Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Effects of a cream containing 5% hyaluronic acid mixed with a bacterial-wall-derived glycoprotein, glycyrretinic acid, piroctone olamine and climbazole on signs, symptoms and skin bacterial microbiota in subjects with seborrheic dermatitis of the face
Authors Puviani M , Campione E , Offidani AM, De Grandi R, Bianchi L, Bobyr I , Giannoni M, Campanati A, Bottagisio M , Bidossi A , De Vecchi E , Eisendle K , Milani M
Received 19 February 2019
Accepted for publication 1 April 2019
Published 2 May 2019 Volume 2019:12 Pages 285—293
DOI https://doi.org/10.2147/CCID.S205904
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Mario Puviani,1 Elena Campione,2 Anna Maria Offidani,3 Roberta De Grandi,4 Luca Bianchi,2 Ivan Bobyr,3 Melania Giannoni,3 Anna Campanati,3 Marta Bottagisio,4 Alessandro Bidossi,4 Elena De Vecchi,4 Klaus Eisendle,5 Massimo Milani5,6
1Medica Plus Dermatology Outpatients Clinic, Modena, Italy; 2Dermatology Clinic, Tor Vergata University, Rome, Italy; 3Dermatology Clinic, Ospedali Riuniti Ancona University, Ancona, Italy; 4Laboratory of Clinical Chemistry and Microbiology, IRCCS Orthopedic Institute Galeazzi, Milan, Italy; 5Dermatology Clinic, Ospedale Centrale di Bolzano (BZ), Bolzano, BZ, Italy; 6Medical Department Cantabria Labs, Difa Cooper, Caronno Pertusella, VA, Italy
Objective: A new cream formulation containing hyaluronic acid 5%, complexed with a mix of a bacterial-wall-derived glycoprotein and peptide glycan complex (EDS), has been recently developed. We evaluated in a prospective, assessor-blinded, 6-week study the efficacy and tolerability of EDS in the treatment of facial seborrheic dermatitis (SD) and the effects on skin microbiota.
Subjects and methods: Seventy-five subjects (mean age 46; 60 men) with moderate-severe SD of the face were enrolled. EDS cream was applied twice daily. The primary outcome was the evolution of the Investigator Global Assessment (IGA) score, evaluating erythema, scale/flaking, grade of seborrhea and itch. Superficial skin bacterial microbiome at baseline and after treatment was assessed, using the 16S rRNA gene methodology, in affected and non-affected face areas. Local tolerability was evaluated checking self-reported side effects at each visit.
Results: Baseline IGA scores (mean±SD) was 10±3. The use of EDS reduced IGA score significantly by 70% at week 3 and by 88% at week 6. An increase in the abundance of Cutibacterium acnes genera associated with a significant drop of Staphylococcus genera presence was detected in affected areas. The ratio of relative abundance of genera Cutibacterium/Staphylococcus increased significantly after treatment in affected areas. The product was very well tolerated.
Conclusion: Treatment with EDS applied twice daily for 6 consecutive weeks was associated with a reduction of the signs and symptoms of SD. Furthermore, after EDS cream treatment, a reequilibrating effect on facial skin microbiota was observed. The product was very well tolerated.
