Back to Journals » Clinical Interventions in Aging » Volume 15
Effects of an Alveolar Recruitment Maneuver During Lung Protective Ventilation on Postoperative Pulmonary Complications in Elderly Patients Undergoing Laparoscopy
Authors Jo YY, Lee KC, Chang YJ, Jung WS, Park J, Kwak HJ
Received 28 May 2020
Accepted for publication 7 August 2020
Published 24 August 2020 Volume 2020:15 Pages 1461—1469
DOI https://doi.org/10.2147/CIA.S264987
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Walker
Youn Yi Jo, Kyung Cheon Lee, Young Jin Chang, Wol Seon Jung, Jongchul Park, Hyun Jeong Kwak
Department of Anesthesiology and Pain Medicine, Gachon University College of Medicine, Gil Hospital, Incheon, Republic of Korea
Correspondence: Hyun Jeong Kwak Email [email protected]
Purpose: Controversy remains over whether alveolar recruitment maneuvers (ARMs) can reduce postoperative pulmonary complications. We hypothesized that performing an ARM in addition to lung protective ventilation (LPV) could improve intraoperative arterial oxygenation and postoperative pulmonary complications (PPCs) in elderly patients undergoing laparoscopy in the Trendelenburg position.
Patients and Methods: Sixty-two patients (aged 65– 85) scheduled for laparoscopic low anterior resection were randomized to receive LPV only (LPV group, n = 32) or LPV with an ARM (ARM group, n = 30). LPV was set to a tidal volume of 6 mL/kg with a positive end expiratory pressure (PEEP) of 5 cmH2O. The ARM was performed by serially increasing the PEEP to 10 cmH2O for 3 breaths, 15 cmH2O for 3 breaths, then 20 cmH2O for 10 breaths, both immediately before and after abdominal insufflation. The primary end-point was the frequency of PPCs such as desaturation (SpO2 < 90%), atelectasis, and pneumonia. Secondary end-points were changes in intraoperative respiratory and gas exchange parameters and hemodynamic variables.
Results: One patient in the LPV group experienced desaturation on the first postoperative day. The frequency of chest X-ray abnormalities such as atelectasis or pleural effusion was comparable between groups (6 (19%) and 5 (17%) patients, respectively, P = 0.676). Changes in other respiratory, gas exchange and hemodynamic parameters over time were not significantly different between the groups. However, vasopressor requirements during surgery were higher in the ARM than the LPV group (9 (30%) and 2 (6%) patients, respectively, P = 0.014).
Conclusion: This study suggests that performing an ARM during LPV may not improve postoperative respiratory outcomes and intraoperative oxygenation compared to LPV alone in geriatric patients undergoing laparoscopy in the Trendelenburg position. In addition, since the ARM could cause a significant deterioration in hemodynamic parameters, applying ARM to elderly patients should be carefully considered.
Keywords: alveolar recruitment maneuver, geriatric, lung protective ventilation, pneumoperitoneum
Introduction
For patients undergoing major abdominal surgery under general anesthesia, a laparoscopic as opposed to an open approach is associated with increased life expectancy due to more rapid recovery.1 Despite the development of minimally invasive surgical procedures, age-related changes in lung physiology such as the deterioration in respiratory system compliance, as well as a diminished response to hypoxemia and a decrease in other protective airway reflexes, still increase the chance of developing postoperative pulmonary complications (PPCs).2 PPCs are associated with a prolonged hospital stay and the need for intensive care, leading to an increase in health care costs.3 Also, the closing volume tends to exceed functional residual capacity with aging,4 and this change leads to abnormal intraoperative gas exchange and postoperative atelectasis.2 Carbon dioxide (CO2) pneumoperitoneum during laparoscopy in the Trendelenburg position decreases lung volume and lung compliance,5 and respiratory compliance might not completely recover even after the cessation of pneumoperitoneum.6
Most lung protective ventilation (LPV) strategies to minimize ventilator-induced lung injury are based upon low tidal volume ventilation and permissive hypercapnia.7 The use of alveolar recruitment maneuvers (ARMs) for recruiting collapsed alveoli is a ventilation strategy involving transient or staircase elevations in transpulmonary pressure. A recent meta-analysis of 12 trials involving a total of 2756 anesthetized patients reported that using an ARM in combination with LPV reduced the incidence of PPCs in non-obese patients.8 Another study showed that the combination of an ARM and positive end-expiratory pressure (PEEP) significantly improved oxygenation and reduced atelectasis, but PEEP or an ARM alone did not improve these parameters in obese patients.9
Despite the clinical importance of perioperative respiratory care in elderly patients, there is little literature examining the reduction of PPCs after laparoscopic surgery in elderly patients. We hypothesized that performing an ARM in addition to LPV might improve intraoperative oxygenation and respiratory parameters, and thus would lead to reducing the PPCs. Therefore, the aim of this prospective randomized study was to evaluate the effect of ARM on PPCs as well as intraoperative oxygenation, respiratory mechanics, and hemodynamic variables in elderly patients undergoing laparoscopic surgery.
