Back to Journals » Veterinary Medicine: Research and Reports » Volume 7
Effectiveness of NEM® brand eggshell membrane in the treatment of suboptimal joint function in dogs: a multicenter, randomized, double-blind, placebo-controlled study
Authors Ruff K , Kopp K, Von Behrens P, Lux M, Mahn M, Back M
Received 8 December 2015
Accepted for publication 11 May 2016
Published 18 August 2016 Volume 2016:7 Pages 113—121
DOI https://doi.org/10.2147/VMRR.S101842
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Young Lyoo
Kevin J Ruff,1 Kenneth J Kopp,2 Pamela Von Behrens,3 Mark Lux,4 Matthew Mahn,5 Matthew Back1
1ESM Technologies LLC, Carthage, 2Kopp Veterinary Consulting, St Louis, 3Clarkson-Wilson Veterinary Clinic, Chesterfield, 4Mackenzie Pointe Animal Hospital, St Louis, 5Midwest Veterinary Referral Center, Chesterfield, MO, USA
Introduction: Sub-optimal joint function is extremely prevalent in dogs. Therefore, a 6-week, prospective, randomized, double-blind, placebo-controlled study was conducted at eight different veterinary clinics to evaluate the efficacy, safety, and tolerability of NEM® brand eggshell membrane (EM), a novel dietary supplement shown in other species to help maintain healthy joints and connective tissues.
Subjects and methods: Fifty-one dogs received oral EM ~13.5 mg/kg (6 mg/lb) or placebo (excipients) once daily for six weeks. The primary outcome measure of this study was to evaluate the change in mean joint function following 1 week and 6 weeks of supplementation as determined via the Canine Brief Pain Inventory (CBPI) questionnaire (Q#5-10) in the treatment group versus the placebo group. Secondary outcome measures were for changes in mean CBPI pain and CBPI quality of life, and mean joint pain, mobility and lameness via Veterinary Canine Scoring Assessments (VCSA). A final secondary outcome measure was for a change in serum levels of the cartilage degradation biomarker, c-terminal cross-linked telopeptide of type-II collagen (CTX-II).
Results: Supplementation with EM produced a significant treatment response versus placebo at 1 week (20.5% improvement, P=0.028), but fell shy of significance at 6 weeks post-treatment (22.5% improvement) for the primary outcome measure (CBPI Function), despite a sizeable treatment effect. Similarly, there was also a significant treatment response versus placebo at 1 week for CBPI Pain (19.4% improvement, P=0.010), but fell just shy of significance at 6 weeks (22.5% improvement), again despite a sizeable treatment effect. Results were not significant versus placebo at 1 week for CBPI quality of life (14.0% improvement), but produced a significant treatment response by the end of the 6-week study (26.8% improvement, P=0.033). Additionally, EM produced a significant treatment response versus placebo at 6 weeks for VCSA pain (23.6% improvement, P=0.012), but fell shy of significance for VCSA mobility and VCSA lameness (walking & trotting). Serum CTX-II levels in EM-supplemented dogs was significantly improved versus placebo at 6 weeks (47.9% improvement, P=0.018). There were no serious adverse events reported during the study and subject dog owners reported that EM was well tolerated by their pets.
Conclusion: Supplementation with EM, ~13.5 mg/kg (6 mg/lb) taken once daily, significantly reduced joint pain and improved joint function rapidly (CBPI 1 week) and demonstrated a lasting improvement in joint pain (VCSA 6 weeks) leading to an improved quality of life (CBPI 6 weeks). Moreover, a profound chondroprotective effect was demonstrated following 6 weeks of supplementation with EM (CTX-II).