Keywords: seborrheic dermatitis, skin microbiota, hyaluronic acid, assessor blinded trial
Introduction
Seborrheic dermatitis (SD) is a common chronic-recurrent skin disease which could affect up to 10% of adult population.1 SD is more common in men between the age of 20 and 50 years.2 Skin regions rich in sebaceous glands are commonly affected. In the pathogenesis of SD, a relevant role seems to be played by the lipophilic yeasts Malassezia spp that are also found in healthy skin.3 So far, a consensus has not been reached regarding the exclusive pathogenetic role of Malassezia spp in SD.4 Recent data have shown that a relevant alteration of superficial bacterial microbiota (reduction of Cutibacterium acnes and increase of Staphylococcus spp, in particular S. epidermidis) is observed in subjects with SD.5,6 Specifically, SD-affected regions present decreased Cutibacterium acnes population and an increase of Staphylococcus spp.7 Therefore, in SD there is a skin microbial disequilibrium which is correlated with the clinical severity of this skin condition. A new corticosteroid-free cream formulation of hyaluronic acid 3.5%, conjugated with a bacterial-wall-derived glycoproteins and peptide glycans complex (GPPG-complex), dimethicone 1%, glycyrrhetinic acid 0.25%, piroctone olamine 0.5% and climbazole 0.5% (EDS) has been recently developed. The cream has shown in a published open uncontrolled trial8 to improve significantly signs and symptoms of SD by 83% in comparison with baseline. However, no data are available, so far, regarding the potential benefits of this cream on facial bacterial microbiota.
Study aim
We performed this trial to evaluate the efficacy and tolerability of EDS in the treatment of facial SD in adult subjects and the effects on skin superficial facial bacterial microbiota. The trial was designed as a prospective, multi-center, open, assessor-blinded study.
Subjects and methods
Population and study design
Between February and December 2017, 115 subjects were screened for inclusion in the trial. Seventy-five subjects, meeting inclusion criteria (mean age 46; 60 men and 15 women), with moderate-severe facial SD were enrolled, after their written informed consent, in a prospective 6-week assessor-blinded study. Permissions for the use, after deidentification procedures, of face pictures of enrolled subjects to document clinical evolution were obtained from each patient. In addition, all the subjects have provided written informed consent for the images to be published. EDS cream was applied twice daily on the most affected areas (mainly face). Local tolerability was evaluated checking self-reported side effects at each visit. Compliance was evaluated counting returned tubes at week 3 and week 6. The trial took place in three Dermatology Clinics in Italy between February 2017 and March 2018. The study protocol was approved by the coordinating center (Investigational Review Board, Tor Vergata University, Rome, Italy) on February 2, 2017. Eligible participants were men and women aged 18 years or above with mild to moderate SD mainly involving the face who met eligibility criteria. Women of child-bearing age should provide a negative pregnancy test at enrollment visit. Exclusion criteria were the presence of other inflammatory skin diseases other than SD, a positive history of allergy to one or more components of the tested cream. The trial was conducted according to the Declaration of Helsinki and the International Conference on Harmonization-Good Clinical Practice Guidelines.9 Trial Registration Number was ISRCTN74021432.
Clinical evaluation
The primary clinical outcome was the evolution of the Investigator Global Assessment (IGA) score evaluating erythema, scale/flaking, grade of seborrhea and itch. For each item, a 4-grade scale was used (from 0: sign/symptom absent to 3: sign/symptom severe). Subjects were assessed at baseline, after 3 and 6 weeks of treatment by an investigator unaware of the type of treatment (investigator-blinded study design).
Skin facial microbiota evaluation
Three pre-specified facial areas were considered for the investigation of the skin microbiota of 48 subjects, randomly selected within the individuals enrolled in this study. In particular, two affected sites, defined as area 1 (intra-glabellar zone) and 2 (one of the nasolabial folds), respectively; and one no-injured site, defined as area 3 (corresponding to mandibular rim zone) were investigated from each area, two standard cotton swabs were collected before and after 6 weeks of cream treatment. Swabs were stored at −80°C until microbiota analysis. Bacterial DNA from each swab was extracted using QIAamp DNA Mini Kit (Qiagen, Germany) following the manufacturer’s instruction. Purified DNA were used to amplify 16S V3-4 regions using barcoded sample-specific primers and 2X KAPA High Fidelity HotStart Ready Mix (Roche) with this thermocycler program: 95°C for 3 mins, 25 cycles of (95°C for 30”, 55°C for 30” and 72°C for 1 mins) and stored at 4°C until usage. The genomic library was prepared using the Illumina protocol 16S Metagenomic Sequencing Library Preparation in order to obtain 0.5 M clusters of fragments of 2x300nt through MiSeq600. After quality filtering, resulting sequences were analyzed with QIIME software (1.6.0), the identification and abundance of microorganisms were investigated at the genus and species levels.