Methods
Patients
This study was approved by the Gachon University Gil Hospital Institutional Review Board (Ref: GBIRB2017-270) and registered at ClinicalTrials.gov (NCT 03331471). Prior written informed consent was obtained from all eligible participants. This study is conducted in accordance with the Declaration of Helsinki. In this prospective observational study, 64 patients scheduled for elective laparoscopic low anterior resection for colorectal cancer were included. This study included patients aged 65–85 with an ASA physical status of 1 or 2, and excluded patients with active infectious lung disease, moderate to severe obstructive or restrictive lung disease on pulmonary function test, symptomatic cardiovascular disease besides proper medication, and cerebrovascular disease with neurologic sequelae. Participants were randomly assigned to the lung protective ventilation group (LPV group; N = 32) or the alveolar recruitment maneuver group (ARM group; N = 32) using the randomization function in Excel 2013 (Microsoft office, Redmond, WA) without stratification.
Anesthesia and Study Setting
Patients were not premedicated with any sedatives or analgesics. In the operating room, routine anesthesia monitors such as a pulse oximeter, a non-invasive blood pressure monitor, and an electrocardiograph were employed. Neuromuscular blockade was monitored using E-NMT module (GE Healthcare, Helsinki, Finland) to obtain a train-of-four (TOF) ratio, which was measured every 15 s. For the induction of anesthesia, lidocaine (1 mg/kg), remifentanil (0.5–1.0 μg/kg), propofol (1–2 mg/kg), and rocuronium (0.8 mg/kg) were administered. For the maintenance of anesthesia, the concentration of sevoflurane used (1.5–2 vol%) was adjusted guided by the bispectral index score aiming to maintain between 40 and 60. To enable blood sampling and continuous blood pressure monitoring, a 22-G radial arterial catheter was inserted after the induction of anesthesia. Target TOF ratio was under 0.3 during anesthesia and ARM. During emergence, extubation was considered after recover the TOF ratio over 0.9. The mechanical ventilator for LPV was set in volume-controlled mode based on a tidal volume of 6 mL/kg of ideal body weight (defined in kg as 0.919 × (height in cm − 152.4) + 45.5 for women, or + 50 for men), an inspiratory to expiratory (I/E) ratio of 1:2, a PEEP of 5 cmH2O, and an inspired oxygen fraction (FiO2) of 0.5. The level of PEEP was determined based on the previous study.10 The respiratory rate was adjusted to maintain an end-tidal carbon dioxide tension (ETCO2) between 35 and 45 mmHg. For the LPV group, the initial ventilator settings were maintained throughout the surgery. For the ARM group, an additional ARM was performed during LPV immediately before and after abdominal insufflation with CO2 gas. This ARM serially increased the PEEP from the initial ventilator settings to 10 cmH2O for 3 breaths, 15 cmH2O for 3 breaths, then 20 cmH2O for 3 breaths. The peak airway pressure (Ppeak) during ARM was limited to 40 cmH2O. The insufflation gas pressure for pneumoperitoneum was limited to 12 mmHg. After gas insufflation, the patient was moved from the supine position into the 30° Trendelenburg position.
