Back to Journals » Journal of Pain Research » Volume 13
Effects of Additional Intraoperative Administration of Sufentanil on Postoperative Pain, Stress and Inflammatory Responses in Patients Undergoing Laparoscopic Myomectomy: A Double-Blind, Randomized, Placebo-Controlled Trial
Authors Liu L , Li B , Cao Q, Zhao B, Gao W, Chen Y , Yu S
Received 8 April 2020
Accepted for publication 11 August 2020
Published 26 August 2020 Volume 2020:13 Pages 2187—2195
DOI https://doi.org/10.2147/JPR.S257337
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Lian Liu,1 Bingyu Li,1 Quan Cao,2 Bo Zhao,1 Wenwei Gao,3 Yuan Chen,4 Shihua Yu5
1Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, People’s Republic of China; 2Department of Ultrasound Imaging, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, People’s Republic of China; 3Critical Care Medicine, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, People’s Republic of China; 4Department of Academic Research, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, People’s Republic of China; 5Department of Anesthesiology, Renmin Hospital of Hannan District, Wuhan, Hubei 430090, People’s Republic of China
Correspondence: Yuan Chen
Department of Academic Research, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, People’s Republic of China
Email [email protected]
Shihua Yu
Department of Anesthesiology, Renmin Hospital of Hannan District, Wuhan, Hubei 430090, People’s Republic of China
Email [email protected]
Purpose: Although pain after laparoscopic surgery is assumed to be minor, many women still suffer from unexpected postoperative pain. Thus, we aimed to assess whether additional intraoperative administration of sufentanil could help to improve postoperative pain and related agitation, stress, and inflammation response in patients undergoing laparoscopic myomectomy.
Patients and Methods: Forty female patients with uterine myoma scheduled for laparoscopic myomectomy under general anesthesia were randomized to receive sufentanil (group T, n=20) or normal saline (group C, n=20) 1h before the end of the surgery. The postoperative pain, agitation, stress, inflammation, and adverse effects were measured.
Results: As the primary outcome, the visual analog scale (VAS) pain score was significantly reduced in group T as compared with group C at each measured time point in a post-anesthesia care unit (PACU), VAS 5 min (31.5 ± 2.7 vs 40.6 ± 5.6) (P< 0.001), VAS 30 min (36.5 ± 4.5 vs 46.0 ± 2.9) (P< 0.001), VAS 1h (37.8 ± 4.0 vs 48.6 ± 5.5) (P< 0.001). The secondary outcomes, including the sedation agitation scale (SAS) scores, plasma concentrations of epinephrine and norepinephrine, and the levels of plasma interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-alpha (TNF-α) in group T were remarkably lower than those in group C (P < 0.001). The cough cases in group T also showed a significant reduction in comparison with group C (P < 0.05). In addition, the anesthetic recovery time, including the spontaneous breathing recovery time and extubation time, were not significantly different between the two groups, as were the cases of respiratory depression and postoperative delirium (P > 0.05).
Conclusion: For patients undergoing laparoscopic myomectomy, administration of sufentanil 1 h before the end of surgery shows excellent analgesic and sedative effects, alleviated postoperative stress and inflammatory responses, reduced incidence of cough, without prolonging anesthetic recovery time and increasing adverse reactions.
