Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Effects of Adjunctive Betahistine Therapy on Lipid Metabolism in Patients with Chronic Schizophrenia: A Randomized Double-Blind Placebo-Controlled Study
Authors Bai L, Liang W, Wang Y, Fan N, zhang Q, Bian Y, Yang F
Received 9 October 2022
Accepted for publication 27 January 2023
Published 27 February 2023 Volume 2023:19 Pages 453—460
DOI https://doi.org/10.2147/NDT.S392770
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Luyuan Bai,1 Weiye Liang,1 Yongqian Wang,2 Ning Fan,1 Qi zhang,3 Yun Bian,1 Fude Yang1
1Peking University Huilongguan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, People’s Republic of China; 2Office of Scientific Research, Peking University Health Science Center, Beijing, People’s Republic of China; 3Department of Psychology, Wuxi Mental Health Center, Wuxi, People’s Republic of China
Correspondence: Fude Yang; Yun Bian, Peking University Huilongguan Clinical Medical School, 7, Nandian Road, Huilongguan, Changping District, Beijing, 100096, People’s Republic of China, Tel +86-010-83024456 ; +86-010-83024572, Email [email protected]; [email protected]
Objective: This study aims to explore the ability of betahistine to inhibit weight gain and abnormal lipid metabolism in patients with chronic schizophrenia.
Methods: A comparison study of betahistine or placebo therapy was conducted for 4 weeks in 94 patients with chronic schizophrenia, who were randomly divided into two groups. Clinical information and lipid metabolic parameters were collected. Positive and Negative Syndrome Scale (PANSS) was used to assess psychiatric symptoms. Treatment Emergent Symptom Scale (TESS) was used to evaluate treatment-related adverse reactions. The differences in lipid metabolic parameters before and after treatment between the two groups were compared.
Results: Repeated measures analysis of variance (ANOVA) revealed that after 4 weeks of betahistine/placebo treatment, the interaction effect of time and group was statistically significant on low-density lipoprotein cholesterol (F = 6.453, p = 0.013) and waist-to-hip ratio (F = 4.473, p = 0.037), but did not reveal any significant interaction effect of time and group on weight, body mass index or other lipid metabolic parameters, as well as the time main effect and group main effect (all p > 0.05). Betahistine had no significant impact on PANSS, and no side effects related to betahistine were detected.
Conclusion: Betahistine may delay metabolic abnormalities in patients with chronic schizophrenia. It does not affect the efficacy of the original antipsychotics. Thus, it provides new ideas for the treatment of metabolic syndrome in patients with chronic schizophrenia.
Keywords: schizophrenia, lipid metabolism, betahistine, histamine, H3-receptor antagonist
Introduction
Schizophrenia is a chronic psychiatric disorder with complex etiology and is associated with severe disease burden and high disability rate.1 Epidemiological studies have shown that the prevalence of metabolic syndrome in patients with schizophrenia is 42.0%, which is approximately 2- to 3-fold higher than in general population.2,3 Several large-scale meta-analyses confirmed that patients with schizophrenia have a significantly increased risk of lipid metabolism and lipid metabolism-related mortality, which may possibly increase over time,4,5 warranting urgent clinical attention. Therefore, comprehensive prevention and treatment of lipid metabolic abnormalities is essential for patients diagnosed with schizophrenia.
