Back to Journals » Drug Design, Development and Therapy » Volume 13
Effects of acarbose and metformin on the inflammatory state in newly diagnosed type 2 diabetes patients: a one-year randomized clinical study
Authors Mo D, Liu S, Ma H, Tian H , Yu H, Zhang X, Tong N, Liao J , Ren Y
Received 11 March 2019
Accepted for publication 8 July 2019
Published 9 August 2019 Volume 2019:13 Pages 2769—2776
DOI https://doi.org/10.2147/DDDT.S208327
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Dan Mo,1 Songfang Liu,1 Hong Ma,1 Haoming Tian,1 Honglin Yu,2 Xiangxun Zhang,2 Nanwei Tong,2 Jiayu Liao,3,4 Yan Ren1
1Division of Endocrinology and Metabolism, West China Hospital of Sichuan University, Chengdu 610041, People’s Republic of China; 2Laboratory of Endocrinology and Metabolism, West China Hospital of Sichuan University, Chengdu 610041, People’s Republic of China; 3Department of Bioengineering, Bourns College of Engineering, University of California, Riverside, CA 92521, USA; 4West China Hospital-California Multiomics Research Center, Key Laboratory of Transplant Engineering and Immunology, National Health Commission of PRC, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China
Objective: This study aimed to investigate the changes in inflammatory biomarkers between newly diagnosed type 2 diabetes (T2DM) patients under one-year acarbose treatments and those under metformin managements.
Methods: Seventy patients with newly diagnosed T2DM and 32 volunteers with normal glucose tolerance (normal controls, NCs) were enrolled. Seventy patients with T2DM were randomly assigned to two subgroups and treated with acarbose (n=34) or metformin (n=36) for 1 year. Blood glucose, insulin, glycosylated hemoglobin (A1C), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and inflammatory biomarker levels (interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-2 (IL-2), and ferritin) were detected at 0, 6 and 12 months.
Results: After adjusting for sex, the waist-to-hip ratio (WHR) and body mass index (BMI), higher fasting plasma glucose (FPG), standard meal test 1/2 hr and 2 hr glucose, TG, TC, LDL-C, IL-6, TNF-α, IL-2 and ferritin levels were observed in T2DM group than in NCs (P<0.05). After 6 months of treatment, TNF-α levels were significantly decreased in both subgroups, and IL-6 and ferritin levels were significantly decreased after 12 months (P<0.05). However, no significant differences in the IL-6, TNF-α and ferritin levels were observed between the two subgroups. Moreover, significantly higher IL-6 and TNF-α levels were detected in the T2DM group than in NCs after 12 months of treatment (P<0.05).
Conclusion: Patients with newly diagnosed T2DM exhibited a marked chronic inflammatory state characterized by increased IL-6, TNF-α, IL-1β, IL-2 and ferritin levels. After 1 year of treatment with acarbose or metformin, IL-6, TNF-α, IL-1β and ferritin levels were significantly decreased compared with the baseline. The anti-inflammatory effects of acarbose and metformin were comparable and required a long-term treatment (1 year), but the characteristics were different. Further investigations are needed to determine whether this effect was independent of the hypoglycemic effects.