When mean arterial pressure (MAP) decreased below 80% of the baseline value during surgery, either 50 μg phenylephrine or 5 mg ephedrine was used, depending on the heart rate (HR). The requirement of vasopressors in each patient was recorded. At 10 min after anesthesia induction, 1 min after the induction of pneumoperitoneum, 60 min after the induction of pneumoperitoneum, the end of pneumoperitoneum, and the end of the operation, respiratory parameters including ETCO2 and Ppeak and plateau airway pressure (Pplat) were recorded, and arterial blood gas analysis was performed. Alveolar oxygen tension was calculated using the following equation: Alveolar oxygen tension = FiO2 × (760 − 47 mmHg) − (PaCO2/0.8), and the alveolar to arterial oxygen tension gradient (AaDO2) was estimated. Alveolar dead space fraction (Vd/Vt) was calculated using the following equation: Vd/Vt = 1.135 × (PaCO2 − ETCO2)/(PaCO2 − 0.005) × 100.11 Dynamic and static lung compliance (Cdyn and Cstat) were calculated using the following equations: Cdyn = tidal volume/(Ppeak − PEEP); Cstat = tidal volume/(Pplat – PEEP).
If the patient subjectively complained of dyspnea in the ward within 48 hours after the operation, oxygen saturation was measured. All enrolled patients were enrolled for chest X-ray on the first and second post-operative day. PPCs are defined as the presence of at least one of the following: the presence of desaturation (defined as SpO2 <90% in room air), clinical signs and symptoms including respiratory infection, respiratory failure and bronchospasm, and abnormal chest X-ray findings including pleural effusion, atelectasis, pneumothorax, aspiration pneumonitis and pulmonary edema.12
Statistical Analysis
The primary outcome was the incidence of PPCs in the first 2 days of the postoperative period. Sample size was calculated based on a previous study13 comparing the prevalence of PPCs after anesthetic protocols with and without an ARM (47% and 14%, respectively). In that study, 29 patients were required in each group to achieve an α-error probability of 0.05 and a power of 80%; we, therefore, recruited 32 patients to each group, considering possible drop-outs.
SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis. Results were presented as mean ± standard deviation (SD) or as the number of patients (%). The normality of variable distribution was tested using the Kolmogorov–Smirnov test. Continuous variables were compared using the independent t-test or the Mann–Whitney U-test, and categorical variables were compared using Fisher’s exact test or the chi square test as appropriate. Changes in hemodynamic and respiratory variables were analyzed using a 2-way repeated measures ANOVA with two factors (group and time). P values <0.05 were considered to be statistically significant.
Results
Participants and Preoperative Data
A total of 64 patients were enrolled in this prospective study; two patients in the ARM group were excluded from analysis due to a change in the surgical plan (Figure 1). Patient characteristics and preoperative respiratory variables (arterial blood gas analysis and pulmonary function test results) were comparable between the groups (Table 1).
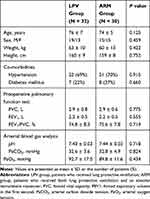 |
Table 1 Patient Characteristics and Preoperative Pulmonary Variables |
 |
Figure 1 Patient allocation flow diagram. |
Intraoperative Data
Intraoperative data including operation time, anesthesia time, and pneumoperitoneum time were comparable between the groups (Table 2).
 |
Table 2 Intraoperative Data |
Intraoperative Changes of Gas Exchange and Dead Space Fraction
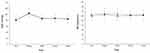
The changes in PaO2, PaCO2, AaDO2, and Vd/Vt during surgery are illustrated in Figure 2. There were no significant differences between the groups in the changes in PaO2, PaCO2, AaDO2, and Vd/Vt over time (group-by-time interaction P = 0.615, 0.818, 0.624, and 0.440, respectively). There were also no significant differences between the groups in the changes in intraoperative respiratory mechanics over time (group-by-time interactions for Ppeak, Pplat, Cdyn, and Cstat: P = 0.988, 0.897, 0.653, and 0.275, respectively, data not shown).
Intraoperative Hemodynamic Changes
The changes in hemodynamics are illustrated in Figure 3. The differences between the groups in the changes in MAP and HR over time were not significant (group-by-time interaction P = 0.736 and 0.599, respectively). However, the number of patients required vasopressor during surgery was significantly higher in the ARM group than in the LPV group (9 (30%) vs 2 (6%), P = 0.014, Table 2).
Postoperative Pulmonary Complications
One patient in the LPV group had the symptom of dyspnea and showed postoperative desaturation and pulmonary edema on the chest X-ray. The detection of abnormalities on chest X-ray such as atelectasis, pneumonia, or pulmonary edema was comparable between the groups (6 patients (19%) in the LPV group vs 5 patients (17%) in the ARM group, P = 0.692). The number of patients who needed admission to the intensive care unit was comparable between the groups (Table 3).