Schizophrenia disease per se, obesity, antipsychotic use and adverse lifestyle are risk factors contributing to lipid metabolism in patients with schizophrenia.6–8 A number of studies have reported that adjunctive medications can improve the metabolic abnormalities of patients with schizophrenia. These medications include metformin, GLP-1RAs, topiramate, sibutramine, aripiprazole, lorcaserin, orlistat, naltrexone, bupropion, amantadine, zonisamide, melatonin and betahistine.9–16 Among them, betahistine, as a regulator of histamine system, has received widespread attention due to its excellent safety profile and unique pharmacological properties as a histaminergic H1 receptor agonist and partial H3 receptor antagonist. Betahistine was studied mainly as a vasodilator for conditions such as cluster headaches, vascular dementia and Meniere’s disease, but in recent years, it was found to be a key neurotransmitter in the regulation of feeding behavior.17 Some clinical trials reported that adjunctive betahistine therapy can inhibit weight gain or obesity caused by antipsychotics.18–20 A possible strategy is to reduce food intake by regulating the histaminergic H1 receptor (H1R) - neuropeptide Y (NPY) - AMP-activated protein kinase (AMPK) pathway of the hypothalamus, increasing the thermogenic effect of brown adipose tissue.21 Another possible approach is to improve AMPK-SREBP-1-PPAR-related pathways in the liver to alleviate blood lipid abnormalities.17
However, using adjunctive betahistine therapy to treat lipid metabolic abnormalities in patients with schizophrenia is not supported by evidence-based medicine. Therefore, this randomized double-blind placebo-controlled study investigated the ability of betahistine to improve weight gain and abnormal lipid metabolism and thus provide evidence for early prevention and treatment of metabolic syndrome in patients with chronic schizophrenia.
Methods
Participants
A total of 94 inpatients with schizophrenia were recruited from Beijing Huilongguan Hospital between September 2019 and September 2021. Researchers fully notified all participants of the purpose and design of this study. All participants voluntarily signed informed consent forms.
All participants met the following inclusion criteria: (1) inpatient status and diagnosis of schizophrenia in accordance with the International Classification of Diseases (ICD)-10 criteria, (2) aged 18~65 years, (3) Han nationality, (4) disease course longer than 10 years and (5) current use of antipsychotics and relatively stable condition.
The exclusion criteria were as follows: (1) use of drugs that affect central nervous system and immune function during the study, (2) current or previous diagnosis of any other mental diseases, (3) history of alcohol or drug abuse within the previous 6 months, (4) pregnancy or lactation and (5) contraindications to betahistine.
Randomization and Blinding
Patients with schizophrenia (n = 94) were randomly assigned to the betahistine group or the placebo group using the PROC PLAN program of SAS package (SAS for Windows, Version 9.4, SAS Institute Inc., Cary, NC). The leader researcher generated a randomization table using block randomization with blocks of size 4, which was preserved by trial designer. Pharmacists participating in the study placed random numbers in envelopes marked with patient numbers. The envelope was left in the pharmacy and was only opened by the pharmacist when randomizing the study participants. Individuals directly involved in the study had no access to these envelopes. Betahistine or placebo was installed in a white opaque plastic bottle. The placebo was the same as betahistine in appearance, characteristics and size. Individuals involved in this study except the trial designer did not know whether betahistine or placebo was administered.
Pharmaceutical Source and Intervention Plan
Betahistine hydrochloride (Xinxiang LepuHengjiuyuan Pharmaceutical Co., Ltd., Henan, China) was used in this study; the specification was 4 mg/tablet. Patients in the betahistine group were administered 48 mg of betahistine hydrochloride per day (16 mg t.i.d.). Patients in the placebo group were given a placebo at the same frequency and dose as the betahistine group. Without affecting the original treatment, all participants were treated with an additional betahistine/placebo for 4 weeks.
Observation Index and Data Collection
This study used a self-made form to record the data of all the participants. The researchers measured participants’ height, weight, waist and hip circumference in the morning at baseline and 4 weeks after the intervention. BMI and waist-to-hip ratio (WHR) were calculated. The Positive and Negative Syndrome Scale (PANSS)22 was used to comprehensively evaluate the severity of psychiatric symptoms of patients with schizophrenia at baseline and 4 weeks after the intervention. The PANSS scale consists of seven positive symptom scores, seven negative symptom scores, and 16 general psychopathological symptom scores. Higher PANSS scores reflect more severe psychiatric symptoms. All researchers participating in the evaluation completed consistency training and the intraclass correlation coefficient (ICC) >0.75 between researchers.
At 7:00 h on the second day after baseline measurements, the venous blood (4 mL) was drawn from all the participants on an empty stomach and immediately centrifuged at 4°C and 3000 r/min for 10 min. The supernatant was transferred to an EP tube for storage at −80°C in a refrigerator until tested. Blood was drawn again from all the participants at 4 weeks after the intervention and processed similarly. Parameters of lipid metabolism analyzed in the blood samples included triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), apolipoprotein a (Apo-A), apolipoprotein b (Apo-b) and lipoprotein (a) [LP (a)].