Keywords: newly diagnosed type 2 diabetes, inflammatory biomarkers, acarbose, metformin
Introduction
Many studies have revealed an important role for the abnormal activation of the inflammatory reaction in the development of T2DM and its macrovascular or microvascular complications.1–3 Interleukins, including IL-6, IL-1β, and IL-2 are important inflammatory factors. IL-6 suppresses the insulin signaling pathway by activating suppressor of cytokine signaling-3 (SOCS-3) and c-Jun N-terminal kinase (JNK), causes endothelial cell dysfunction and promotes the development of chronic micro- and macrovascular complications by stimulating monocyte chemotactic protein-1 and cell adhesion molecules.4 IL-1β is mainly regulated by diet-induced metabolic stress. Its binding to interleukin-1 receptor type I (IL-1RI) reduces the expression of insulin receptor substrate-1 (IRS-1), leads to islet cell damage and reduces the sensitivity of peripheral tissues to insulin.5 IL-1β is related to retinal dysfunction in subjects with T2DM.6 IL-2 is mainly secreted by Helper T Lymphocyte(TH) after antigen or mitogen stimulation and induces IL-1 secretion. Changes in the IL-2 signaling pathway have been reported to cause Treg dysfunction in subjects with T2DM.7 In addition, tumor necrosis factor-α (TNF-α), an adipocytokine, has been reported to induce T2DM by activating nuclear factor kappa-B (NF-κB), Jun NH2-terminal kinase (JNK) and other pathways, and may contribute to the development and progression of renal injury in individuals with T2DM.8 Ferritin is an index of the iron stores in the body. High ferritin levels are associated with chronic inflammation, which might cause metabolic disorder, including islet beta cell dysfunction and insulin resistance.9
Several hypoglycemic agents have been reported to ameliorate chronic inflammation and protect vessels in patients with T2DM. Metformin is a commonly used basic hypoglycemic agent, and its anti-inflammatory effects have been confirmed in numerous studies. Andrews M, et al observed had lower levels of C-reactive protein (CRP) and TNF-α in obese patients with diabetes who were treated with metformin. However, no differences in the glucose levels or lipid profiles were observed.10 A randomized, placebo-controlled trial showed significantly reduced CRP levels in 390 patients with T2DM after treatment with metformin; these patients were followed for 4.3 years.11 In these studies, the effects of metformin on IL-6, IL-1β, IL-2 and ferritin levels were not observed. However, the effect of metformin on vascular complications remains uncertain.12,13 Acarbose, an α-glycosidase inhibitor, is commonly used to treat Chinese patients with diabetes. Acarbose significantly decreases glucose and lipid levels and influences gastrointestinal functions.14 However, only one study showed that acarbose reduced IL-6 and CRP levels in patients with T2DM after 7 months of treatment compared with a placebo.15 Subgroup analyses in the STOP-NIDDM trial showed that acarbose administration reduces the intima media thickness and the risk of cardiovascular disease.16,17
In this prospective randomized controlled trial, we studied the effects of metformin and acarbose on the levels of the inflammatory factors IL-6, TNF-α, IL-1β, IL-2 and ferritin in patients with newly diagnosed T2DM to determine whether these two anti-hyperglycemic agents improve the chronic inflammatory state, a definite risk factor for diabetic complications.
Study design
Subjects
Newly diagnosed T2DM group (T2DM): Seventy patients were recruited from the outpatient department of West China Hospital, which is a MARCH research site.18,19 The first subject was recruited to our study on December the first, 2008. All patients were diagnosed with T2DM within 6 months and were not treated with any hypoglycemic agent. The average patient age ranged from 30 to 70 years, and the average BMI ranged from 19 to 30 kg/m2. The A1C level ranged from 7.0% to 10%, and the FPG level was ≤11.1 mmol/L. None of the subjects had an infection during the two weeks prior to the study, liver or kidney diseases, severe cardiovascular and hematological system diseases, and other endocrine or mental system diseases.
Normal control (NC) group: Thirty-two healthy volunteers with normal glucose tolerance were recruited.
Methods
After 4 weeks of lifestyle therapy administered according to Chinese diabetes management guidelines, all patients with newly diagnosed T2DM completed a standard meal test (70 g of instant noodles equivalent to 500 kilocalories of energy intake). Blood was collected at 0, 1/2 and 2 hrs after the standard meal. Then, patients in the T2DM group were randomly assigned (1:1) to the metformin-treated subgroup, which was administered 1500 mg of metformin once daily (500 mg per tablet, Beijing Double Crane Pharms, Beijing, China) or the acarbose-treated subgroup, which was administered 300 mg of acarbose once daily (50 mg per tablet, Bayer Healthcare, Beijing, China). Acarbose was initially administered at a dose of 50 mg once a day at dinner during the first week and titrated up to 50 mg twice a day at lunch and dinner during the second week, 50 mg three times a day at three meals during the third week, and 100 mg three times a day from the fourth week onward. Metformin was initially administered at a dose of 500 mg once a day after dinner during the first 2 weeks and titrated up to 1000 mg once a day after dinner during the third week and 1500 mg once a day after dinner from the fourth week onward. The T2DM group was scheduled for follow-up every 2 weeks during the first 4 weeks and then every 4 weeks thereafter.
