Back to Journals » International Journal of General Medicine » Volume 7
Effects and tolerability of betahistine in patients with vestibular vertigo: results from the Romanian contingent of the OSVaLD study
Authors Băjenaru OA, Roceanu A, Albu S , Zainea V, Pascu A, Georgescu M, Cozma S, Mărceanu L, Mureşanu D
Received 11 July 2014
Accepted for publication 3 September 2014
Published 4 December 2014 Volume 2014:7 Pages 531—538
DOI https://doi.org/10.2147/IJGM.S71015
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ovidiu Băjenaru,1 Adina Maria Roceanu,2 Silviu Albu,3 Viorel Zainea,4 Alexandru Pascu,4 Mădălina Gabriela Georgescu,4 Sebastian Cozma,5 Luigi Mărceanu,6 Dafin Fior Mureşanu7
1Department of Neurology, Carol Davila University of Medicine and Pharmacy, 2Department of Neurology, University Hospital, Bucharest, 3IInd Department of Otolaryngology, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, 4Institute of Phono-Audiology and ENT Functional Surgery Prof Dr Dorin Hociota, Carol Davila University of Medicine and Pharmacy, Bucharest, 5ENT Department, University of Medicine and Pharmacy Grigore T Popa, Audiology and Vestibulogy Department, Rehabilitation Clinical Hospital, Iasi, 6Transilvania University, Faculty of Medicine, Department: Doctoral Program (PhD), Brasov, 7"RoNeuro" Institute for Neurological Research and Diagnostic Cluj-Napoca, University of Medicine and Pharmacy "Iuliu Haţieganu", Department of Clinical Neurosciences, Cluj-Napoca, Romania
Background and methods: An efficacy population of 245 patients with vertigo of peripheral vestibular origin was recruited in Romania as part of a 3-month multinational, post-marketing surveillance study of open-label betahistine 48 mg/day (OSVaLD). Endpoints were changes in the Dizziness Handicap Index (primary endpoint), Medical Outcome Study Short-Form 36 (SF-36v2®), and the Hospital Anxiety and Depression Scale.
Results: During treatment, the total Dizziness Handicap Index score improved by 41 points (on a 100-point scale). Statistically significant improvements of 12–14 points were recorded in all three domains of the Dizziness Handicap Index scale (P<0.0001). Betahistine therapy was also accompanied by progressive improvements in mean Hospital Anxiety and Depression anxiety and depression scores (P<0.0001) and significant improvements in both the physical and mental component summary of the SF-36v2 (P<0.0001). Betahistine was well tolerated, with only one suspected adverse drug reaction recorded in the Romanian safety population (n=259).
Conclusion: Betahistine 48 mg/day was associated with improvements in multiple measures of health-related quality of life and had a good tolerability profile in these Romanian patients with recurrent peripheral vestibular vertigo.
Keywords: betahistine, vertigo, dizziness, anxiety, depression, Romania
Introduction
OSVaLD (Observational Study in patients suffering from recurrent peripheral vestibular Vertigo to assess the effect of betahistine 48 mg/day on quality of Life and Dizziness symptoms) documented a positive effect of betahistine therapy on health-related quality of life (HRQoL) in a multinational population of patients with recurrent peripheral vestibular vertigo.1,2 OSVaLD was conducted in 13 countries, with the single largest patient contingent recruited in Romania. This supplementary report of findings from OSVaLD examines HRQoL trends in the Romanian participants.
Materials and methods
Details of the methodology of OSVaLD have been published previously,1,2 and readers are referred to those sources for particulars of statistical methods and sample size calculations. OSVaLD was a post-marketing surveillance study of open-label betahistine in patients with vertigo of peripheral vestibular origin, and was undertaken in primary care centers in 13 countries with a planned duration of 3 months. The daily dose of betahistine was 48 mg/day, administered as 24 mg twice daily or 16 mg three daily as specified by the relevant product information for each country.