Keywords: EM, canine, pain, stiffness, lameness, CTX-II
Introduction
Suboptimal joint function (stiffness, inflexibility, and lameness) is extremely prevalent in dogs, often arising from either congenital abnormalities or traumatic injury. Hip dysplasia is the most common orthopedic condition, leading to suboptimal joint function. Estimates of its rate of incidence are quite variable throughout the world, with larger studies centering around ~20% across all breeds and having a range of anywhere from a few percent to ~50% depending upon breed.1–4 Joint laxity, femoral head misalignment, and uneven weight distribution while walking produce chronic localized inflammation in hip dysplasia, frequently leading to the development of osteoarthritis (OA) secondarily. Epidemiological evidence of the prevalence of primary and/or secondary OA in dogs is sparse, but some estimate that up to 20% of adult dogs and 80% of geriatric dogs (>8 years old) suffer from OA,5,6 which is in line with estimates of the prevalence of OA in humans (21%).7
Cartilage is primarily composed of extracellular matrix, a composite network of proteins such as type II collagen interacting with negatively charged polysaccharides such as hyaluronic acid and chondroitin sulfate (CS), all of which are synthesized and secreted by chondrocytes. Pathological conditions such as OA are characterized by an imbalance in cartilage turnover, in which catabolic processes predominate over anabolic processes. Extracellular matrix synthesis cannot keep pace with degradation, resulting in a loss of the structural integrity of the articular cartilage. This cartilage metabolism imbalance coupled with biomechanical stress in the joint resulting from laxity, misalignment, or other malformations leads to chronic inflammation and ultimately irreversible joint destruction. Products of this cartilage degradation process can be found in both blood and urine of arthritic subjects.
Of these cartilage degradation biomarkers, c-terminal cross-linked telopeptide of type II collagen (CTX-II) has been shown to have a strong correlation with the histological severity of destructive joint diseases in animal models.8 Because CTX-II is highly conserved genetically within vertebrate species, it has shown an exceptional correlation between animal model research and human clinical evaluation. CTX-II has been associated with both the incidence and progression of human OA in multiple clinical trials.9–11 However, relatively few studies have assessed the utility of CTX-II in the evaluation of canine joint disease;12 and to our knowledge, CTX-II has never been used to evaluate the chondroprotective effect of a treatment for naturally occurring canine joint disease.
The pain and inflammation associated with canine maladies such as hip dysplasia and OA can be quite debilitating, and few treatment options exist outside of easing symptoms. Traditional nonsurgical treatments for these disorders usually involve the use of nonsteroidal anti-inflammatory drugs (NSAIDs, eg, carprofen, meloxicam, etc).13 However, as in humans, there are safety concerns with long-term use of NSAIDs. Complementary and alternative medicines such as dietary supplements are also sometimes used in the management of canine OA,14 although there have been few well-controlled trials demonstrating their efficacy in dogs. Glucosamine (GluN), chondroitin, and omega-3 fatty acids (eg, fish oil, green-lipped mussel, etc) alone and in combination are widely marketed as canine dietary supplements to treat joint pain. The discovery of eggshell membrane (ESM) as a natural source of immune-modulating components has prompted the evaluation of this material as a treatment for suboptimal joint function in dogs.
ESM, found between the calcified shell and the albumin in chicken eggs, is primarily composed of fibrous proteins such as collagen type I,15 which form the mesh-like structure of the bilayered material. However, ESMs have also been shown to contain other bioactive components, namely glycosaminoglycans (ie, dermatan sulfate,16 CS,16 hyaluronic acid,17 etc). ESM is known to reduce the expression of various proinflammatory cytokines both in vitro18 and in vivo,19 including the key mediators of inflammation, interleukin-1beta and tumor necrosis factor-alpha. A proprietary form of ESM, commercially available as the branded product NEM® brand eggshell membrane (EM) (ESM Technologies LLC, Carthage, MO, USA), has demonstrated safety and efficacy in multiple clinical trials in relieving joint pain and stiffness in humans with OA20–22 and has been investigated for similar uses in various species of animals, including cranes,23 camels,24 and horses.25 However, EM has not been previously evaluated in dogs.
Although it is generally agreed that animals lack a significant placebo effect, it can nonetheless be difficult to evaluate subjective measures of their health and well-being. The Canine Brief Pain Inventory (CBPI) is a validated owner-administered test instrument (questionnaire) that is designed to assess the severity of chronic pain in dogs with OA and its impact on their function during daily activities. The CBPI has been shown to be appropriate and sufficiently sensitive to reliably detect treatment responses in multiple studies.26–29 In addition to the dog owner’s assessment of treatment effect, it is also important to obtain a clinician’s assessment, as a veterinarian will generally be more objective regarding the dog’s condition than will the dog owner. Over the past decade, various ordinal-scale clinician assessment tools have been developed, which provide a good basis for the inclusion of veterinarian evaluation of study dogs.30–32
The multicenter trial reported herein was designed to evaluate the efficacy and safety of the natural joint treatment, EM in dogs. Therefore, a 6-week, multicenter, randomized, controlled trial was conducted to evaluate the efficacy and tolerability of EM for the treatment of suboptimal joint function (eg, stiffness, inflexibility, lameness) in dogs.