Statistical analysis and sample size calculation
Statistical analysis was performed using GraphPad statistical software ver. 13.0 (La Jolla, CA, USA). Continuous variables were expressed as mean±SD. The primary endpoint of the trial was the evolution of IGA score from baseline to week 6 (end of treatment). The Wilcoxon was used for the analysis of the study outcomes. To determine the significant differences in microbial taxa nonparametric tests based on the Kruskal–Wallis and Wilcoxon rank-sum tests were used, and the statistical analysis was performed using the Vegan 2.4.3 package for R Software V.3.3.1 for Windows. Adjustment for multiple testing was evaluated with Benjamin-Hochberg FDR correction. Differences were considered significant when P<0.05 and are indicated as *P<0.05, **P<0.01, ***P<0.001. The efficacy analysis tested the hypothesis if EDS cream would be able to reduce significantly the IGA score (the primary endpoint of the study). Therefore, sample size calculation was performed on the hypothesis that the tested treatment could reduce the IGA score, in comparison with baseline value, with an effect size of at least 0.4. With an alpha value of 0.05 and a power of 95%, a total of at least 70 subjects should be enrolled to detect this difference. The sample size was calculated using G-Power statistical software version 3.9 (Kiel, Germany). The analysis was performed based on the intention-to-treat principle. We summarized continuous variables by mean±standard deviation. We tested for differences in the outcomes by using the non-parametric Wilcoxon test. Confidence intervals for the absolute differences in IGA score comparing baseline with week 6 were calculated. Regarding skin microbiota analysis the Benjamini–Hochberg method for multiple testing was used.
Results
Participating subjects’ flow is presented in Figure 1. All subjects were enrolled and treated during winter and spring months. All but one subject completed the study. Table 1 reports baseline demographic and clinical characteristics of the enrolled subjects. Figure 2 reports the area of the face where swabs were performed.
 | Table 1 Baseline demographic and clinical characteristics |
 | Figure 1 Study flow. |
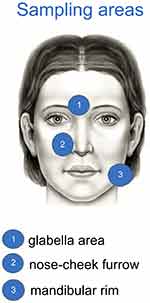 | Figure 2 Sites for swabs skin bacterial microbiome assessment. Areas 1 and 2 were considered lesional sites; area 3 non-lesional site. |
Clinical efficacy
Baseline IGA scores (mean±SD) was 10±3 (range:4–16). The use of EDS reduced IGA score significantly to 3±2.5 at week 3 (a 70% reduction; P=0.001 vs baseline; Wilcoxon test) and to 1.2±1.5 at week 6 (88% reduction; P=0.0001 vs baseline; Wilcoxon test). (Figure 3). The absolute difference of IGA score at week 6 in comparison with baseline was 8.5 (95% confidence interval from 7.4 to 9.5) with an effect size of 2.6. Scaling score was reduced from 2.5 at baseline to 1.5 after 3 weeks (−60%) and to 0.35 (−86%) after 6 weeks of treatment. Erythema score at baseline was 2.7. It was reduced to 1.2 (−57%) after 3 weeks and to 0.3 at week 6 (−89%; P=0.001). Similar reductions were observed for pruritus, from 2.2 at baseline to 0.10 and 0.25 at weeks 3 and 6, respectively (P=0.05) and for seborrhea, from 2.9 at baseline to 0.10 and 0.25 at weeks 3 and 6, respectively (P=0.05) scores. Figure 4 documents three subjects (1, 2 and 3) at baseline (A) and after 6 weeks (B) of treatment. The product was very well tolerated. No local side effects were reported.