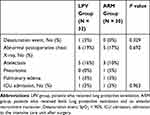 |
Table 3 Pulmonary Complications Within the First Two Days Postoperatively |
Discussion
This study suggests that the additional use of an ARM during LPV may not significantly improve postoperative respiratory outcomes or intraoperative oxygenation compared to LPV alone in elderly patients undergoing laparoscopy in the Trendelenburg position. In addition, the ARM may cause a significant deterioration in hemodynamic parameters during the surgery.
Nearly 100% of patients experience atelectasis during general anesthesia; this could contribute to postoperative pulmonary morbidity rates.14,15 Decreases in lung compliance and functional residual capacity, as well as a high concentration of inspired oxygen, contribute to anesthesia-induced atelectasis.14 A previous study of LPV reported that low tidal volume ventilation with low PEEP during laparoscopy could reduce the incidence of PPCs as compared to conventional high tidal volume ventilation with an ARM.13 Another clinical study reported that the postoperative radiological atelectasis score after robot-assisted laparoscopic radical prostatectomy was significantly lower in patients maintained with a low tidal volume of 6 mL/kg and a PEEP of 5cmH2O than in patients maintained with a high tidal volume of 10 mL/kg and without PEEP.16 In a single-group study, the performance of an ARM followed by 5 cmH2O PEEP after pneumoperitoneum was found to increase transpulmonary pressure and lead to improved alveolar recruitment and gas exchange during laparoscopy in the Trendelenburg position.17 In this study, the level of PEEP was decided based on previous studies.10,18 The study by Meininger et al10 showed that the application of 5cmH2O of PEEP significantly improved arterial oxygenation without hemodynamic deterioration during prolonged pneumoperitoneum in elderly patients. In addition, in the meta-analysis of the effect of PEEP on postoperative pulmonary complication, the usual level of PEEP studied was from 5 cmH2O to 10 cmH2O during anesthesia.18
The ARM reopens collapsed alveoli and induces the release of surfactant at an even distribution across the alveolar surface.11,14,15 There are several techniques in ARM method, such as sustained inflation followed by decremental PEEP, step-wise recruitment (incremental PEEP), airway pressure release ventilation, or high-frequency oscillation ventilation. A previous review reported that ARMs could improve intraoperative PaO2 and lung compliance regardless of the ARM method used; this review concluded that an ARM followed by PEEP after the induction of anesthesia might reduce PPCs.19 Meanwhile, a recent meta-analysis reported that ARM combining with LPV reduced the occurrence of PPCs and improved oxygenation in non-obese patients.8 Although they did not conclude the ideal strategy as the variable techniques of ARM, they suggested that sustained inflation might be better than step-wise recruitment on the development of PPC.8
We applied ARM immediately before and after pneumoperitoneum based on previous studies.20,21 A previous clinical study that examined the effect of preemptive ARM only before pneumoperitoneum reported that preemptive ARM improved arterial oxygenation and AaDO2 during the laparoscopic hysterectomy.20 Also, in the thoracic surgery, preemptive ARM before one lung ventilation effectively improves arterial oxygenation during the surgery.21 However, in this study, performing an ARM during LPV did not improve intraoperative oxygenation profiles and respiratory mechanics in elderly patients as compared to LPV alone. These results were consistent with those of Choi et al,22 who found that a single ARM before the induction of pneumoperitoneum did not improve intraoperative respiratory mechanics and oxygenation during robotic-assisted radical prostatectomy in elderly patients. There are a few possible explanations for the decreased effect of ARMs in elderly patients. The first explanation is that, with aging, the loss of elastic recoil associated with the reduction in power of expiratory muscles can lead to alveolar hyperinflation and faster re-collapse of alveoli recruited by the ARM. This means that the effect of ARMs in elderly patients can be diminished.22 Furthermore, the loss of elastic recoil leads to an increase in residual volume and a decrease in vital capacity.23 In addition, a previous animal study suggested that protein deficiency, which can develop in old age, might lead to the degradation of surfactant and therefore impair lung function.24 In another animal study, decreased phospholipid content of surfactant was noticed with age; this might be caused by age-related pulmonary changes.25 Thus, surfactant protein deficiency or a decrease in phospholipid content may impair lung function in old age, which could limit the effect of the ARM in this study. There are several options for ARM. We only applied ARM before and after pneumoperitoneum, but if we applied it with other options, such as hourly or every 30 min applying of ARM,26 we could have a more favorable effect on PPC or arterial oxygenation in elderly patients. Thus, further studies might be needed to find the ideal moments to re-open the de-recruited lungs.