Keywords: anesthesia, laparoscopic myomectomy, postoperative outcomes, sufentanil
Introduction
In the past few decades, laparoscopic surgery has played an ever-increasing important role in the gynecological treatment of many benign and malignant diseases.1 Uterine myoma is the most common benign tumors in the female reproductive tract, with a lifetime risk of approximately 70% to 80%.2 Especially for women who want to retain the uterus and fertility, if surgery is required, uterine myomectomy must be performed without removing the entire uterus.3 Therefore, the laparoscopic myomectomy under general anesthesia is a preferred option for uterine myoma treatment instead of laparotomy.4,5
Compared with laparotomy, laparoscopic surgery shows many advantages, such as lower perioperative morbidity, quicker recovery, and shorter hospital stay without reducing efficacy.6 However, pain after laparoscopic surgery is often underestimated, pain control is comparatively inadequate.7–9 It has been reported that pain is mainly resulted from nerve injury, central sensitization, and inflammatory damage.10 Incisions in the skin and subcutaneous tissue, stretching of the abdominal wall structures, combined with transabdominal suture and laparoscopic tacks applied during the procedure, may stimulate the peripheral nociceptors, leading to pain sensation. Furthermore, ischemia-reperfusion–inflammation processes in the peritoneum also arouses the pain receptors to increase pain sensation.11–13 In addition, women are more sensitive to pain; many women patients who underwent laparoscopic surgery may suffer unexpected considerable postoperative pain.14
Given that perioperative comfort depends primarily on the patient’s self-reporting, providing timely, safe, and adequate analgesia remains a topic of concern for anesthesiologists. Various interventions, such as medication patches and percutaneous pump devices, have been used to alleviate postoperative pain after laparoscopic surgery, but the effect is limited. Therefore, exploring a preferable way to improve postoperative pain has been the focus of laparoscopic surgery.15,16 Sufentanil, a cheap synthetic opioid with a high therapeutic index and a quick response, is an attractive drug for postoperative pain.17,18 Sufentanil has been widely used for general anesthesia induction due to its potent analgesic properties, quick onset, and excellent cardiovascular stability.19 Recently, sufentanil has been proved effective in some laparoscopic surgery studies, but rarely in laparoscopic myomectomy.15,20,21 Intraoperative sufentanil has been reported has certain advantages for the patients undergoing laparoscopic cholecystectomy, including reducing stress response, improving recovery effect, and decreasing adverse reactions.21 Hence, we hypothesized that, except for the administration in anesthesia induction, additional intraoperative administration of sufentanil might be a preferable choice for the patients undergoing laparoscopic myomectomy. This study was designed to explore whether additional intraoperative administration of sufentanil 1h before the end of the surgery is beneficial to reduce postoperative pain, stress, and inflammatory response in the patients with laparoscopic myomectomy.
Patients and Methods
This prospective, randomized, and double-blind, placebo-controlled clinical trial was approved by the Ethics Committee of Renmin Hospital of Wuhan University (WDRY2018-K009) and registered at the Chinese Clinical Trial Registry (ChiCTR) with registration number ChiCTR1800019949. The trial was conducted in accordance with the Declaration of Helsinki. We included female patients with the American Society of Anesthesiologists (ASA) class I and II, aged between 35 and 55 years, diagnosed as uterine myoma by the history of present illness and combined with ultrasound, scheduled for laparoscopic myomectomy under general anesthesia. The trial was performed at Renmin Hospital of Wuhan University during the period of January 1, 2019 and April 30, 2019. Informed consent was obtained from all patients prior to enrollment in the study.
Exclusion criteria were patients with other systemic diseases; serious brain injury; stroke or other central nervous system diseases; severe adverse events happened during the operation, such as cardiac arrest or cardiopulmonary resuscitation; a history of hypertension; patients were unable to communicate correctly; patients with a reactive airway disease (asthma, or a history of chronic coughing); a history of mental illness, alcohol addiction or drug abuse.
The standard visual analogue scale (VAS), ranging from 0 to 10 cm (0 means no pain;10 cm describes the worst pain imaginable),22 was explained to the patients during the preoperative visit and the patients should slide the ruler every time the pain is assessed. A statistician who was unaware of the design of the study randomly and equally divided patients into two groups by using a computer-generated random number table: Test group (group T), 0.2μg/kg sufentanil (prepared by a independent anesthesiologist and diluted into 10mL) was given 1h before the end of the surgery; and Control group (group C), an equivalent volume of normal saline (prepared by the independent anesthesiologist and diluted into 10mL). (n=20 each). After allocation, both patients and investigators were blinded to the interventions. Sufentanil takes nearly 45 minutes to achieve a steady-state between plasma concentrations and effect-site concentrations, so we chose to administrate sufentanil 1h before the end of the surgery (usually when the last myomas was begin to be removed)23,24
Patients were sent to the operation room without any premedication, then the left lower limb peripheral veins were opened and compound sodium lactate solution 4–6 mL/kg/h was infused. Standard monitoring, including five-lead electrocardiography (ECG), pulse oxygen saturation (SpO2), and noninvasive blood pressure (NBP), was performed. The participating anesthesiologists, surgeons, and nurses were blinded to the study. All data were collected by trained observers who were blinded to the study and were not involved in patient care.