Safety Assessment
The Treatment Emergent Symptom Scale (TESS)23 was used to comprehensively evaluate adverse effects of treatment at baseline and 4 weeks after the intervention. We defined “severity” greater than or equal to 1, and “relationship with drugs” as “possibly” a side effect of betahistine.
Statistical Analysis
Data analysis was based on intent-to-treat method, and the missing observations were treated using the last observation carried forward (LOCF). Statistical analysis was conducted using SPSS 25.0 software package. Quantitative data were expressed as mean ± standard deviation, and qualitative data were expressed as frequencies. Normality of the data was tested using the Shapiro–Wilk test. Comparisons between groups were made using the independent sample t-test when the data were normally distributed; otherwise, Wilcoxon rank-sum test was used as an alternative method. Categorical data were compared using the χ2 test. Repeated measures analysis of variance (ANOVA) was performed to analyze the differences in metabolism indicators of different groups over time. When the bilateral p value was <0.05, the difference was considered statistically significant.
Ethical Considerations
The trial was approved by the ethics committee of the Beijing Huilongguan Hospital (number: 2018–47) and registered at the China Clinical Test Center (CHICTR1900021078). This study was conducted according to the Declaration of Helsinki.
Results
Participants’ Characteristics
Two participants in betahistine group and three participants in placebo group were lost to follow-up due to discharge from hospital. Data at discharge were used to replace that at 4 weeks after the intervention. Betahistine group included 28 male and 19 female patients with schizophrenia, with an average age of 46.49 ± 9.00 years, and the average course of disease was 22.55 ± 10.71 years. Placebo group included 29 male and 18 female patients with schizophrenia, with an average age of 46.28 ± 10.17 years, and an average disease course of 22.13 ± 11.37 years. There was no significant difference with regard to types of antipsychotics between two groups (χ2 = 0.42, p = 0.667), and olanzapine was the most commonly used drugs (31.9% in betahistine group, 36.2% in placebo group, respectively). No statistically significant differences existed between two groups in gender, age, course, chlorpromazine equivalent and lipid metabolic indicators (p > 0.05) as shown in Table 1.
 |
Table 1 Baseline Characteristics of Participants and Comparison Between Betahistine Group and Placebo Group |
Efficacy Evaluation
The results of ANOVA showed that after 4 weeks of betahistine/placebo treatment, the interaction effect of time and group was statistically significant on LDL-C (F = 6.453, p = 0.013). A further simple effect analysis revealed that the time main effect of placebo group was significant (F = 5.602, p = 0.023), and the time main effect of betahistine group was not significant (F = 1.391, p = 0.245). No obvious time main effects were found in the two groups (F = 0.878, p = 0.351) and no obvious group differences were detected (F = 0.012, p = 0.912).
The interaction effect of time and group was statistically significant on WHR (F = 4.473, p = 0.037). No obvious time main effects were found in the two groups (F = 0.142, p = 0.708) and no obvious group differences were detected (F = 0.226, p = 0.636). After 4 weeks of treatment with betahistine/placebo, ANOVA did not reveal any significant interaction effect of time and group on weight, BMI and other lipid metabolic parameters, as well as the time main effect and group main effect (all p > 0.05), as shown in Table 2.
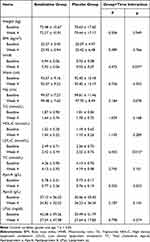 |
Table 2 Comparison of Weight and Lipid Metabolism in Betahistine Group and Placebo Group at Baseline and 4 Weeks |
Psychiatric Symptoms
Changes in psychiatric symptoms after 4 weeks of intervention are shown in Table 3. There were no time-group effects involving positive symptom scores, negative symptom scores, general psychopathological symptom scores, or total scores in the betahistine group.
 |
Table 3 Changes in Psychiatric Symptoms of Patients in Betahistine and Placebo Groups at Baseline and 4 Weeks Later |
Adverse Effects
During the 4-week intervention, the TESS items in both betahistine and placebo groups were not greater than the “basic relationship.” Thus, all patients involved in this study showed no betahistine-related adverse effect. In the first week of treatment, a patient in betahistine group showed increased antipsychotic levels in the blood. A complete evaluation revealed that it had no basic relationship with betahistine.