Scores on the Dizziness Handicap Index (DHI), the Medical Outcome Study Short-Form 36 (SF-36v2®), and the Hospital Anxiety and Depression Scale (HADS) were to be measured at baseline, at regular intervals during the study, and at study completion in the efficacy population (ie, patients who were prescribed betahistine at baseline and who attended at least one subsequent clinic visit and generated at least one score for at least one endpoint scale at baseline and at one or more later visits). The primary efficacy outcome was the change from baseline in total DHI score at 3 months. Statistical treatments of all three endpoints, including provisions for missing data, have been reported in detail elsewhere.1,2 All the endpoint indices used have been extensively evaluated and validated.3–12
The safety population, from which reports of adverse drug reactions were collated, consisted of all patients who received a prescription of betahistine at baseline and who made at least one clinic visit after baseline.
The design and conduct of OSVaLD conformed to international principles of Good Clinical Practice and the Declaration of Helsinki. The study protocol was submitted to independent institutional review as required by local regulatory provisions, for approval prior to starting the study. Informed consent was obtained from each patient as stipulated by local laws and regulations before they participated in the study. Patients were advised that they could withdraw from the study at any time if they wished to, that they could do so for any reason, and that they were not obliged to explain their reason. They were also assured that withdrawing from the study would have no effect on other treatments they might receive. The FOVEA Group (Rueil Malmaison, France) was responsible for data management and statistical analysis.
Results
In Romania, a total of 262 patients were recruited at 84 centers. Participating practitioners are named in Supplementary material. From this initial contingent was derived a safety population of 259 patients and an efficacy population of 245 patients; these patients were almost exclusively (>99%) white/Caucasian. Other salient demographic features of each contingent are shown in Table 1.
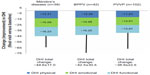
Betahistine was most often introduced in response to a new diagnosis (n=135, 55.1%) or inefficacy of existing therapy, which accounted for most of the remainder of the patients (n=97, 39.6%). The single most often recorded diagnosis associated with use of betahistine was peripheral vestibular vertigo of unknown pathophysiology (PVVP; n=102, 41.6% of cases); other prominent diagnoses included benign paroxysmal positional vertigo (BPPV; n=42, 17.1%) and Ménière’s disease (n=38, 15.5%). Across these three diagnoses, the proportions of cases attributed to a new diagnosis or inefficacy of current therapy were similar to those for the Romanian contingent as a whole. Multiple diagnoses were recorded in 21 cases (8.6%).
Almost 12% of patients in the efficacy contingent (n=29) had a diagnosis of ear infection and 14.7% had records of psychosomatic or psychiatric disorders, such as hyperventilation or panic disorder (n=36). Cerebrovascular diseases (≈30%), cardiac diseases (≈25%), and metabolic disorders (≈14%) were widely recorded.
Betahistine was almost exclusively prescribed at baseline at a dosage of 24 mg twice daily (n=240, 98%) in the Romanian contingent, and there was almost no change in betahistine dosage distribution during the study. Mean treatment duration was 90±14.5 days.
Combination antivertiginous therapy (meaning betahistine plus at least one other drug) was prescribed at baseline for 77 patients (31.4%), with gingko biloba being the single most widely used additional drug recorded (n=23). Use of combination therapy had declined to 24.1% (n=59) by the end of the study, with gingko biloba still the most frequently recorded agent used in this context (n=21, 8.6%). Among the single-diagnosis categories, combination therapy was more likely to be associated with Ménière’s disease (≈37%) than with PVVP or BPPV (both ≈29%). Forty-seven of the patients prescribed combination therapy were also taking cardiovascular drugs judged capable of having an impact on vertigo and 27 were taking psychotropic drugs judged capable of having an impact on vertigo.
Efficacy outcomes
DHI score
Net changes in the total DHI score and the three dimensions of that score are illustrated in Figure 1. All those indices changed significantly from baseline (P<0.0001). Changes in all elements of the DHI score were similar among the three major diagnostic categories of PVVP, BPPV, and Ménière’s disease, as shown in Figure 2. DHI responses were similar in both sexes. DHI responses were slightly larger in patients (n=168) prescribed betahistine monotherapy than in those (n=77) prescribed combination therapy, but these differences were small. In both these categories, the reductions in overall and dimension-specific DHI scores from baseline were highly statistically significant (P<0.0001).
HADS score
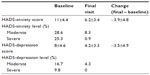
Baseline, end of study, and in-study changes in HADS scores are summarized in Table 2. Changes in mean HADS scores for both anxiety and depression between the baseline and final visits were highly statistically significant (P<0.0001). These findings were observed consistently in men and women and in patients with different diagnoses for the origins of vertigo.