 | Figure 3 Evolution of IGA score from baseline and after 3 and 6 weeks of treatment (mean and SD). *P<0.05; **P<0.01. |
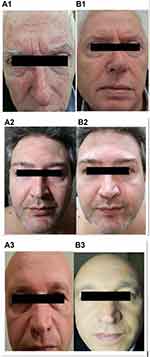 | Figure 4 Color pictures of three subjects (1, 2 and 3) at baseline (A) and after 6 weeks of treatment with EDS (B). |
Skin microbiota
To identify changes in skin microbiota induced by treatment with the product under study, 144 skin swabs (corresponding to 48 samples for each area analyzed) were used for 16S rRNA-based amplicon sequencing. A significant drop of Staphylococcus spp. was detected, mainly in glabellar and nasolabial fold sites (areas 1 and 2) after 6 weeks of topical treatment if compared to baseline (Figure 5A–C). This staphylococcal reduction was accompanied by an increase, although not statistically significant, of Cutibacterium spp. The set of Operational Taxonomic Units (OTUs) detected in both SD-affected areas for Staphylococcus spp. and Cutibacterium spp. were used to investigate more in detail the abundance trends of these microorganisms; Figure 6 reports the results deriving from the comparisons at different time-points and sampling areas. A significant boost of Cutibacterium spp. was observed in the SD-affected areas after 6 weeks treatment if compared to the baseline, whereas an opposite trend of Staphylococcus spp. abundances was highlighted. Interestingly, Cutibacteria and Staphylococci were detected in low amounts in the no SD-affected area than in the SD ones, both before and after treatment. This difference was statistically significant for staphylococci at all-time points. Furthermore, the microbial prevalence of Cutibacteria versus Staphylococci was assessed calculating the OTU abundances ratio at different time points (ratio T1/T0). These microbial abundances ratios have underlined a significant prevalence of Cutibacterium spp. in SD-affected areas, as shown in Figure 7.
Discussion
SD is a very common skin disease with a chronic evolution.10 SD is characterized by the presence of flaking, red, greasy area of the skin, involving mainly scalp, nasolabial folds, eyebrows and chest.11 Relapses are common.12 A key pathogenetic role in SD seems to be played by Malassenzia yeasts,13 even if there are contradictory data regarding the real involvement of these organisms in this skin disease.14 A relevant role of Malassezia is further supported by the fact that both oral and topical anti-fungal drugs, like azole compounds, are very effective in the treatment of SD.15 Topical or oral anti-fungal products, together with topical corticosteroids, are considered the first-line treatment approach.16,17 For these reasons, studies on SD and dandruff predominantly focused on Malassezia species.18 However, recent data have demonstrated that in scalp of SD subjects there is an alteration of skin microbiota. Park et al19 demonstrated that bacterial and fungal communities are different between subjects with SD in comparison with healthy group. Clavaud et al5 demonstrated that an increase of Staphylococcus epidermidis and a reduction in Cutibacterium acnes (formerly Propionibacterium acnes) are observed in skin microbiota of subjects with scalp SD. Xu et al20 further demonstrated that a disequilibrium of bacterial microbiota has a stronger relationship with the severity of SD than fungi. Their results show that in SD/dandruff skin the abundance of Staphylococcus spp and the reduction of Cutibacterium acnes were correlated with the severity of SD. Staphylococcus spp and Cutibacterium acnes are considered dominant but reciprocally inhibited bacterial genera. In fact, Cutibacterium acnes secretes bacteriocins to suppress the growth of Staphylococcus spp21 and on the other hand, Staphylococcus spp can ferment glycerol inhibiting the overgrowth of Cutibacetium acnes.22 Furthermore, these authors suggest that enhancing the presence of Cutibacterium acnes and suppressing Staphylococcus epidermidis could be a potential solution to lessen SD and dandruff.16 In our knowledge, this trial is the first to evaluate the effect of a corticosteroid-free hyaluronic acid-based cream on clinical evolution and on modification of skin bacterial microbiota. After 6-week treatment with the tested product an increase of the presence of Cutibacterium acnes in SD affected areas and an significantly decrease of the presence of Staphylococcus epidermidis in both affected and non-affected areas was observed. We observed also that the ratio of relative abundance of Cutibacterium acnes/Staphylococus