Anesthesia induction was achieved with propofol 2.5 mg/kg, sufentanil 0.4 µg/kg, and cisatracurium 0.3 mg/kg. Anesthesia maintenance was intravenous-inhalation combined with 1% (inspiratory concentration) sevoflurane and a continuous intravenous infusion of remifentanil 0.2 µg/kg/min. Before general anesthesia, patients were given 100% oxygen for pre-oxygenated before induction, which was delivered through a facial mask. General anesthesia was provided, as mentioned above. Manual facemask ventilation was continued for no less than 4 minutes until the jaw was relaxed, and the bispectral index (BIS) was less than 50. The endotracheal tube was inserted with the help of a direct laryngoscope. After intubation, a ventilator (Primus, Dräger, Germany) was connected immediately. Respiratory parameters were set at a tidal volume (TV) 8–10 mL/kg, respiratory rate (RR) 12–16 times/min, and the fraction of inspired oxygen (FiO2) 80% to maintain end-tidal carbon dioxide (ETCO2) in the normal range. The sevoflurane and remifentanil were adjusted if needed, to maintain BIS within 40–60, and keep the fluctuation of mean arterial pressure (MAP) and heart rate (HR) not exceed 20% of the baseline values.
When the final suture of the skin was completed, all the anesthetics were stopped to allow the patient to emerge from anesthesia. All the patients received dexamethasone 5 mg and metoclopramide 5 mg before the end of surgery to prevent postoperative nausea and vomiting. Before the patient resumed spontaneous breathing and responded to simple commands, gentle manual ventilatory assistance was provided. The criteria of pulling out the endotracheal tube were: (1) recovery of consciousness, muscle tension returned to normal, fist clenched firmly according to the instruction; (2) steady spontaneous breathing, ETCO2 < 45 mmHg, TV > 7 mL/kg; (3) SpO2 > 97% after stopping to receive oxygen for 5 min; (4) swallowing reflex recovered. The anesthetic recovery time, including spontaneous breathing recovery time and extubation time, were recorded, and the cases of cough and respiratory depression (RR <10 times/min and/or SpO2 < 90% for 1 min)25 on emergence from anesthesia were also measured in two groups.
Following extubation, the patients were transferred to the post-anesthesia care unit (PACU) and monitored for 60 min. The VAS score was used to evaluate postoperative pain, and the sedation agitation scales (SAS) score was used to assess the agitation of the patients.26 CAM-ICU Scales (1, acute onset or repeated fluctuations of symptoms; 2, insufficient attention; 3, disorganized thinking; 4, abnormal consciousness levels. The patient was diagnosed with postoperative delirium when the symptoms were 1+2+3 or 1+2+4).27 The VAS and SAS scores were recorded at the following time points: T3, 5 min in the PACU; T4, 30 min in the PACU; T5, 1 h in the PACU. CAM-ICU Scale was recorded at T5. Upon arrival at the PACU, 100 µg of sufentanil and 5 mg of dezocine diluted in 100 mL saline were started to infusion with a flow of 2 mL/h by an intravenous analgesia device. If patients with VAS > 60 mm, 5 mg dezocine was intravenously injected for rescue analgesia. When patients back to the ward, diclofenac suppositories of no more than 150 mg per day were administered if needed.
Three milliliters of venous blood were collected at the following time points: T1 (baseline), T3 (5 min in the PACU), and T5 (1h in the PACU). The blood was added to tubes without anticoagulant, completely static until the serum separation, the serum precipitation was taken with centrifugal to centrifuge at 4000 rpm in 4°C for 10 min, and then the supernatant was sucked out to be placed in −80°C cryogenic refrigerator for the test of the levels of epinephrine, norepinephrine, interleukin-6 (IL-6), interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α). All the detecting parameters were measured by enzyme-linked immunosorbent assay (Boster Biotechnology Co., Ltd).
Our primary outcomes were postoperative pain score assessed at 5min, 30min, and 1h in the PACU. We also calculated the mean pain score from these three measurements, which represented the average pain score within the first hour after surgery in the PACU; the secondary outcomes were postoperative agitation, stress hormones, and inflammatory markers levels, as well as the side effects. The sample size was calculated based on the VAS score at T5, which is a commonly used method to evaluate the pain of patients. A pilot study of 5 patients undergoing laparoscopic myomectomy under the group T regimen showed that the mean ± standard deviation of the postoperative VAS score at T5 was 3.38 ± 0.6. To demonstrate one mean difference of the VAS score at T5 after operation between the two groups with a two-tailed α of 0.01 and β of 0.05, 13 patients per group were required. Finally, we recruited 20 patients per group for consideration of the possible dropout.