SF-36v2 score
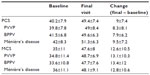
At the baseline visit, the mean physical component summary (PCS) score was 40.2±7.9 and the mean mental component summary (MCS) score was 35±11. These scores were indicative of reduced HRQoL status (below the norm of the general US population). Improvements in both scores were recorded during the treatment period (P<0.0001 versus baseline, Table 3). As shown in Figure 3, there were consistent improvements in all domains of the SF-36v2 instrument. Some numerical differences were noted between men and women in some domain responses, but these were small and were not subjected to statistical comparison. PCS and MCS scores improved to a similar extent in patients stratified by diagnostic category, as shown in Table 3.
Body weight
The mean ± standard deviation change in weight between baseline and the final visit was 0.4±3.7 kg in the efficacy population, with identical mean changes between men and women. The average weight gain was smaller in patients with PVVP (n=102, 0.2±5.2 kg) than in those with BPPV or Ménière’s disease (n=80, ≈0.6±2 kg).
Subjective assessment of efficacy
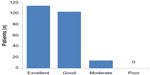
Patients’ assessments of treatment are illustrated in Figure 4, stratified by recorded cause of vertigo. Regardless of diagnostic category, almost all patients rated betahistine therapy as “excellent” or “good”. No material differences in assessment levels were seen between men and women. Physicians’ and patients’ impressions of treatment showed close alignment (r=0.76, P<0.0001).
Safety and tolerability
The only suspected adverse drug reaction (ADR) recorded in the Romanian contingent was a case of dyspepsia in a 46-year-old male patient. The relationship to study medication was recorded as “probable” but the event was not classified as either severe or serious.
In the study as a whole, 49 patients were reported as having a total of 76 ADRs. Twenty of the patients affected by suspected ADRs were in the Brazilian contingent and 12 in Slovenia and Spain. More patients experienced a suspected ADR at a dosage of 24 mg twice daily than at 16 mg three times daily (25 versus seven; dosage data not recorded for 28 suspected ADRs).
The most frequently reported suspected ADRs were gastrointestinal disorders (33 events in 27 patients, principally abdominal pain upper, nausea or dyspepsia) and nervous system disorders (14 events in 13 patients, principally headaches). The majority of suspected ADRs were characterized as mild (47 events in 33 patients) or moderate (28 events in 19 patients). No deaths were reported during the study.
Discussion
This secondary analysis, based on the Romanian contingent of the OSVaLD population, replicates the demonstration of improved HRQoL with betahistine that was recorded in the primary results.
Diseases of the peripheral vestibular system can have a significant adverse impact on the lives of patients.13,14 Data from Romanian participants in OSVaLD provided some corroboration of that view, with relatively high baseline mean scores on the HADS.15–17 The baseline SF-36v2 data are also consistent with the premise of low HRQoL in this subpopulation.18
The primary efficacy criterion evaluated was the absolute change in mean total DHI score between the baseline and final (3-month) visits. Substantial improvements were registered on this measure, with a 41-point reduction in the mean total score and reductions of 12, 14, and 14 points, respectively, in the physical, emotional, and functional domains of the scale. These improvements were statistically robust (P<0.0001) and were broadly consistent across various subgroups, including sex and disease status at baseline. The improvement in total DHI score greatly exceeded the threshold for a minimally important change.19,20
Statistically significant (P<0.0001) and clinically meaningful improvements were also recorded with the HADS questionnaire and the PCS and MCS components of the SF-36v2. This consistency of effect across scales gives confidence that the responses seen are authentic, although the exploratory nature of the analysis must be borne in mind.
Our findings are compatible with evidence from a meta-analysis supporting the beneficial effects of betahistine in Ménière’s disease and vestibular vertigo21 and with observations and findings from trials in which betahistine has in several instances been used as the reference therapy.22–25 The potential for use of betahistine in conjunction with vestibular rehabilitation maneuvers has recently been the subject of a local case report26 and should be noted, although the OSVaLD data offer no direct experiences on that point.
