessed in addition to MDSCs. We understand that our results would be more convincing if the changes in NK, T cell subsets, and immunosuppressive cytokines were also discussed. However, in the present study, we mainly focused on the effects of anesthetic technique and surgery on MDSCs and prognosis in women underwent breast cancer surgery, and we think that should be enough draw a conclusion to some degree. We would love to add the analyzation of changes in NK, T cell subsets, and immunosuppressive cytokines in our following follow-up and the future studies.
In conclusion, the ratio of either MDSCs subtype, (PMN)-MDSCs and monocytic (Mo)-MDSCs, correlates to tumor stage. Although many previous works have suggested the potential benefits of propofol over volatile agent in cancer surgery, our study showed propofol was not superior to sevoflurane, on the aspects of serological MDSCs and prognosis after breast cancer surgery. Compared to BCS, patients who underwent breast mastectomy had significantly lower VAS score and fewer MDSCs, while there is no significant difference in prognosis.
Acknowledgments
This study was supported by the grants from Beijing Municipal Science and Technology Project (No. Z151100003915109), Beijing Hope Run Special Fund of Cancer Foundation of China (No. LC2013A18), Beijing Hope Run Special Fund of Cancer Foundation of China (No. LC2017B25), Teaching Reform Project of Peking Union Medical College (10023201800307), and Sanming Project of Medicine in Shenzhen, Cancer Pain Treatment and Perioperative Medical Team of Professor Li Sun in Cancer Hospital, Chinese Academy of Medical Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130:1237–1250. doi:10.1002/ijc.26448
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi:10.3322/caac.21442
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338
4. Zheng R, Zeng H, Zhang S, Chen W. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer. 2017;36:66. doi:10.1186/s40880-017-0234-3
5. Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 2018;16:8. doi:10.1186/s12967-018-1389-7
6. Kim R. Anesthetic technique for cancer surgery: harm or benefit for cancer recurrence? Eur J Surg Oncol. 2018;44:557–558. doi:10.1016/j.ejso.2018.02.207
7. Seo EH, Namgung JH, Oh CS, Kim SH, Lee SH. Association of chemokines and chemokine receptor expression with monocytic-myeloid-derived suppressor cells during tumor progression. Immune Netw. 2018;18:e23. doi:10.4110/in.2018.18.e23
8. Ma M, Huang W, Kong D. IL-17 inhibits the accumulation of myeloid-derived suppressor cells in breast cancer via activating STAT3. Int Immunopharmacol. 2018;59:148–156. doi:10.1016/j.intimp.2018.04.013
9. Markowitz J, Wesolowski R, Papenfuss T, Brooks TR, Carson WE
10. Lim JA, Oh CS, Yoon TG, et al. The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: an in vitro analysis. BMC Cancer. 2018;18:159. doi:10.1186/s12885-018-4242-8
11. Kim R, Kawai A, Wakisaka M, et al. Differences in immune response to anesthetics used for day surgery versus hospitalization surgery for breast cancer patients. Clin Transl Med. 2017;6:34. doi:10.1186/s40169-017-0163-4
12.
13. Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–281. doi:10.1016/j.semcancer.2012.01.011
14. O‘Connor MA, Fu WW, Green KA, Green WR. Subpopulations of M-MDSCs from mice infected by an immunodeficiency-causing retrovirus and their differential suppression of T- vs B-cell responses. Virology. 2015;485:263–273. doi:10.1016/j.virol.2015.07.020
15. Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–220. doi:10.1159/000127412
16. Malik HZ, Prasad KR, Halazun KJ, et al. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246:806–814. doi:10.1097/SLA.0b013e318142d964
17. Neal CP, Mann CD, Sutton CD, et al. Evaluation of the prognostic value of systemic inflammation and socioeconomic deprivation in patients with resectable colorectal liver metastases. Eur J Cancer. 2009;45:56–64. doi:10.1016/j.ejca.2008.08.019
18. Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi:10.1007/s00262-008-0523-4
19. Khaled YS, Ammori BJ, Elkord E. Increased levels of granulocytic myeloid-derived suppressor cells in peripheral blood and tumour tissue of pancreatic cancer patients. J Immunol Res. 2014;2014:879897. doi:10.1155/2014/394127
20. Verma C, Eremin JM, Robins A, et al. Abnormal T regulatory cells (Tregs: FOXP3+, CTLA-4+), myeloid-derived suppressor cells (MDSCs: monocytic, granulocytic) and polarised T helper cell profiles (Th1, Th2, Th17) in women with large and locally advanced breast cancers undergoing neoadjuvant chemotherapy (NAC) and surgery: failure of abolition of abnormal treg profile with treatment and correlation of treg levels with pathological response to NAC. J Transl Med. 2013;11:16.
21. Yap A, Lopez-Olivo MA, Dubowitz J, Hiller J, Riedel B, Global Onco-Anesthesia Research Collaboration Group. Anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia. Can J Anaesth. 2019;66(5):546–561. doi: 10.1007/s12630-019-01330-x.
22. Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124:69–79. doi:10.1097/ALN.0000000000000936
23. Lai R, Peng Z, Chen D, et al. The effects of anesthetic technique on cancer recurrence in percutaneous radiofrequency ablation of small hepatocellular carcinoma. Anesth Analg. 2012;114:290–296. doi:10.1213/ANE.0b013e318239c2e3
24. Mundy-Bosse BL, Thornton LM, Yang HC, Andersen BL, Carson WE. Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol. 2011;270:80–87. doi:10.1016/j.cellimm.2011.04.003
25. Koo KC, Park SU, Kim KH, et al. Prognostic impacts of metastatic site and pain on progression to castrate resistance and mortality in patients with metastatic prostate cancer. Yonsei Med J. 2015;56:1206–1212. doi:10.3349/ymj.2015.56.5.1206
26. Mercadante S, Porzio G, Adile C, et al. Pain intensity as prognostic factor in cancer pain management. Pain Pract. 2015;15:E1–E8. doi:10.1111/papr.12259
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.