The height, weight, waist circumference, hip circumference, and blood glucose, insulin, A1C, TG, TC, HDL-C, LDL-C, IL-6, TNF-α, IL-1β, IL-2 and ferritin levels were measured at baseline, 24 weeks, and 48 weeks after treatment. The blood glucose, insulin, A1C, TG, TC, HDL-C, LDL-C, IL-6, TNF-α, IL-1β, IL-2 and ferritin levels were detected using standard laboratory procedures. Glucose was measured using the glucose oxidase method, insulin was measured using a radioimmunoassay, TG and TC were measured using enzymatic methods, and HDL-C and LDL-C were measured using the phosphor-tungstic acid precipitation method. IL-6, TNF-α, IL-2, and IL-1β were measured using enzyme immunoassay kits (R&D systems, Minneapolis, minn), and ferritin was measured using a radioimmunoassay kit (Beijing Research Institute of biotechnology of the North, Beijing, China).
Statistical analyses
The datasets analyzed during the current study are available from the corresponding author on reasonable request within ten years of the publication. The data were analyzed using Excel and presented as means ± standard deviations. An independent samples t-test or variance-covariance analysis was used to compare the data between the two subgroups. A paired t-test was used to compare the pre- and post-treatment data in the same subgroup. All P-values were two-tailed, and P<0.05 was considered statistically significant. All statistical analyses mentioned above were performed using SPSS version 25.0 for Windows software.
Compliance with ethical guidelines
This study’s design was approved by the Medical ethics committee of West China Hospital of Sichuan university. All procedures performed in the study were conducted in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants who were included in the study.
Results
Comparison of the biochemical parameters of the T2DM and NC groups
The average age was comparable between the two groups, while the sex distribution, BMI and WHR significantly differed between the two groups (P<0.05). After adjusting for sex, BMI and WHR, the levels of the 0 hr, 1/2 hr and 2 hr glucose, A1C, TG, TC, and LDL-C in the T2DM group were significantly higher than those in the NC group (P<0.05); 1/2 hr and 2 hr insulin levels were detected in the T2DM group than in the NC group (P<0.05). After adjusting for sex, BMI and WHR, the levels of TG, TC and LDL-C levels, IL-6, TNF-α, IL-2 and ferritin in the T2DM group were higher than in the NC group (P<0.05), but IL-1β levels were comparable between the two groups (Table 1).
 |
Table 1 Comparison of the baseline parameters of the T2DM and NC groups |
Comparison of the parameters between the acarbose-treated T2DM subgroup and metformin-treated T2DM subgroup
The age and sex distributions were comparable between the two subgroups. Greater waist circumferences and WHR were observed in the metformin subgroup than in the acarbose subgroup (P<0.05). The 0 hr, 1/2 hr, and 2 hr glucose, insulin, A1C, TG, TC, HDL-C, LDL-C, IL-6, TNF-α, IL-1β, IL-2 and ferritin levels at baseline were comparable between the two subgroups (Table 2).
 |
Table 2 Comparison of the parameters at baseline between the acarbose group and metformin group |
Comparison of the levels of glucose, insulin and inflammatory factors in the T2DM group before and after treatment
Compared with the baseline, the 0 hr, 1/2 hr, and 2 hr glucose, A1C and TNF-α levels were significantly decreased in the T2DM group after 6 months of acarbose and metformin treatment (P<0.05). Compared with the baseline levels, IL-6 levels were significantly reduced in all patients with T2DM after 12 months of treatment compared with the levels recorded after 6 months of treatment (P<0.05), but significant differences were not observed between the two subgroups (P>0.05) (Table 3).
 |
Table 3 Comparison of glucose, insulin and inflammatory factors before and after treatment |
In the acarbose-treated T2DM subgroup, lower 0 hr, 1/2 hr and 2 hr insulin levels were measured after 6 months than at baseline (P<0.05). After 12 months of treatment, lower 2 hr insulin and ferritin levels were observed than at baseline (P<0.05). In the metformin-treated T2DM subgroup, a significantly lower ferritin level was observed after 6 months of treatment than at baseline (P<0.05). However, a further decrease was not observed after 12 months of treatment. After 6 months or 12 months of treatment, the 0 hr, 1/2 hr and 2 hr insulin levels did not significantly differ from the levels recorded at baseline (Table 3). After 6 months of treatment, the 0 hr, 1/2 hr and 2 hr glucose, IL-1β, TNF-α and ferritin levels were significantly lower than the baseline levels in the T2DM group treated with acarbose or metformin (P<0.05), and the IL-6 level was also significantly decreased after 12 months of treatment (P<0.05) (Table 3).