The finding of improved HRQoL among patients with BPPV who received betahistine during this study was considered at some length in the report of primary findings of this study,1 and some further remarks on this subject may be offered. Particle repositioning is the preferred and recommended treatment for BPPV,27 and nothing in our present data or the overall findings of OSVaLD contradict that advice. However, in OSVaLD as a whole, in our subpopulation and in the REVERT registry,28 a high percentage of patients with a diagnosis of BPPV were treated with betahistine. Hence, the reality of primary care as revealed in these data appears to be that physicians make extensive use of betahistine in this situation, regardless of expert recommendations. Whether or not betahistine is effective in this indication cannot be confirmed from our data, although the fact that the impact of betahistine on HRQoL in BPPV patients was similar to that in patients with other diagnoses suggests to us that it confers some benefits; an alternative explanation of this similarity would require an extensive placebo effect operating across all diagnostic categories. As noted in the report of the primary data, the scale of the patient database for HRQoL-related effects of betahistine in BPPV generated by OSVaLD compares favorably with the dataset for canalith repositioning.1 That in itself does not prove benefit from betahistine but, combined with our own observations, it leads us to believe that there is perhaps some benefit from this intervention in at least a proportion of patients with BPPV. Another possibility is that the prescription of betahistine in this indication28 is indicative of a lack of confidence on the part of primary care doctors in their ability to successfully perform repositioning maneuvers; such a conclusion is not per se incompatible with a favorable effect of betahistine, although it would imply other primary motives for using drug therapy. The lack of robust direct comparisons between repositioning and drug therapy has been noted elsewhere29 and remains deserving of attention.
Also untested in OSVaLD was the possible effect of higher doses of betahistine in Ménière’s disease. It is widely held (see, for example, Strupp et al30) that the appropriate dosage of betahistine in this condition is 48 mg three times daily, ie, three times the dose examined in OSVaLD. Even higher doses (up to 480 mg/day) have been used with benefit in severe cases.31 These data suggest the possibility that the beneficial effects of betahistine on HRQoL seen in patients with Ménière’s disease in the Romanian contingent of OSVaLD may underrepresent the full effect that might be achieved with a larger dose.
OSVaLD was an open-label observational study; there are acknowledged limitations to studies of this type,32 but it was a practical and appropriate format for a multinational trial conducted within the framework of routine care and compliant with the provisions and principles of the Strengthening the Reporting of Observational Studies in Epidemiology methodology.33 Adrion and Mansmann34 have recently discussed the merits of Bayesian methodologies for the analysis of clinical trials with longitudinal count data as the primary endpoint, specifically vertigo. This is an approach that may find application in future studies.
Safety experience with betahistine in OSVaLD was generally satisfactory, with ADRs affecting <2.5% of the study population. Many of these suspected ADRs were predominance of gastrointestinal and nervous system disorders, which is compatible with other reports about betahistine;35 the drug appears to retain a good tolerability profile even when administered for Ménière’s disease at doses ten times higher than those used in OSVaLD.31 There was distinct national variation in rates of suspected ADR reporting, with only one suspected ADR being reported in the Romanian contingent, compared with reports of suspected ADRs in 20 patients in Brazil.
Conclusion
In 245 Romanian patients diagnosed with recurrent peripheral vestibular vertigo, betahistine 48 mg/day for 3 months was associated with sustained and statistically significant improvements in multiple indices of HRQoL. The safety and tolerability of the treatment were good in this cohort, with only one reported suspected ADR.
Acknowledgments
The authors wish to thank the physicians and patients who participated in the OSVaLD survey. This report is published on behalf of the OSVaLD investigators, a full list of whom is provided in Appendix 1.
Author contributions
All the named authors made substantial contributions to the acquisition of data, and to its analysis and interpretation. They also contributed to drafting the article and/or revising it critically for important intellectual content. All named authors provided final approval of the version to be published and were accountable for ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Disclosure
Preparation of this report was assisted by Hughes associates, Oxford, UK. OSVaLD is supported financially by Abbott Products Operations AG, Allschwil, Switzerland. The authors report no other conflicts of interest in this work.