In contrast to an earlier study of elderly patients undergoing laparoscopy,22 this study found that the ARM did not reduce the rate of PPCs. This discrepancy may result from differences in the age of enrolled patients and in anesthetic protocols. As mentioned earlier, age is a major risk factor for PPCs, and the average patient age in this study was about 10 years older than in the previous study.22 The anesthesia method can also affect postoperative pulmonary function and complications, and it is important to note that propofol was used in the previous study22 while sevoflurane was used in this study. It has been found that the postoperative decrease in forced vital capacity is greater after intravenous anesthesia with propofol than after balanced anesthesia with sevoflurane in patients undergoing surgery in the prone position.27 In addition, another study found that in comparison to propofol anesthesia, sevoflurane anesthesia for lung resection surgery was associated with a reduced incidence of PPCs. This reduction was linked to the attenuation of systemic and pulmonary inflammatory responses.28
The increased intrathoracic pressure induced by ARMs significantly reduces venous return, decreases right ventricular filling, and consequently decreases stroke volume.29 In various lung injury models including pneumonia, oleic acid injury, and ventilator-induced lung injury, ARM has been shown to profoundly decrease cardiac output.30 Furthermore, restoration of cardiac output after ARMs in that study took 5–15 minutes. In addition, the increase in intra-abdominal pressure induced by the pneumoperitoneum itself compresses the inferior vena cava and reduces venous return.31 In the ARDS patients, a stepwise recruitment maneuver was preferred over sustained inflation because of the benefit of less hemodynamic compromise.32 Although we applied a stepwise ARM considering the hemodynamic vulnerabilities in old age, vasopressor use was significantly higher in the ARM group than in the LPV group in our study. It seems that the negative hemodynamic effects of the ARM were enhanced by old age and pneumoperitoneum in this study.
There are some limitations in the present study that warrant consideration. First, we excluded patients with uncontrolled obstructive respiratory disease, active inflammatory lung disease, or cerebrovascular disease. Considering the high frequency of these morbidities in the elderly population, our results cannot be generalized to all elderly patients. Second, we assessed PPCs only in the first 48 hours of the postoperative period. The European Perioperative Clinical Outcome (EPCO) definitions and other published definitions for PPCs also include clinical symptoms and laboratory findings, and do not limit the time period of assessment.33 Though we studied the effectiveness of an ARM in reducing atelectasis up to 2 days postoperatively, as this is the time period in which most cases of atelectasis occur, we did not assess PPCs that occurred thereafter. Further studies conducted in a larger population over a longer time period may be needed to obtain more definitive results. Third, we only recorded postoperative desaturation via pulse oximeter in the patients with dyspnea in the general ward. However, SpO2 might not reliably predict the equivalent change in arterial oxygenation in critically ill patients,34 and dyspnea and desaturation are not always consistent in elderly people.35 In this study, because the definition of PPCs does not include the sign of dyspnea and all patients had chest X-rays at 1st and 2nd postoperative day regardless of symptoms, no patient may be missing from the data of PPCs. Fourth, although we calculated sample size based on a previous report,13 our calculation may be short for the detection of severe complications, because this previous study did not involve elderly patients. We may have overlooked the fact that the risk of PPC doubles from 60 to 69 years and triples from 70 to 79 years.36