Discussion
This study found no impact of betahistine on weight and BMI in patients with chronic schizophrenia, which is inconsistent with other studies reported previously. Lian et al25 found that betahistine treatment in animal models showed reversal in olanzapine-induced body weight gain mediated via H1R-NPY and H1R-AMPK-alpha phosphorylation. Activation of H1 receptors increased the amount of histamine released, causing a series of physiological reactions, weight reduction, and energy metabolism.26 However, blocking H3 receptor can indirectly enhance the role of H1 receptor,27 and H1 receptor is the main cause of antipsychotic-induced weight gain/obesity. Barak et al28 showed that after 4 weeks of treatment, the average weight increase in the betahistine group 37% less than in placebo group. Although the foregoing studies indicate that betahistine can reduce patients’ weight, other studies found that betahistine has no effect on weight. A proof-of-concept, randomized, double-blind, placebo-controlled, dose-ranging study was performed by Ali AH et al29 to analyze the effects of betahistine on class I or II obesity in women. The results showed that after a 24-h placebo run-in period, 144 mg betahistine/d did not induce any effect on food intake or appetite. In a randomized, placebo-controlled, dual-center (China and the United States) study by Smith et al,30 betahistine was significantly better than placebo in preventing increases in weight, BMI, and waist circumference only in a subgroup of patients being treated with olanzapine or clozapine but not other antipsychotics. Kang D et al31 reported that 1000 mg metformin/d was more efficacious than 36 mg betahistine/d in preventing and reversing the weight gain induced by antipsychotics. The inconsistent results may be attributed to the treatment duration, the treatment dose, and the type of antipsychotics. More potent histaminergic modulators may be required to elucidate the possible role of histaminergic pathways in human obesity.
In this study, the interaction effect of time and group was statistically significant on WHR after the 4-week double-blind betahistine/placebo treatment, but no obvious time main effects and no obvious group differences were detected. Smith et al30 also did not find statistically significant changes in WHR before and after betahistine treatment, consistent with the final results of this study. This study also found that the interaction effect of time and group was statistically significant on LDL-C after the 4-week double-blind betahistine/placebo treatment, and the time main effect in the placebo group was significant. Therefore, we believe that the differences between the two groups of LDL-C after treatment are mainly due to the increased LDL-C levels in placebo group, rather than betahistine group. Antipsychotics with significant H1 receptor affinity in the hypothalamus, particularly in the ventromedial nucleus, may cause weight gain in psychotic patients.32 Previous studies have shown that mice administered with clozapine and olanzapine, potent H1 receptor antagonists, demonstrate greater activation of AMPK in hypothalamus,33,34 which increases glucose levels and alters lipid metabolism.35 Comparatively, antipsychotics are less likely to induce weight gain, eg ziprasidone, haloperidol, lurasidone and aripiprazole and have a minimal effect on AMPK activity and lipid metabolism.33 The results of this study indicate that betahistine may delay the elevation of LDL-C in patients with chronic schizophrenia, which are consistent with the results of Kotańska et al.34 To reveal the mechanisms underlay, Lian et al26 found that olanzapine significantly enhanced the mRNA and protein expressions of H1R, the NPY mRNA expression, and AMPK-alpha activation; these changes were significantly reversed by co-treatment of olanzapine and betahistine compared with olanzapine only treatment.
Finally, this study also evaluated the impact of betahistine intervention on PANSS and TESS. The results showed that betahistine group showed no significant difference in psychiatric symptoms, compared with the placebo group, and no side effects related to betahistine occurred, which is consistent with previous studies.25,28
Limitations
The small sample size and short intervention period are limitations of this study. Different antipsychotics used at baseline may have different effects on lipid metabolism. In the future, targeted studies involving larger populations with a longer follow-up period are required. Nevertheless, the dosage and duration of betahistine therapy is also an important factor affecting results. Further studies are needed to improve the research design.