References
Bennecke H, Pérez-Garrigues H, Bin Sidek D, et al. Effects of betahistine on patient-reported outcomes in routine practice in patients with vestibular vertigo and appraisal of tolerability: experience in the OSVaLD study. Int Tinnitus J. 2010;16:14–24. | |
Pérez-Garrigues H, Kuessner D, Benecke H. Patient baseline characteristics in a multinational study of betahistine in recurrent peripheral vestibular vertigo: the OSVaLD study. Curr Med Res Opin. 2007;23:2753–2761. | |
Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–427. | |
Enloe LJ, Shields RK. Evaluation of health-related quality of life in individuals with vestibular disease using disease-specific and general outcome measures. Phys Ther. 1997;77:890–903. | |
Jarlsäter S, Mattson E. Test of reliability of the dizziness handicap inventory and the activities-specific balance confidence scale for use in Sweden. Adv Physiother. 2003;5:137–144. | |
Kammerlind AS, Ledin TE, Skargren EI, Odkvist LM. Reliability of clinical balance tests and subjective ratings in dizziness and disequilibrium. Adv Physiother. 2005;7:96–107. | |
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. | |
Horney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. | |
McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. | |
Saris-Baglama RN, Dewey CJ, Chisholm GB, et al. SF Health Outcomes™ Scoring Software User’s Guide. Lincoln, RI, USA: QualityMetric Inc.; 2004. | |
SF-36v2™. Scoring SF-36 Scales. Lincoln, RI, USA: QualityMetric Inc.; 2000. | |
Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. | |
Yardley L, Dibb B, Osborne G. Factors associated with quality of life in Menière’s disease. Clin Otolaryngol Allied Sci. 2003;28:436–441. | |
Kinney SE, Sandridge SA, Newman CW. Long-term effects of Ménière’s disease on hearing and quality of life. Am J Otol. 1997;18:67–73. | |
Michopoulos I, Douzenis A, Kalkavoura C, Christodoulou C, Michalopoulou P, Kalemi G. Hospital Anxiety and Depression Scale (HADS): validation in a Greek general hospital sample. Ann Gen Psychiatry. 2008;7:4. | |
Mozaffarieh M, Sacu S, Benesch T, Wedrich A. Mental health measures of anxiety and depression in patients with retinal detachment. Clin Pract Epidemiol Ment Health. 2007;3:10. | |
Pallant JF, Bailey CM. Assessment of the structure of the Hospital Anxiety and Depression Scale in musculoskeletal patients. Health Qual Life Outcomes. 2005;3:82. | |
Farivar SS, Cunningham WE, Hays RD. Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.I. Health Qual Life Outcomes. 2007;5:54. | |
Whitney SL, Hudak MT, Marchetti GF. The activities-specific balance confidence scale and the Dizziness Handicap Inventory: a comparison. J Vestib Res. 1999;9:253–259. | |
Tamber AL, Wilhelmsen KT, Strand LI. Measurement properties of the Dizziness Handicap Inventory by cross-sectional and longitudinal designs. Health Qual Life Outcomes. 2009;7:101. | |
Nauta JJ. Meta-analysis of clinical studies with betahistine in Ménière’s disease and vestibular vertigo. Eur Arch Otorhinolaryngol. 2014;271:887–897. | |
Scholtz AW, Steindl R, Burchardi N, Bognar-Steinberg I, Baumann W. Comparison of the therapeutic efficacy of a fixed low-dose combination of cinnarizine and dimenhydrinate with betahistine in vestibular neuritis: a randomized, double-blind, non-inferiority study. Clin Drug Investig. 2012;32:387–399. | |
Maslovara S, Soldo SB, Puksec M, Balaban B, Penavic IP. Benign paroxysmal positional vertigo (BPPV): influence of pharmacotherapy and rehabilitation therapy on patients’ recovery rate and life quality. NeuroRehabilitation. 2012;31:435–441. | |
Djelilovic-Vranic J, Alajbegovic A, Tiric-Campara M, et al. Betahistine or cinnarizine for treatment of Meniere’s disease. Med Arch. 2012;66:396–398. | |