Conclusion
In conclusion, performing an ARM during LPV may not significantly improve respiratory parameters or postoperative respiratory outcomes compared to LPV alone in elderly patients undergoing laparoscopy in the Trendelenburg position. In addition, since the ARM could cause significant deterioration in hemodynamic parameters, applying ARM to elderly patients should be carefully considered.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Weber DM. Laparoscopic surgery: an excellent approach in elderly patients. Arch Surg. 2003;138(10):1083–1088. doi:10.1001/archsurg.138.10.1083
2. Sprung J, Gajic O, Warner DO. Review article: age related alterations in respiratory function – anesthetic considerations. Can J Anaesth. 2006;53(12):1244–1257. doi:10.1007/BF03021586
3. Shander A, Fleisher LA, Barie PS, Bigatello LM, Sladen RN, Watson CB. Clinical and economic burden of postoperative pulmonary complications: subject safety summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med. 2011;39(9):2163–2172. doi:10.1097/CCM.0b013e31821f0522
4. Zaugg M, Lucchinetti E. Respiratory function in the elderly. Anesthesiol Clin North Am. 2000;18(1):47–58. doi:10.1016/s0889-8537(05)70148-6
5. Jo YY, Kim JY, Chang YJ, Lee S, Kwak HJ. The effect of equal ratio ventilation on oxygenation, respiratory mechanics, and cerebral perfusion pressure during laparoscopy in the trendelenburg position. Surg Laparosc Endosc Percutan Tech. 2016;26(3):221–225. doi:10.1097/SLE.0000000000000276
6. Oikkonen M, Tallgren M. Changes in respiratory compliance at laparoscopy: measurements using side stream spirometry. Can J Anaesth. 1995;42(6):495–497. doi:10.1007/BF03011687
7. Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354. doi:10.1056/NEJM199802053380602
8. Cui Y, Cao R, Li G, Gong T, Ou Y, Huang J. The effect of lung recruitment maneuvers on post-operative pulmonary complications for patients undergoing general anesthesia: a meta-analysis. PLoS One. 2019;14(5):e0217405. doi:10.1371/journal.pone.0217405
9. Reinius H, Jonsson L, Gustafsson S, et al. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: a computerized tomography study. Anesthesiology. 2009;111(5):979–987. doi:10.1097/ALN.0b013e3181b87edb
10. Meininger D, Byhahn C, Mierdl S, Westphal K, Zwissler B. Positive end-expiratory pressure improves arterial oxygenation during prolonged pneumoperitoneum. Acta Anaesthesiol Scand. 2005;49(6):778–783. doi:10.1111/j.1399-6576.2005.00713.x
11. Dietl P, Frick M, Mair N, Bertocchi C, Haller T. Pulmonary consequences of a deep breath revisited. Biol Neonate. 2004;85(4):299–304. doi:10.1159/000078176
12. Canet J, Gallart L, Gomar C, et al. ARISCAT group. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–1350. doi:10.1097/ALN.0b013e3181fc6e0a
13. Park SJ, Kim BG, Oh AH, Han SH, Han HS, Ryu JH. Effects of intraoperative protective lung ventilation on postoperative pulmonary complications in patients with laparoscopic surgery: prospective, randomized and controlled trial. Surg Endosc. 2016;30(10):4598–4606. doi:10.1007/s00464-016-4797-x
14. Hedenstierna G, Edmark L. Mechanisms of atelectasis in the perioperative period. Best Pract Res Clin Anaesthesiol. 2010;24(2):157–169. doi:10.1016/j.bpa.2009.12.002
15. Güldner A, Kiss T, Serpa Neto A, et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology. 2015;123(3):692–713. doi:10.1097/ALN.0000000000000754
16. Haliloglu M, Bilgili B, Ozdemir M, Umuroglu T, Bakan N. Low tidal volume positive end-expiratory pressure versus high tidal volume zero-positive end-expiratory pressure and postoperative pulmonary functions in robot-assisted laparoscopic radical prostatectomy. Med Princ Pract. 2017;26(6):573–578. doi:10.1159/000484693
17. Cinnella G, Grasso S, Spadaro S, et al. Effects of recruitment maneuver and positive end-expiratory pressure on respiratory mechanics and transpulmonary pressure during laparoscopic surgery. Anesthesiology. 2013;118(1):114–122. doi:10.1097/ALN.0b013e3182746a10