Conclusions
Betahistine may potentially delay LDL-C increase in Chinese patients diagnosed with chronic schizophrenia. It does not affect the efficacy of the original antipsychotic, which provides new ideas for the treatment of metabolic syndrome in patients with chronic schizophrenia. Further research with a longer follow-up period designs is necessary to determine the safety and effectiveness of betahistine in antipsychotic-treated patients.
Data Sharing Statement
The datasets presented in this study are available from the corresponding authors (Fude Yang, [email protected]; Yun Bian, [email protected]) upon reasonable request.
Acknowledgments
The authors acknowledge the help of all the study participants.
Funding
This study was funded by Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20182001) and Beijing Municipal Administration of Hospitals Incubating Program (PX2018066). The funding source had no role in study design; collection, analysis and interpretation of data; writing the report; or decision to submit the article for publication.
Disclosure
All authors declare that they have no competing interests in this work.
References
1. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. doi:10.1016/S0140-6736(15)01121-6
2. Sun MJ, Jang MH. Risk Factors of Metabolic Syndrome in Community-Dwelling People with Schizophrenia. Int J Environ Res Public Health. 2020;18:17. doi:10.3390/ijerph18010017
3. Wu X, Huang Z, Han H, et al. The comparison of glucose and lipid metabolism parameters in drug-naive, antipsychotic-treated, and antipsychotic discontinuation patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1361–1368. doi:10.2147/NDT.S63140
4. Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163–180. doi:10.1002/wps.20420
5. Oakley P, Kisely S, Baxter A, et al. Increased mortality among people with schizophrenia and other non-affective psychotic disorders in the community: a systematic review and meta-analysis. J Psychiatr Res. 2018;102:245–253. doi:10.1016/j.jpsychires.2018.04.019
6. Scigliano G, Ronchetti G. Antipsychotic-induced metabolic and cardiovascular side effects in schizophrenia: a novel mechanistic hypothesis. CNS Drugs. 2013;27:249–257. doi:10.1007/s40263-013-0054-1
7. Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--A systematic review and meta-analysis. Schizophr Bull. 2013;39:306–318. doi:10.1093/schbul/sbr148
8. Schreurs MDEH. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8:15–22. doi:10.1002/j.2051-5545.2009.tb00199.x
9. Marteene W, Winckel K, Hollingworth S, et al. Strategies to counter antipsychotic-associated weight gain in patients with schizophrenia. Expert Opin Drug Saf. 2019;18:1149–1160. doi:10.1080/14740338.2019.1674809
10. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40:1385–1403. doi:10.1093/schbul/sbu030
11. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299:185–193. doi:10.1001/jama.2007.56-b
12. Fan X, Borba CP, Copeland P, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand. 2013;127:217–226. doi:10.1111/acps.12009
13. Deberdt W, Winokur A, Cavazzoni PA, et al. Amantadine for weight gain associated with olanzapine treatment. Eur Neuropsychopharmacol. 2005;15:13–21. doi:10.1016/j.euroneuro.2004.03.005
14. Ko YH, Joe SH, Jung IK, Kim SH. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28:169–175. doi:10.1097/01.wnf.0000172994.56028.c3
15. Romo-Nava F, Alvarez-Icaza Gonzalez D, Fresan-Orellana A, et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 2014;16:410–421. doi:10.1111/bdi.12196
16. Ghanizadeh A, Nikseresht MS, Sahraian A. The effect of zonisamide on antipsychotic-associated weight gain in patients with schizophrenia: a randomized, double-blind, placebo-controlled clinical trial. Schizophr Res. 2013;147:110–115. doi:10.1016/j.schres.2013.03.021