Statistical Analysis
The statistical analyses were performed with Statistical Package for Social Sciences (version 17.0 for Windows; SPSS Inc., Chicago, IL). The normal distribution of continuous data was first evaluated using the Shapiro–Wilk test (P >0.05). The normally distributed data were expressed as mean ± standard deviation (Mean ± SD) and analyzed using Independent-Samples t-test; the non-normally distributed data were expressed as Median (min, max) and analyzed using Mann–Whitney U-test. Independent-Samples t-test was used to compare the patients’ characteristic data, VAS score, levels of stress hormones and inflammatory cytokines, spontaneous breathing recovery time and extubation time; while the Mann–Whitney U-test was used for the SAS score. Categorical variables were presented as numbers. Pearson’s χ2 test was used to analyze cough cases, and the Fisher exact test was used to analyze the cases of respiratory depression and postoperative delirium. All reported P values were two-sided, and P values less than 0.05 were considered significant.
Results
During the study, 53 female patients (36–55 years old) were assessed for eligibility, of which seven patients did not meet the inclusion criteria, and six patients refused (Figure 1). Finally, 40 patients completed the study. Randomization produced two groups with similar characteristics. All patients underwent their scheduled surgery procedure and received their assigned regimen of drug use. There were no significant differences between the two groups regarding age, height, weight, the number of removed myomas, and operative time (P > 0.05; Table 1).
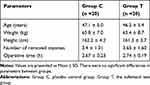 |
Table 1 Patients’ Characteristics and Surgical Procedures |
 |
Figure 1 Flow diagram of patient recruitment. Abbreviations: Group C, placebo control group; Group T, the sufentanil test group. |
A comparison of the postoperative VAS score between the two groups is shown in Table 2. We found that the VAS scores were gradually increased with the prolongation of postoperative time in the two groups. The group T showed a significant reduction of VAS pain score at all measured time points as compared with the group C (P < 0.001). In addition, the mean overall VAS score in group T was significantly lower than that in group C (35.3 ± 4.6 vs 45.0 ± 5.8, P < 0.001).
 |
Table 2 The Postoperative VAS Scores in the Two Groups |
The postoperative SAS scores are listed in Table 3. The SAS score tended lower in group T as compared with group C at all measured time points (P < 0.001). 5min and 30min after patients’ arrivals in the PACU (T3, T4), the median SAS score was 3 in group T, while 4 in group C (P < 0.001). When reaching T5 (1h in the PACU), the median SAS was 4 in group T, while 5 in group C (P < 0.001). Meanwhile, the median overall SAS score in the first 1h in the PACU in group T was significantly lower than that in group C (3 (2, 4) vs 4 (3, 6)) (P < 0.001).
 |
Table 3 The Postoperative SAS Scores in the Two Groups |
The postoperative changes in stress hormones are displayed in Table 4. The levels of epinephrine and norepinephrine were of no noticeable difference between the two groups at T1 and T3 (P > 0.05). However, when reaching T5, the level of epinephrine was lower in group T than that in group C (24.8 ± 2.7 vs 32.4 ± 2.5, P < 0.001). Meanwhile, the norepinephrine level was also significantly reduced in group T as compared with group C at T5 (302.3 ±17.6 vs 485.5 ± 22.5, P < 0.001).
 |
Table 4 The Stress Hormones in the Two Groups |
The postoperative changes of inflammatory cytokines are presented in Table 5. Plasma concentrations of IL-6, IL-8, and TNF-œ were of no marked difference between two groups at T1 and T3 (P > 0.05). However, there was a significant reduction of the three inflammatory cytokines (IL6, 10.4 ± 0.8 vs 15.9 ± 1.1; IL8, 184.5 ± 15.5 vs 291.5 ± 18.5; TNF-α, 11.7 ± 1.0 vs 19.5 ± 1.3) at T5 in group T in comparison with group C (P < 0.001).
 |
Table 5 The Inflammation Level in the Two Groups |
The anesthetic recovery time (spontaneous breathing recovery time and extubation time) and the incidence of adverse effects (cough, respiratory depression, and postoperative delirium) in the two groups are shown in Table 6. The anesthetic recovery time in group C and group T were of no remarkable differences (P > 0.05). There were four cases of respiratory depression and one case of postoperative delirium occurred in group C while there were three cases of respiratory depression and zero case of postoperative delirium occurred in group T (P = 1.000). However, the cough cases in group T was significantly reduced as compared with group C (12 vs 5, P = 0.025).
 |
Table 6 The Recovery Time and Adverse Effects in Two Groups |
Discussion
In the present study, we have demonstrated that additional administration of sufentanil 1h before the end of the surgery is safe and effective for patients undergoing laparoscopic myomectomy. The additional intraoperative application of sufentanil showed significantly reduced pain and agitation, decreased occurrence of cough, alleviated stress and inflammatory responses, without the prolonged anesthetic recovery time and elevated adverse events during the early postoperative period.