Comparison of inflammatory factors in the NC group and T2DM group after 12 months of treatment
In the NC group and T2DM group, the age, BMI, waist circumference, and hip circumference were comparable, but sex and WHR differed (P<0.05). After adjusting for sex and WHR, the levels of IL-6, TNF-α, IL-2 and ferritin in the T2DM group were still higher after 12 months than in the NC group (P<0.05), but no significant differences in IL-1β levels were observed between these two groups (Table 4).
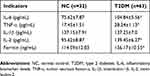 |
Table 4 Comparison of the inflammation factors in the NC and T2DM groups after 12 months of treatment |
Comparison of the degree of variability in glucose, A1C, insulin, IL-6, IL-2, TNF-α, IL-1β and ferritin levels in the two T2DM subgroups before and after treatment
Compared with the metformin subgroup, the 1/2 hr glucose and insulin levels measured in the standard meal test exhibited a more significant decrease after 6 months of treatment and the 1/2 hr and 2 hr glucose and insulin levels measured in the standard meal test were significantly decreased after 12 months of treatment with acarbose (P<0.05). After 6 months or 12 months of treatment, the variation margins of IL-2, IL-6, IL-1β, TNF-α and ferritin levels were similar between individuals treated with the two drugs (Table 5).
 |
Table 5 Comparison of the variation margin of glucose, insulin and inflammatory factors in the two groups |
Discussion
The levels of inflammatory biomarkers, such as IL-6, TNF-α and IL-2, are increased in patients with T2DM.2,18 Our study revealed the same results in patients with newly diagnosed T2DM, who exhibited significantly higher IL-6, TNF-α, IL-2 and ferritin levels than the NCs, indicating that T2DM is characterized by a chronic inflammatory state.
Based on accumulating evidence, the inflammatory state is involved in the development of microvascular and macrovascular complications in patients with T2DM.8,19,20 Theoretically, anti-inflammatory therapy can prevent cardiovascular complications and improve the patients’ quality of life. Metformin, a basic, widely used hypoglycemic agent, has been found to reduce the levels of two major inflammatory biomarkers, ie, CRP and TNF-α.10,11 Compared with a placebo, acarbose decreases the concentrations of IL-6 and TNF-α.15,21 However, no studies investigated the effects of long-term treatment with metformin or acarbose on the levels of three other major inflammatory cytokines, IL-1β, IL-2 and ferritin, or compared the effects of these two drugs on the levels of inflammatory biomarkers.22 In this prospective randomized controlled study, compared to the baseline, the IL-6, TNF-α and ferritin levels were significantly decreased in the T2DM group after treatment, suggesting that acarbose and metformin both improved the chronic inflammatory state in patients with T2DM. Additional long-term, large-scale clinical trials are needed to determine whether these treatments prevent the development of complications.
Metformin may exert its anti-inflammatory effects by activating AMP-activated protein kinase, antioxidant activity, and insulin sensitization.23–25 Acarbose can indirectly improve insulin resistance.15,19,26 As shown in the study by Fukaya N, et al, the alpha-glucosidase inhibitor miglitol reduces the hyperglycemia-induced mRNA expression of inflammatory cytokines in peripheral blood leukocytes.27 However, researchers have not clearly determined whether the anti-inflammatory mechanisms of metformin and acarbose rely on indirect effects, such as improving insulin sensitivity and reducing glucose, or direct anti-inflammatory effects. In the present study, the TNF-α and ferritin levels decreased significantly after 6 months of treatment with metformin, while ferritin levels decreased at 12 months after treatment with acarbose, indicating these two drugs may improve inflammation through different mechanisms. Considering their distinct hypoglycemic and anti-inflammatory mechanisms, we speculated that a combination therapy including metformin and acarbose may exert a synergistic effect on inflammation.