17. Barak N. Betahistine: what’s new on the agenda? Expert Opin Investig Drugs. 2008;17:795–804. doi:10.1517/13543784.17.5.795
18. Poyurovsky M, Pashinian A, Levi A, Weizman R, Weizman A. The effect of betahistine, a histamine H1 receptor agonist/H3 antagonist, on olanzapine-induced weight gain in first-episode schizophrenia patients. Int Clin Psychopharmacol. 2005;20:101–103. doi:10.1097/00004850-200503000-00007
19. Poyurovsky M, Fuchs C, Pashinian A, Levi A, Weizman R, Weizman A. Reducing antipsychotic-induced weight gain in schizophrenia: a double-blind placebo-controlled study of reboxetine-betahistine combination. Psychopharmacology. 2013;226:615–622. doi:10.1007/s00213-012-2935-2
20. Naguy A, AlShalabi SR, AlKhadhari S. Betahistine-Associated Weight Loss and Improved Cognitive and Negative Symptoms: domain in Early-Onset Schizophrenia. Am J Ther. 2019;26:e790–e2. doi:10.1097/MJT.0000000000000965
21. Wankhade UD, Shen M, Yadav H, Thakali KM. Novel Browning Agents, Mechanisms, and Therapeutic Potentials of Brown Adipose Tissue. Biomed Res Int. 2016;2016:2365609. doi:10.1155/2016/2365609
22. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi:10.1093/schbul/13.2.261
23. Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology, National Institute of Mental Health, Rockville. 1976. 217–222. doi: 10.1037/e591322011-001
24. Lian J, Huang XF, Pai N, Deng C. Effects of olanzapine and betahistine co-treatment on serotonin transporter, 5-HT2A and dopamine D2 receptor binding density. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:62–68. doi:10.1016/j.pnpbp.2013.08.005
25. Lian J, Huang XF, Pai N, Deng C. Betahistine ameliorates olanzapine-induced weight gain through modulation of histaminergic, NPY and AMPK pathways. Psychoneuroendocrinology. 2014;48:77–86. doi:10.1016/j.psyneuen.2014.06.010
26. Kumar A, Pasam VR, Thakur RK, et al. Novel Tetrahydroquinazolinamines as Selective Histamine 3 Receptor Antagonists for the Treatment of Obesity. J Med Chem. 2019;62:4638–4655. doi:10.1021/acs.jmedchem.9b00241
27. Barak N, Beck Y, Albeck JH. Betahistine decreases olanzapine-induced weight gain and somnolence in humans. J psychopharmacol. 2016;30:237–241. doi:10.1177/0269881115626349
28. Ali AH, Yanoff LB, Stern EA, et al. Acute effects of betahistine hydrochloride on food intake and appetite in obese women: a randomized, placebo-controlled trial. Am J Clin Nutr. 2010;92:1290–1297. doi:10.3945/ajcn.110.001586
29. Smith RC, Maayan L, Wu R, et al. Betahistine effects on weight-related measures in patients treated with antipsychotic medications: a double-blind placebo-controlled study. Psychopharmacology. 2018;235:3545–3558. doi:10.1007/s00213-018-5079-1
30. Kang D, Jing Z, Li R, et al. Effect of Betahistine and Metformin on Antipsychotic-Induced Weight Gain: an Analysis of Two Clinical Trials. Front Psychiatry. 2018;9:620. doi:10.3389/fpsyt.2018.00620
31. Pertti P, Paul LC, Marlon C, et al. International Union of Basic and Clinical Pharmacology. Pharmacol Rev. 2015;67:601–655.
32. Kim SF, Huang AS, Snowman AM, et al. From the Cover: antipsychotic drug induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci U S A. 2007;104(9):3456–3459. doi:10.1073/pnas.0611417104
33. He M, Zhang Q, Deng C, et al. Hypothalamic histamine H1 receptor-AMPK signaling time-dependently mediates olanzapine-induced hyperphagia and weight gain in female rats. Psychoneuroendocrinology. 2014;42:153–164. doi:10.1016/j.psyneuen.2014.01.018
34. Carli M, Kolachalam S, Longoni B, et al. Atypical antipsychotics and metabolic syndrome: from molecular mechanisms to clinical differences. Pharmaceuticals. 2021;14:238. doi:10.3390/ph14030238
35. Kotanska M, Mika K, Regula K, et al. KSK19 - Novel histamine H3 receptor ligand reduces body weight in diet induced obese mice. Biochem Pharmacol. 2019;168:193–203. doi:10.1016/j.bcp.2019.07.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