Laparoscopic myomectomy is commonly recommended for the treatment of uterine myoma. Although laparoscopic surgery is a less invasive operative treatment, it may cause severe pain and anxiety.7 Postoperative pain is a common complaint of patients after the surgery. Adequate postoperative analgesia can not only improve patient satisfaction but also reduce the incidence of postoperative complications and shorten the length of hospital stay.28 However, there is no consensus on the analgesics and anesthetic regimens during laparoscopic surgery. The most appropriate therapeutic regimen should provide adequate analgesia and sedation, as well as fast recovery and minimal adverse effects.26
Opioids can effectively relieve moderate to severe postoperative pain. Sufentanil, a synthetic opioid analgesic that mainly acts at the μ receptor, shows some advantages over other opioids.29,30 It has been reported that sufentanil infusion before extubation reduces the postoperative analgesic requirement without delaying extubation time.31,32 Consistent with these studies, we found that patients in group T consistently showed lower VAS scores at the timepoint of 5min, 30min, and 1h and demonstrated significantly lower mean overall VAS scores compared with group C (45.0 vs 35.3). Previous studies reported that “minimum clinically important difference” between 9.4 and 13 mm on a 10 cm VAS would cause changes in pain perception.16,33 In our present study, there is a difference of 9.7 mm on a 10 cm VAS, which indicates the clinical relevance of the result. Recently, combined spinal-general anesthesia applied in laparoscopic gynecological surgery has been reported to obviously decrease the pain severity and increase the quality of analgesic strategies.13 The application of this strategy into our regimen may further improve pain management in patients undergoing laparoscopic myomectomy.
Inadequate sedation may affect postoperative pain sensitivity, thereby aggravating patients’ subjective perception of postoperative pain and leading to increased demand for postoperative analgesics.34 In turn, insufficient pain control after surgery may adversely affect patients, leading to unnecessary physical and psychological manifestations.35,36 Favorable postoperative pain management makes for adequate analgesia and sedation.37–39 Our present study showed attenuated agitation as demonstrated by significantly reduced SAS score in the group T compared with group C, which was in line with our results of the postoperative pain score, indicating that additional intraoperative administration of sufentanil is helpful for proper postoperative pain management in patients undergoing laparoscopic myomectomy.
The release of stress factors and inflammatory mediators caused by surgical or anesthetic operations may directly cause pain and affect patients’ postoperative recovery.40–42 Stress and inflammatory mediators play an essential role in pain sensitization.40 Proper analgesic regimens can prevent both peripheral and central sensitization, thereby attenuating the postoperative amplification of pain sensation and consequently reducing postoperative stress and inflammatory response.43,44 The levels of stress hormones and inflammatory mediators in our study were significantly lower in group T compared with group C at 1h in the PACU. This result is consistent with our findings of postoperative VAS pain scores. Patients in group T also showed significantly lower VAS pain scores in the first 1h of the PACU stay period. Therefore, our results suggest that the sufentanil administered 1h before the end of surgery would be effective in reducing the stress and inflammatory responses, so that reduces postoperative pain sensitization.45
Given the safety must be considered to evaluate a novel treatment, we assessed the adverse effect of our regimen in the present study. We found that the sufentanil administration in group T reduced the occurrence of cough compared to group C during the recovery period after general anesthesia. In recent studies, sufentanil has been reported to have a higher therapeutic index and a lower respiratory depressive property compared to other opioids.25,46,47 Consistent with these studies, patients in group T showed no apparent respiratory depression after extubation, and most of the patients could maintain oxygen saturation above 95% without the oxygen mask. Additionally, the two groups showed no noticeable difference in the anesthetic recovery time, as well as the incidence of postoperative delirium. These phenomena are in line with the previous studies.27,31
There are some limitations to this study. Firstly, only female patients with ASA class I or II were included in our study, it would be better if this study were performed on patients with various demographic and clinical characteristics. Secondly, a single dose of sufentanil was used in our study. It is worth to further explore the relationship between the dose of sufentanil and postoperative pain. Thirdly, sufentanil was intraoperatively administered at a single timepoint. The protocol with more time points will be helpful to confirm the optimal administration time in the future study. Fourthly, the generalizability of our study may be limited as it was a single-center study. Fifthly, due to its relatively small sample size, the study may have a potential bias. Therefore, studies with larger sample sizes and multicenter may help draw more definitive conclusions.