18. Imberger G, McIlroy D, Pace NL, Wetterslev J, Brok J, Møller AM. Positive end-expiratory pressure (PEEP) during anaesthesia for the prevention of mortality and postoperative pulmonary complications. Cochrane Database Syst Rev. 2010;8(9):CD007922. doi:10.1002/14651858.CD007922.pub2
19. Hartland BL, Newell TJ, Damico N. Alveolar recruitment maneuvers under general anesthesia: a systematic review of the literature. Respir Care. 2015;60(4):609–620. doi:10.4187/respcare.03488
20. Park HP, Hwang JW, Kim YB, et al. Effect of pre-emptive alveolar recruitment strategy before pneumoperitoneum on arterial oxygenation during laparoscopic hysterectomy. Anaesth Intensive Care. 2009;37(4):593–597. doi:10.1177/0310057X0903700419
21. Park SH, Jeon YT, Hwang JW, Do SH, Kim JH, Park HP. A preemptive alveolar recruitment strategy before one-lung ventilation improves arterial oxygenation in patients undergoing thoracic surgery: a prospective randomised study. Eur J Anaesthesiol. 2011;28(4):298–302. doi:10.1097/EJA.0b013e3283436fdb
22. Choi ES, Oh AY, In CB, Ryu JH, Jeon YT, Kim HG. Effects of recruitment manoeuvre on perioperative pulmonary complications in patients undergoing robotic assisted radical prostatectomy: a randomised single-blinded trial. PLoS One. 2017;12(9):e0183311. doi:10.1371/journal.pone.0183311
23. Oyarzún GM. Pulmonary function in aging. Rev Med Chil. 2009;137(3):411–418. doi:/S0034-98872009000300014
24. Walski M, Pokorski M, Antosiewicz J, et al. Pulmonary surfactant: ultrastructural features and putative mechanisms of aging. J Physiol Pharmacol. 2009;60 Suppl 5(Suppl50):121–125.
25. Christmann U, Hite RD, Witonsky SG, et al. Influence of age on surfactant isolated from healthy horses maintained on pasture. J Vet Intern Med. 2009;23(3):612–618. doi:10.1111/j.1939-1676.2009.0298.x
26. Neuschwander A, Futier E, Jaber S, et al. The effects of intraoperative lung protective ventilation with positive end-expiratory pressure on blood loss during hepatic resection surgery: a secondary analysis of data from a published randomised control trial (IMPROVE). Eur J Anaesthesiol. 2016;33(4):292–298. doi:10.1097/EJA.0000000000000390
27. Tiefenthaler W, Pehboeck D, Hammerle E, Kavakebi P, Benzer A. Lung function after total intravenous anaesthesia or balanced anaesthesia with sevoflurane. Br J Anaesth. 2011;106(2):272–276. doi:10.1093/bja/aeq321
28. de la Gala F, Piñeiro P, Reyes A, et al. Postoperative pulmonary complications, pulmonary and systemic inflammatory responses after lung resection surgery with prolonged one-lung ventilation. Randomized controlled trial comparing intravenous and inhalational anaesthesia. Br J Anaesth. 2017;119(4):655–663. doi:10.1093/bja/aex230
29. Fessler HE, Brower RG, Wise RA, Permutt S. Effects of positive end-expiratory pressure on the gradient for venous return. Am Rev Respir Dis. 1991;143(1):19–24. doi:10.1164/ajrccm/143.1.19
30. Lim SC, Adams AB, Simonson DA, et al. Transient hemodynamic effects of recruitment maneuvers in three experimental models of acute lung injury. Crit Care Med. 2004;32(12):2378–2384. doi:10.1097/01.ccm.0000147444.58070.72
31. Srivastava A, Niranjan A. Secrets of safe laparoscopic surgery: anaesthetic and surgical considerations. J Minim Access Surg. 2010;6(4):91–94. doi:10.4103/0972-9941.72593
32. Hess DR. Recruitment maneuvers and PEEP titration. Respir Care. 2015;60(11):1688–1704. doi:10.4187/respcare.04409
33. Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118(3):317–334. doi:10.1093/bja/aex002
34. Perkins GD, McAuley DF, Giles S, Routledge H, Gao F. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit Care. 2003;7(4):R67–R71. doi:10.1186/cc2339
35. Hjalmarsen A, Hykkerud DL. Severe nocturnal hypoxaemia in geriatric inpatients. Age Ageing. 2008;37(5):526–529. doi:10.1093/ageing/afn110
36. Sieber FE, Barnett SR. Preventing postoperative complications in the elderly. Anesthesiol Clin. 2011;29(1):83–97. doi:10.1016/j.anclin.2010.11.011
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.