spp at affected areas was very low at baseline and significantly improved after treatment. Furthermore, our data are in accordance with the results of Tanaka15 et al who demonstrated in SD patients that Cutibacterium acnes was abundant in non-affected sites whereas Staphylococcus spp predominated at affected sites. In our study, the modifications of skin microbiota composition were associated with a clinically relevant and strong improvement of SD with a reduction of the IGA score >80%, in comparison with baseline. It is not possible to demonstrate that the positive effects on skin microbiota could be a direct action of the cream on skin bacterial flora or, otherwise, the reequilibrium between Staphylococcus spp and Cutibacterium acnes was secondary to an improvement of skin barrier or other unspecific anti-inflammatory effects of the tested product. The skin microbiota varies by the layer of the skin and changes with skin barrier alteration or disruption.23 Hyaluronic acid alone has shown to improve signs and symptoms in subjects with SD of the face.24 Topical hyaluronic acid has a potent skin hydrating effect,25 therefore indirectly influencing skin microbiome.26 A recent systematic review on hyaluronic acid and wound healing highlighted the general safety and the efficacy of hyaluronic acid-based products when used in skin repair therapeutic approaches.27It is reasonable to suppose that the major beneficial effects of topical hyaluronic acid may be due to its great hydrating properties useful to favoring the natural homeostasis and spontaneous regeneration of the skin.28 Also, modification on sebum content can affect skin superficial microbiome29 and seborrhea was significantly reduced by the treatment with the tested cream. Moreover, the tested product contains low concentrations of piroctone olamine and climbazole, two antifungal compounds used for their preservative function only. Youn et al’s study shows that the above antifungal agents are effective to inhibit the growth of several Malassezia species including M. restricta and M. globose.30 Despite the fungal component of the skin microbiota was not investigated in this study, it cannot be excluded that the beneficial properties of piroctone olamine and climbazole may contribute to alleviating the SD symptomatology. The tested cream contains also of topical glycyrrhetinic acid. Therapeutic potential of glycyrrhetinic acids was resumed in a patent review published not long ago.31 Topical glycyrretinic acid exerts anti-inflammatory action.32 A shampoo with glycyrretinic acid was effective in the treatment of dandruff.33 Finally, the cream contains a bacterial-wall-derived glycoproteins and peptide glycans complex which could exert an immunomodulator action34 with a direct influence on the skin microbiome composition. In evaluating the results of our study, we must take into account some trial’s limitations. First, this was uncontrolled, not randomized trial. However, in order to reduce the investigator bias, we adopted an assessor-blinded study design for the evaluation of the primary clinical end-point. In addition, the skin microbiota evaluation should be considered as an operator-independent study outcome. Another study limitation was that we did not evaluate the modification of yeast microbiome. However, one of the main goals of our study was to confirm or not the role of the bacterial population of the skin microbiota in SD. As stated before, in SD subjects, bacterial disequilibrium of skin microbiome seems to be more related to the severity of the disease than Malassezia spp.16 Finally, our study was not a comparative trial (ie, a comparison with an antifungal product or a corticosteroid) and therefore is not possible to exclude that clinical efficacy of SD reference standard treatments could be associated with an improvement of skin bacterial microbiota. In this regards, future comparative trials are warranted. However, the main strength of our study was that for the first time we were able to demonstrate that a corticosteroid-free cream together with a significant clinical positive effect was able to riequilibrate the facial skin bacterial microbiome.
Conclusion
Significative reduction of signs and symptoms of SD of the face was observed after treatment with EDS cream, applied twice daily for 6 consecutive weeks. Furthermore, treatment with EDS cream was associated with a reequilibrating effect on facial skin microbiota. The product was very well tolerated.