In summary, additional administration of sufentanil 1h before the end of the surgery improved postoperative pain and agitation, reduced inflammatory and stress responses, without delaying anesthetic recovery time and increasing adverse effects during the early postoperative period in patients underwent laparoscopic myomectomy surgery.
Data Sharing Statement
The data used to support the findings of this study are included in the article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tamrakar SR, Dongol A, Shakya S, Kayastha B. Minimal invasive gynaecological surgeries in dhulikhel hospital: one and half decade long experience. Kathmandu Univ Med J. 2018;16(64):333–337.
2. Stewart EA, Lytle BL, Thomas L, et al. The comparing options for management: patient-centered results for uterine fibroids (COMPARE-UF) registry: rationale and design. Am J Obstet Gynecol. 2018;219(1):95 e9195 e10. doi:10.1016/j.ajog.2018.05.004
3. Lee D, Kim SK, Kim K, Lee JR, Suh CS, Kim SH. Advantages of single-port laparoscopic myomectomy compared with conventional laparoscopic myomectomy: a randomized controlled study. J Minim Invasive Gynecol. 2018;25(1):124–132. doi:10.1016/j.jmig.2017.08.651
4. Vargas MV, Larson KD, Sparks A, et al. Association of operative time with outcomes in minimally invasive and abdominal myomectomy. Fertil Steril. 2019;111(6):1252–1258 e1251. doi:10.1016/j.fertnstert.2019.02.020
5. Vargas MV, Robinson JK, Schwab TD, Opoku-Anane J, Marfori CQ, Moawad GN. Feasibility and safety of a minimally invasive surgical (MIS) approach in complex myomectomies. J Minim Invasive Gynecol. 2015;22(6S):S38. doi:10.1016/j.jmig.2015.08.106
6. Tsuzuki Y, Tsuzuki S, Wada S, Fukushi Y, Fujino T. Recovery of quality of life after laparoscopic myomectomy. J Obstet Gynaecol Res. 2019;45(1):176–181. doi:10.1111/jog.13808
7. Sjovall S, Kokki M, Kokki H. Laparoscopic surgery: a narrative review of pharmacotherapy in pain management. Drugs. 2015;75(16):1867–1889.
8. Fields AC, Gonzalez DO, Chin EH, Nguyen SQ, Zhang LP, Divino CM. Laparoscopic-assisted transversus abdominis plane block for postoperative pain control in laparoscopic ventral hernia repair: a randomized controlled trial. J Am Coll Surg. 2015;221(2):462–469. doi:10.1016/j.jamcollsurg.2015.04.007
9. Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934–944. doi:10.1097/ALN.0b013e31828866b3
10. Jarrell J, Ross S, Robert M, et al. Prediction of postoperative pain after gynecologic laparoscopy for nonacute pelvic pain. Am J Obstet Gynecol. 2014;211(4):360 e361368. doi:10.1016/j.ajog.2014.04.010
11. Golzari SE, Nader ND, Mahmoodpoor A. Underlying mechanisms of postoperative pain after laparoscopic surgery. JAMA Surg. 2016;151(3):295–296. doi:10.1001/jamasurg.2015.3934
12. Joe-Ikechebelu NN, Eleje GU, Ugwu EO, et al. A randomized controlled trial on efficacy and safety of trocar-site infiltration with lidocaine for postoperative pain relief after diagnostic laparoscopy. Gynecol Obstet Invest. 2019;84(1):71–78. doi:10.1159/000490565
13. Zdravkovic M, Kamenik M. A prospective randomized controlled study of combined spinal-general anesthesia vs. general anesthesia for laparoscopic gynecological surgery: opioid sparing properties. J Clin Anesth. 2020;64:109808. doi:10.1016/j.jclinane.2020.109808
14. Joo J, Moon HK, Moon YE. Identification of predictors for acute postoperative pain after gynecological laparoscopy (STROBE-compliant article). Medicine. 2019;98(42):e17621. doi:10.1097/MD.0000000000017621
15. Zhang XK, Chen QH, Wang WX, Hu Q. Evaluation of dexmedetomidine in combination with sufentanil or butorphanol for postoperative analgesia in patients undergoing laparoscopic resection of gastrointestinal tumors: A quasi-experimental trial. Medicine. 2016;95(50):e5604. doi:10.1097/MD.0000000000005604