The changes in the levels of these inflammatory cytokines were different in our study. TNF-α and IL-1β levels were significantly decreased in all patients with newly diagnosed T2DM after 6 months of treatment, and the IL-2, IL-6, and ferritin levels only showed a decreasing trend. After 12 months of treatment, the IL-6 levels were significantly reduced. Based on these results, a separate analysis of IL-1β levels in the metformin and acarbose subgroups was performed, and a significant reduction was not observed after treatment. Based on these results, the chronic inflammatory status was not improved as quickly as glucose metabolism and required a long-lasting therapy. Further research is needed to determine the duration of treatment required take to significantly improve the inflammatory status.
Furthermore, even after one year of hypoglycemic treatment and good glucose control, the patients still presented higher levels of these inflammatory cytokines than the NC group, suggesting that the inflammatory state in patients with T2DM was severe and persistent. Further study should consider whether direct anti-inflammatory drugs should be administered in the early therapeutic regimen, even in patients with newly diagnosed T2DM.
Aspirin, a nonsteroidal anti-inflammatory drug (NSAID), has been reported to inhibit the rolling and adhesion of T cells induced by TNF-α and reduce the levels of cytokines and other inflammatory markers, such as CRP and IL-6,28–30 Statins significantly reduce the levels of inflammatory factors independent of their effects on lipids.31,32Angiotensin-converting enzyme inhibitors (ACEIs) and adrenergic receptor-binding agents (ARBs) might exert anti-inflammatory effects since angiotensin II is a precursor inflammatory peptide, and ARBs inhibit the generation of oxygen species and inflammatory mediators.33,34 Two large studies investigating cardiovascular events as endpoints showed that ramipril and losartan reduce the incidence of T2DM, potentially due to their anti-inflammatory effects.35,36 In addition, the inflammatory factor inhibitors, including a TNF-α inhibitor (infliximab) and interleukin-1 receptor antagonist (IL-1Ra), have been confirmed to exert both anti-inflammatory and hypoglycemic effects on an animal model of T2DM.3,37,38 However, aspirin applications are limited since the absolute benefits in reducing risk of vascular complications in patients with diabetes who do not present an evident cardiovascular disease are largely counterbalanced by the risk of bleeding.33 Additionally, the administration of statins, ACEIs and ARBs should depend on the patient’s blood lipid levels and blood pressure. Further studies are needed to determine whether these drugs are able to be used earlier to control the chronic inflammatory state and prevent the development of chronic complications of diabetes.
This study has several limitations. First, the sample size was a bit small. Second, this study did not include a placebo control group due to ethical considerations. Finally, the treatment duration was one year, and we did not clearly determine whether the effect of a subsequent longer term treatment on the levels of inflammatory factors reduced vascular complications.
In summary, the IL-6, TNF-α, IL-2 and ferritin levels were significantly increased in patients with newly diagnosed T2DM. One year of treatment with metformin or acarbose significantly improved the blood glucose levels and reduced the levels of these inflammatory factors. However, the anti-inflammatory effects of metformin and acarbose differed. Additionally, the anti-inflammatory effects of these two hypoglycemic drugs were only detected after a long-term treatment (1 year). These results must be confirmed and clarified in additional large-sample prospective studies.
Ethics and consent statement
The Metformin and Acarbose in Chinese as the initial Hypoglycemic treatment (MARCH) Study (Registry number: ChiCTR-TRC-08000231) compared the medical outcomes of two different glycemic treatment approaches. This study was a non-inferiority, multicenter randomized controlled trial that tested two drug interventions (metformin and acarbose). Our study was performed at one of the 11 clinical sites in China invited to participate, which had received ethical approval. All trial participants who were enrolled in the present study provided written informed consent (no additional registration of RCT), and the study was performed in accordance with the principles of the Declaration of Helsinki.