Data Sharing Statement
Trial Registration Number was ISRCTN74021432. Study protocol was approved by the competent Ethic Committee. All participants provided written informed consent before starting the study. Individual participant data of the study, after deidentification, is not being shared with others. We do not share any specific data, and no other study-related documents will be made available. Our patients’ data were recorded in a specific case record form. The case record form and essential documents will be kept in a designated place for 15 years. The data and documents are available if requested by relevant authorities.
Acknowledgments
The present trial was supported by an unrestricted grant of Cantabria Labs Difa Cooper.
Author contributions
MP, EC, AMO and RDG were the principal investigators of the trial writing the study protocol. All other authors conducted the trial performing visits and instrumental evaluations. MM was involved in study protocol design. All the authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
MM is an employee of Cantabria Labs Difa Cooper. All other authors declare no conflicts of interest in this work.
References
1. Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3(2):1–22.
2. Naldi L, Rebora A. Seborrheic dermatitis. N Engl J Med. 2009;360(4):387–396. doi:10.1056/NEJMcp0806464
3. Faergemann J, Jones TC, Hettler O, Loria Y. Pityrosporum ovale (Malassezia furfur) as the causative agent of seborrhoeic dermatitis: new treatment options. Br J Dermatol. 1996;134:12–15.
4. Kim GK. Seborrheic dermatitis and Malassezia species: how are they related? J Clin Aesthet Dermatol. 2009;2(11):14.
5. Tanaka A, Cho O, Saito M, Tsuboi R, Kurakado S, Sugita T. Molecular characterization of the skin fungal microbiota in patients with seborrheic dermatitis. J Clin Exp Dermatol Res. 2014;5:239.
6. Clavaud C, Jourdain R, Bar-Hen A, et al. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS One. 2013;8(3):e58203. doi:10.1371/journal.pone.0058203
7. Tanaka A, Cho O, Saito C, Saito M, Tsuboi R, Sugita T. Comprehensive pyrosequencing analysis of the bacterial microbiota of the skin of patients with seborrheic dermatitis. Microbiol Immunol. 2016;60(8):521–526. doi:10.1111/1348-0421.12398
8. Milani M, Barcella A, Puviani M. Efficacy of a corticosteroid-free, 5% hyaluronic-based facial cream in the treatment of seborrheic dermatitis. A Proof-of-Concept Study. J Clin Exp Dermatol Res. 2017;8:6–10.
9. Puri KS, Suresh KR, Gogtay NJ, et al. Declaration of Helsinki, 2008: implications for stakeholders in research. J Postgrad Med. 2009;55:131. doi:10.4103/0022-3859.52846
10. Gupta AK, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18(1):13–26.
11. Gupta AK, Madzia SE, Batra R. Etiology and management of seborrheic dermatitis. Dermatology. 2004;208(2):89–93. doi:10.1159/000076478
12. Peter RU, Richarz‐Barthauer U. Successful treatment and prophylaxis of scalp seborrhoeic dermatitis and dandruff with 2% ketoconazole shampoo: results of a multicentre, double‐blind, placebo‐controlled trial. Br J Dermatol. 1995;132(3):441–445.
13. DeAngelis YM, Gemmer CM, Kaczvinsky JR, Kenneally DC, Schwartz JR, Dawson TL
14. Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013;31(4):343–351. doi:10.1016/j.clindermatol.2013.01.001
15. Gupta AK, Nicol K, Batra R. Role of antifungal agents in the treatment of seborrheic dermatitis. Am J Clin Dermatol. 2004;5(6):417–422. doi:10.2165/00128071-200405060-00006
16. Johnson BA, Nunley JR. Treatment of seborrheic dermatitis. Am Fam Physician. 2000;61(9):2703–2710.
17. Park T, Kim HJ, Myeong NR, et al. Collapse of human scalpmicrobiome network in dandruff and seborrhoeicdermatitis. ExpDermatol. 2017;26(9):835–838. doi:10.1111/exd.13293
18. Stratigos JD, Antoniou CHR, Katsambas A, et al. Ketoconazole 2% cream versus hydrocortisone 1% cream in the treatment of seborrheic dermatitis: a double-blind comparative study. J Am Acad Dermatol. 1988;19(5):850–853.