16. Kundu S, Weiss C, Hertel H, Hillemanns P, Klapdor R, Soergel P. Association between intraabdominal pressure during gynaecologic laparoscopy and postoperative pain. Arch Gynecol Obstet. 2017;295(5):1191–1199. doi:10.1007/s00404-017-4325-9
17. Nie Y, Tu W, Shen X, et al. Dexmedetomidine added to sufentanil patient-controlled intravenous analgesia relieves the postoperative pain after cesarean delivery: a prospective randomized controlled multicenter study. Sci Rep. 2018;8(1):9952. doi:10.1038/s41598-018-27619-3
18. Turi S, Deni F, Lombardi G, Marmiere M, Nisi FG, Beretta L. Sufentanil Sublingual Tablet System (SSTS) for the management of postoperative pain after major abdominal and gynecological surgery within an ERAS protocol: an observational study. J Pain Res. 2019;12:2313–2319. doi:10.2147/JPR.S214600
19. Li J, Li Y, Huang Z. Effect of dexmedetomidine on analgesia and sedation of sufentanil during anesthesia induction period of gynecological surgery. Pak J Pharm Sci. 2020;33(1(Special)):429–432.
20. Jung SM, Yang CW, Oh JY, et al. Predicted effect-site concentration of propofol and sufentanil for gynecological laparoscopic surgery. Acta Anaesthesiol Scand. 2011;55(1):110–117. doi:10.1111/j.1399-6576.2010.02327.x
21. Damen SL, Nieuwenhuijs VB, Joosten W, Houweling PL, Clevers GJ. The effects of remifentanil and sufentanil on the quality of recovery after day case laparoscopic cholecystectomy: a randomized blinded trial. J Laparoendosc Adv Surg Tech A. 2004;14(2):87–92. doi:10.1089/109264204322973853
22. Son I, Oh CS, Choi JW, Kim SH. The effect of sufentanil administration on remifentanil-based anaesthesia during laparoscopic gynaecological surgery: a double-blind randomized controlled trial. ScientificWorldJournal. 2014;2014:701329. doi:10.1155/2014/701329
23. Song IK, Lee JH, Jung S, Kim JT, Kim HS. Estimation of the plasma effect site equilibration rate constant of sufentanil in children using the time to peak effect of heart rate and blood pressure. Indian J Pharmacol. 2015;47(4):360–364. doi:10.4103/0253-7613.161251
24. Song IK, Ji SH, Kim EH, Lee JH, Kim JT, Kim HS. Comparison of the effect of different infusion rates of sufentanil on surgical stress index during cranial pinning in children under general anaesthesia: a randomized controlled study. BMC Anesthesiol. 2017;17(1):167. doi:10.1186/s12871-017-0448-6
25. Oh SK, Lee IO, Lim BG, et al. Comparison of the analgesic effect of sufentanil versus fentanyl in intravenous patient-controlled analgesia after total laparoscopic hysterectomy: a randomized, double-blind, prospective study. Int J Med Sci. 2019;16(11):1439–1446. doi:10.7150/ijms.34656
26. Bingol Tanriverdi T, Koceroglu I, Devrim S, Gura Celik M. Comparison of sedation with dexmedetomidine vs propofol during hysteroscopic surgery: single-centre randomized controlled trial. J Clin Pharm Ther. 2019;44(2):312–317. doi:10.1111/jcpt.12793
27. Liu L, Yuan Q, Wang Y, et al. Effects of dexmedetomidine combined with sufentanil on postoperative delirium in young patients after general anesthesia. Med Sci Monit. 2018;24:8925–8932. doi:10.12659/MSM.911366
28. Blumenthal S, Borgeat A, Nadig M, Min K. Postoperative analgesia after anterior correction of thoracic scoliosis: a prospective randomized study comparing continuous double epidural catheter technique with intravenous morphine. Spine. 2006;31(15):1646–1651. doi:10.1097/01.brs.0000224174.54622.1b
29. Soltesz S, Biedler A, Silomon M, Schopflin I, Molter GP. Recovery after remifentanil and sufentanil for analgesia and sedation of mechanically ventilated patients after trauma or major surgery. Br J Anaesth. 2001;86(6):763–768. doi:10.1093/bja/86.6.763
30. Sun S, Huang SQ. Effects of pretreatment with a small dose of dexmedetomidine on sufentanil-induced cough during anesthetic induction. J Anesth. 2013;27(1):25–28. doi:10.1007/s00540-012-1470-y