Lepcha A, Amalanathan S, Augustine AM, Tyagi AK, Balraj A. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol. October 29, 2013. [Epub ahead of print.] | |
Georgescu M, Stoian S, Mogoantă CA, Ciubotaru GV. Vestibulary rehabilitation – election treatment method for compensating vestibular impairment. Rom J Morphol Embryol. 2012;53:651–656. | |
Bhattacharyya N, Baugh RF, Orvidas L, et al; American Academy of Otolaryngology-Head and Neck Surgery Foundation. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008;139(5 Suppl 4):S47–S81. | |
Agus S, Benecke H, Thum C, Strupp M. Clinical and demographic features of vertigo: findings from the REVERT Registry. Front Neurol. 2013;4:48. | |
Hilton M, Pinder D. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2004;2:CD003162. | |
Strupp M, Kremmyda O, Brandt T. Pharmacotherapy of vestibular disorders and nystagmus. Semin Neurol. 2013;33:286–296. | |
Lezius F, Adrion C, Mansmann U, Jahn K, Strupp M. High-dosage betahistine dihydrochloride between 288 and 480 mg/day in patients with severe Menière’s disease: a case series. Eur Arch Otorhinolaryngol. 2011;268:1237–1240. | |
Gauthier S, Juby A, Morelli L, Rehel B, Schecter R. A large, naturalistic, community-based study of rivastigmine in mild-to-moderate AD: the EXTEND Study. Curr Med Res Opin. 2006;22:2251–2265. | |
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS One. 2007;4:e296. | |
Adrion C, Mansmann U. Bayesian model selection techniques as decision support for shaping a statistical analysis plan of a clinical trial: an example from a vertigo phase III study with longitudinal count data as primary endpoint. BMC Med Res Methodol. 2012;12:137. | |
Jeck-Thole S, Wagner W. Betahistine: a retrospective synopsis of safety data. Drug Saf. 2006;29:1049–1059. |
Supplementary material
Participating investigators from 88 centers in Romania
Alaicescu M (Bucharest), Augustin A (Bucharest), Bădescu A (Bucharest), Baltag D (Iasi), Bărbos C (Timişoara), Becuş T (Târgu-Mureş), Bucan L (Bucharest), Călăraşu R (Bucharest), Cămpeanu A (Bucharest), Chirileanu RD (Timişoara), Comşa GI (Constanţa), Constantinescu D (Bucharest), Cotulbea S (Timişoara), Cozma S (Iaşi), Cucoş L (Iaşi), Docu AA (Constanţa), Dulămea A (Bucharest), Enache N (Bucharest), Ene A (Bucharest), Fischer TS (Cluj Napoca), Floare L (Cluj Napoca), Frăsineanu A (Bucharest), Geană I (Bucharest), Georgescu E (Bucharest), Georgescu M (Bucharest), Georgescu M-J (Bucharest), Gherman E (Bucharest), Hâncu A (Constanţa), Iliescu I (Constanţa), Ionescu-Mihăiţă ER (Bucharest), Ionita E (Craiova), Ionita I (Craiova), Iovănescu D (Craiova), Ladea M (Bucharest), Loghin V (Constanţa), Marceanu L (Braşov), Mărginean I (Cluj Napoca), Marian G (Bucharest), Marin M (Constanţa), Mariş C (Bucharest), Mârţu D (Iaşi), Matcău L (Timişoara), Muhlfay G (Târgu-Mureş), Muică L (Târgu-Mureş), Naconecinîi D (Iaşi), Nireştean A (Târgu-Mureş), Niţă A (Constanţa), Niţu L (Bucharest), Oană N (Cluj Napoca), Oancea A (Bucharest), Oşanu M (Bucharest), Panea N (Cluj Napoca), Pascu A (Bucharest), Pastia M (Bucharest), Pavel R (Cluj Napoca), Pendefunda L (Iaşi), Petruţiu S (Târgu-Mureş), Plăviţu I (Bucharest), Poenaru M (Timişoara), Popa GC (Cluj Napoca), Popa G (Bucharest), Popi S (Constanţa), Popovici A (Timişoara), Prelipceanu D (Bucharest), Radu L (Bucharest), Rădulescu L (Iaşi), Roceanu A (Bucharest), Rusu A (Cluj Napoca), Sabău MS (Târgu-Mureş), Safta D (Bucharest), Sarafoleanu D (Bucharest), Stanciu M (Bucharest), Stănciulescu R (Bucharest), Ştefanache F (Iaşi), Stefanescu EH (Timişoara), Szatmari S (Târgu-Mureş), Szocs M (Târgu-Mureş), Tomescu L (Cluj Napoca), Tudorache B (Bucharest), Tudose C (Bucharest), Ursu C (Iaşi), Vasilescu L (Bucharest), Vasu I (Cluj Napoca), Vioreanu M (Constanţa), Zaboş D (Timişoara), Zaharia C (Craiova), Zainea V (Bucharest), Zarie G (Timişoara).
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.