19. Park HK, Ha MH, Park SG, Kim MN, Kim BJ, Kim W. Characterization of the fungal microbiota (mycobiome) in healthy and dandruff-afflicted human scalps. PLoS One. 2012;7(2):e32847. doi:10.1371/journal.pone.0032847
20. Xu Z, Wang Z, Yuan C, et al. Dandruff is associated with the conjoined interactions between host and microorganisms. Sci Rep. 2016;6:srep24877. doi:10.1038/srep24877
21. Shu M, Wang Y, Yu J, et al. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS One. 2013;8(2):e55380. doi:10.1371/journal.pone.0055380
22. Wang Y, Kuo S, Shu M, et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol. 2014;98(1):411–424. doi:10.1007/s00253-013-5394-8
23. Zeeuwen PL, Boekhorst J, van Den Bogaard EH, et al. Microbiome dynamics of human epidermis following skin barrier disruption. Genome Biol. 2012;13(11):R101. doi:10.1186/gb-2012-13-11-r101
24. Schlesinger T, Powell CR. Efficacy and safety of a low-molecular weight hyaluronic Acid topical gel in the treatment of facial seborrheic dermatitis. J Clin Aesthet Dermatol. 2012;5(10):20.
25. Manuskiatti W, Maibach HI. Hyaluronic acid and skin: wound healing and aging. Int J Dermatol. 1996;35(8):539–544.
26. SanMiguel A, Grice EA. Interactions between host factors and the skin microbiome. Cell Mol Life Sci. 2015;72(8):1499–1515. doi:10.1007/s00018-014-1812-z
27. Neuman MG, Nanau RM, Oruña-Sanchez L, Coto G. Hyaluronic acid and wound healing. J Pharm Pharm Sci. 2015;18(1):53–60.
28. Mast BA, Flood LC, Haynes JH, et al. Hyaluronic acid is a major component of the matrix of fetal rabbit skin and wounds: implications for healing by regeneration. Matrix. 1991;11(1):63–68.
29. Mukherjee S, Mitra R, Maitra A, et al. Sebum and hydration levels in specific regions of human face significantly predict the nature and diversity of facial skin microbiome. Sci Rep. 2016;6:36062. doi:10.1038/srep36062
30. Youn HJ, Kim SY, Park M, et al. Efficacy and safety of cream containing climbazole/piroctone olamine for facial seborrheic dermatitis: a single-center, open-label split-face clinical study. Ann Dermatol. 2016;28(6):733–739. doi:10.5021/ad.2016.28.6.733
31. Hussain H, Green IR, Shamraiz U, et al. Therapeutic potential of glycyrrhetinic acids: a patent review (2010–2017). Expert Opin Ther Pat. 2018;28(5):383–398. doi:10.1080/13543776.2018.1455828
32. Teelucksingh S, Mackie ADR, Burt D, Edwards CRW, Mclntyre MA, Brett L. Potentiation of hydrocortisone activity in skin by glycyrrhetinic acid. Lancet. 1990;335(8697):1060–1063. doi:10.1016/0140-6736(90)92633-S
33. Turlier V, Viode C, Durbise E, et al. Clinical and biochemical assessment of maintenance treatment in chronic recurrent seborrheic dermatitis: randomized controlled study. Dermatol Ther (Heidelb). 2014;4(1):43–59. doi:10.1007/s13555-014-0047-0
34. Hanisch FG, Schwientek T, Von Bergwelt‐Baildon MS, Schultze JL, Finn O. O‐Linked glycans control glycoprotein processing by antigen‐presenting cells: a biochemical approach to the molecular aspects of MUC1 processing by dendritic cells. Eur J Immunol. 2003;33(12):3242–3254. doi:10.1002/eji.200324189
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