Acknowledgments
This study was supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant No. ZYGD18022), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant No. ZYJC18023), and Sichuan Provincial Science and Technology Foundation (grant No. 2015SZ0228).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Akash MSH, Rehman K, Liaqat A, Numan M, Mahmood Q, Kamal S. Biochemical investigation of gender-specific association between insulin resistance and inflammatory biomarkers in types 2 diabetic patients. Biomed Pharmacother. 2018;106:285–291. doi:10.1016/j.biopha.2018.06.044
2. Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine. 2016;86:100–109. doi:10.1016/j.cyto.2016.06.028
3. Kanwal R, Akash MSH. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? Biomed Sci. 2016;23:87. doi:10.1186/s12929-016-0303-y
4. Wegner M, Araszkiewicz A, Piorunska-Stolzmann M, Wierusz-Wysocka B, Zozulinska-Ziolkiewicz D. Association between IL-6 concentration and diabetes-related variables in DM1 patients with and without microvascular complications. Inflammation. 2013;36:723–728. doi:10.1007/s10753-013-9598-y
5. D’Amico AG, Maugeri G, Rasà DM, et al. Modulation of IL-1β and VEGF expression in rat diabetic retinopathy after PACAP administration. Peptides. 2017;97:64–69. doi:10.1016/j.peptides.2017.09.014
6. Capitão M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem. 2016;117:2443–2453. doi:10.1002/jcb.25575
7. Sheikh V, Zamani A, Mahabadi-Ashtiyani E, Tarokhian H, Borzouei S, Alahgholi-Hajibehzad M. Decreased regulatory function of CD4CD25CD45RA T cells and impaired IL-2 signalling pathway in patients with T2DM. Scand J Immunol. 2018;88(4):e12711. doi:10.1111/sji.2018.88.issue-4
8. Domingueti CP, Dusse LMS, das Graças Carvalho M, et al. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. Diabetes Complicat. 2016;30:738–745. doi:10.1016/j.jdiacomp.2015.12.018
9. Mascitelli L, Goldstein MR, Zacharski LR, et al. Association of multiple biomarkers of iron metabolism and type 2 diabetes: the EPIC-interact study. Diabetes Care. 2016;39:572–581. doi:10.2337/dc15-0257
10. Andrews M, Soto N, Arredondo M, et al. Effect of metformin on the expression of tumor necrosis factor-α, toll like receptors 2/4 and C reactive protein in obese type-2 diabetic patients. Rev Med Chil. 2012;140(11):1377–1382. doi:10.4067/S0034-98872012001100001
11. Jager J, Kooy A, Schalkwijk C, et al. Long-term effects of metformin on endothelial function in type 2 diabetes: a randomized controlled trial. J Intern Med. 2014;275(1):59–70. doi:10.1111/joim.12128
12. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
13. Griffin Simon J, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017;60:1620–1629. doi:10.1007/s00125-017-4337-9
14. Derosa G, Maffioli P, D’Angelo A, et al. Acarbose on insulin resistance after an oral fat load: a double-blind, placebo-controlled study. J Diabetes Complications. 2011;25:258–266. doi:10.1016/j.jdiacomp.2011.01.003
15. Giuseppe D, Pamela M, Ilaria F, et al. Acarbose actions on insulin resistance and inflammatory parameters during an oral fat load. Eur J Pharmacol. 2011;651(1–3):240–250. doi:10.1016/j.ejphar.2010.11.015
16. Chiasson J-L. Acarbose for the prevention of diabetes, hypertension, and cardiovascular disease in subjects with impaired glucose tolerance: the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) trial. Endocr Pract. 2006;12(Supplement 1):25–30. doi:10.4158/EP.12.S1.25
17. Markolf H. Treatment of impaired glucose tolerance with acarbose and its effect on intima-media thickness: a substudy of the STOP-NIDDM trial (study to prevent non-insulin-dependent diabetes mellitus). Endocr Pract. 2006;12(Supplement 1):56–59. doi:10.4158/EP.12.S1.56
18. Phosat C, Panprathip P, Chumpathat N, et al. Elevated C-reactive protein, interleukin 6, tumor necrosis factor alpha and glycemic load associated with type 2 diabetes mellitus in rural thais: a cross-sectional study. BMC Endocr Disord. 2017;17(1):44. doi:10.1186/s12902-017-0189-z
19. Lowe G, Woodward M, Hillis G, et al. Circulating inflammatory markers and the risk of vascular complications and mortality in people with type 2 diabetes and cardiovascular disease or risk factors: the ADVANCE study. Diabetes. 2014;63(3):1115–1123. doi:10.2337/db12-1625
20. Hsieh MC, Tien KJ, Chang SJ, et al. High sensitivity C-reactive protein and silent myocardial ischemia in Chinese with type 2 diabetes mellitus. Metabolism. 2008;57(11):1533–1538. doi:10.1016/j.metabol.2008.06.007
21. Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6, expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280(5):E745–E751. doi:10.1152/ajpendo.2001.280.5.E745
22. Kothari V, Galdo JA, Mathews ST. Hypoglycemic agents and potential anti-inflammatory activity. J Inflamm Res. 2016;9:27–38.