31. Lee JY, Lim BG, Park HY, Kim NS. Sufentanil infusion before extubation suppresses coughing on emergence without delaying extubation time and reduces postoperative analgesic requirement without increasing nausea and vomiting after desflurane anesthesia. Korean J Anesthesiol. 2012;62(6):512–517. doi:10.4097/kjae.2012.62.6.512
32. Scott JC, Cooke JE, Stanski DR. Electroencephalographic quantitation of opioid effect: comparative pharmacodynamics of fentanyl and sufentanil. Anesthesiology. 1991;74(1):34–42. doi:10.1097/00000542-199101000-00007
33. Olsen MF, Bjerre E, Hansen MD, et al. Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 2017;15(1):35. doi:10.1186/s12916-016-0775-3
34. Rehberg B, Mathivon S, Combescure C, Mercier Y, Savoldelli GL. Prediction of acute postoperative pain following breast cancer surgery using the pain sensitivity questionnaire: a cohort study. Clin J Pain. 2017;33(1):57–66. doi:10.1097/AJP.0000000000000380
35. Czarnecki ML, Turner HN, Collins PM, Doellman D, Wrona S, Reynolds J. Procedural pain management: a position statement with clinical practice recommendations. Pain Manag Nurs. 2011;12(2):95–111. doi:10.1016/j.pmn.2011.02.003
36. Yang Y, Wu J, Li H, et al. Prospective investigation of intravenous patient-controlled analgesia with hydromorphone or sufentanil: impact on mood, opioid adverse effects, and recovery. BMC Anesthesiol. 2018;18(1):37. doi:10.1186/s12871-018-0500-1
37. Choi JW, Joo JD, Kim DW, et al. Comparison of an intraoperative infusion of dexmedetomidine, fentanyl, and remifentanil on perioperative hemodynamics, sedation quality, and postoperative pain control. J Korean Med Sci. 2016;31(9):1485–1490. doi:10.3346/jkms.2016.31.9.1485
38. Jung HS, Joo JD, Jeon YS, et al. Comparison of an intraoperative infusion of dexmedetomidine or remifentanil on perioperative haemodynamics, hypnosis and sedation, and postoperative pain control. J Int Med Res. 2011;39(5):1890–1899. doi:10.1177/147323001103900533
39. Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg. 2013;26(3):191–196. doi:10.1055/s-0033-1351138
40. Tang C, Huang X, Kang F, et al. Intranasal dexmedetomidine on stress hormones, inflammatory markers, and postoperative analgesia after functional endoscopic sinus surgery. Mediators Inflamm. 2015;2015:939431. doi:10.1155/2015/939431
41. Cabanero D, Puig MM. Immediate and delayed remifentanil-induced hypersensitivity. Anesth Analg. 2012;115(4):977–978. (). doi:10.1213/ANE.0b013e318263ca82
42. Hu LG, Pan JH, Li J, Kang F, Jiang L. Effects of different doses of sufentanil and remifentanil combined with propofol in target-controlled infusion on stress reaction in elderly patients. Exp Ther Med. 2013;5(3):807–812. doi:10.3892/etm.2013.900
43. Gottschalk A, Ochroch EA. Preemptive analgesia: what do we do now? Anesthesiology. 2003;98(1):280–281. (). doi:10.1097/00000542-200301000-00047
44. Qi Y, Yao X, Zhang B, Du X. Comparison of recovery effect for sufentanil and remifentanil anesthesia with TCI in laparoscopic radical resection during colorectal cancer. Oncol Lett. 2016;11(5):3361–3365. doi:10.3892/ol.2016.4394
45. Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia II: recent advances and current trends. Canadian J Anaesthesia. 2001;48(11):1091–1101. doi:10.1007/BF03020375
46. Bailey PL, Streisand JB, East KA, et al. Differences in magnitude and duration of opioid-induced respiratory depression and analgesia with fentanyl and sufentanil. Anesth Analg. 1990;70(1):8–15. doi:10.1213/00000539-199001000-00003
47. Subrahmanyam M, Sreelakshmi B. Comparison of total intravenous anaesthesia using propofol with or without sufentanil in laparoscopic cholecystectomies. Indian J Anaesth. 2009;53(4):467–474.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