23. Saisho Y. Metformin and inflammation: its potential beyond glucose lowering effect. Endocr Metab Immune Disord Drug Targets. 2015;15:196–205. doi:10.2174/1871530315666150316124019
24. Zheng Z, Chen H, Li J, et al. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes. 2012;61:217–228. doi:10.2337/db11-1296
25. Han X
26. Chen P-H, Tsai Y-T, Wang J-S, et al. Post-meal β-cell function predicts the efficacy of glycemic control in patients with type 2 diabetes inadequately controlled by metformin monotherapy after addition of glibenclamide or acarbose. Diabetol Metab Syndr. 2014;6:68.
27. Fukaya N, Mochizuki K, Shimada M, et al. The alpha-glucosidase inhibitor miglitol decreases glucose fluctuations and gene expression of inflammatory cytokines induced by hyperglycemia in peripheral leukocytes. Nutrition. 2009;25:657–667. doi:10.1016/j.nut.2008.11.015
28. Khajehdehi P, Roozbeh J, Mostafavi H, et al. A comparative randomized and placebo-controlled short-term trial of aspirin and dipyridamole for overt type 2 diabetic nephropathy. Scand J Urol Nephrol. 2002;36(2):145–148. doi:10.1080/003655902753679454
29. Sun X, Han F, Yi J, Han L, Wang B. Effect of aspirin on the expression of hepatocyte NF-kappa B and serum TNF-alpha in streptozotocin-induced type 2 diabetic rats. J Korean Med Sci. 2011;26:765–770. doi:10.3346/jkms.2011.26.6.765
30. Jiang XZ, Zhe G, Bin Z, et al. Effects of aspirin on inflammatory factors of type 2 diabetes mellitus patients. Chin J New Drugs and Clin Remedies. 2009;28(4):297–300.
31. Shu Z, Jinshan W, Qing Z, et al. Effect of atorvastatin and rosiglitazone on inflammatory factors in patients with type 2 diabetes mellitus association with unstable angina. J Qingdao Univ. 2009;45(3):274–276.
32. Ukinc K, Ersoz HO, Erem C, Hacihasanoglu AB, Karti SS. Effects of one year simvastatin and atorvastatin treatments on acute phase reactants in uncontrolled type 2 diabetic patients. Endocrine. 2009;35:380–388. doi:10.1007/s12020-009-9157-3
33. Dandona P, Kumar V, Aljada A, et al. Angiotensin II receptor blocker valsartan suppresses reactive oxygen species(ROS)generation in leucocytes, nuclear factor-KB (NF-KB), and activator protein-l (AP-1) in mononuclear cells (MNC) of normal subjects: evidence of an anti-inflammation action. J Clin Endocrinol Metab. 2003;88(9):496–4501.
34. Pavlatou MG, Mastorakos G, Margeli A, et al. Angiotensin blockade in diabetic patients decreases insulin resistance-associated low-grade inflammation. Eur J Clin Invest. 2011;41:652–658. doi:10.1111/j.1365-2362.2010.02453.x
35. DREAM on Investigators, Gerstein HC, Mohan V, et al. Long-term effect of rosiglitazone and/or ramipril on the incidence of diabetes. Diabetologia. 2011;54(3):487–495. doi:10.1007/s00125-010-1985-4
36. Shahinfar S, Lyle PA, Zhang Z, Keane WF, Brenner BM. Losartan: lessons learned from the RENAAL study. Expert Opin Pharmaco Ther. 2006;7(5):623–630. doi:10.1517/14656566.7.5.623
37. Araujo EP, De Souza CT, Ueno M, et al. Infliximab restores glucose homeostasis in an animal model of diet‐induced obesity and diabetes. Endocrinology. 2007;148:5991–5997. doi:10.1210/en.2007-0132
38. Akash MSH, Rehman K, Sun H, Chen S. Interleukin-1 receptor antagonist improves normoglycemia and insulin sensitivity in diabetic goto-kakizaki-rats. Eur J Pharmacol. 2013;701:87–95. doi:10.1016/j.ejphar.2013.01.008
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