Subjects and methods
Study design
The study was conducted according to a prospective, randomized, double-blind, placebo-controlled design and was conducted across eight veterinary clinics in the Saint Louis, MO metropolitan area. The study design was approved by the Institutional Animal Care and Use Committee (IACUC) of Missouri State University (Springfield, MO USA) (Study #14-035.0) in accordance with the Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, D.C. USA, 1996). Dog owners provided their written informed consent for their dogs to participate. Eligible subjects were centrally randomized to receive either EM or placebo in the order in which they were enrolled in the study using a permuted-block randomization table consisting of four subjects per block. Dog owners, clinical investigators, subinvestigators, clinical site personnel, and the clinical coordinator (performed central randomization) were all blinded to treatment. Treatment consisted of tablets (once daily, orally) providing either 150 mg of EM or 150 mg of additional excipients for every 25 pounds of a subject dog’s body weight (equating to ~6 mg/lb or 13.5 mg/kg). This dose was based upon an allometric conversion33 from the human dose of 500 mg/d that has been shown to be effective in previous clinical trials.20–22 Both treatment and placebo tablets were provided by United Pet Group, a division of Spectrum Brands (Earth City, MO, USA), and were identical in appearance and odor and were stored in closed containers at ambient temperature. Inactive excipients (ie, brewer’s yeast, maltodextrin, silicon dioxide) were used to produce both treatment tablets, and additional excipients replaced the EM in the placebo tablets. The placebo tablets were tested to verify that they did not contain EM. Clinic visits were scheduled for subject dogs at study initiation and at 6 weeks following the onset of treatment. Subject dog owners were given an owner-assessment diary to be filled out daily for 6 weeks and were instructed to record any changes in the overall subject health, changes in exercise routine, and any apparent discomfort associated with ingestion of either treatment or placebo tablets. Treatment compliance was checked at the final clinic visit by owner interview and by counting the number of unused doses of the study medications.
Subjects
All privately owned dogs, 18 months of age or older, weighing 10–100 pounds whose owners were seeking relief of mild to moderate suboptimal joint function were considered for enrollment in the study. In order to be eligible, subject dogs must have had mild-to-moderate persistent suboptimal joint function (eg, difficulty in getting up from a laying position, a noticeable limp, impaired gait, difficulty in climbing stairs) lasting for at least 3 months with a mean baseline function score between 2.0 and 8.5 on Q#5–10 of the CBPI questionnaire. Dogs that were currently receiving prescription or over-the-counter analgesic medications or NSAIDs daily were only eligible to participate in the study following a 14-day washout period for NSAIDs, a 7-day washout period for narcotics, and a 90-day washout period for injected steroids. Dogs currently receiving joint health supplements or consuming a joint health diet (ie, those containing glucosamine, CS, methylsulfonylmethane, curcumin, etc) were only eligible to participate in the study following a 3-month washout period. Subjects were excluded if they were currently receiving remission-inducing drugs such as methotrexate or immunosuppressive medications or had received them within the past 3 months. They were also excluded if they had a known confounding immune-mediated (eg, lupus), known infectious (eg, Lyme disease), known neurological, or known neoplastic disease or condition that would interfere with assessment of joint function. Other exclusionary criteria were a known allergy to eggs or egg products, a significant injury to the affected joint within the past 3 months, or pregnant or nursing female dogs. Subjects participating in any other research study involving an investigational product (drug, device, or biologic) or a new application of an approved product, within 30 days of screening, were also excluded from participating in the trial.
Treatment response
The primary outcome measure of this study was to evaluate the change in mean joint function as determined via CBPI questionnaire (Q#5–10) in the treatment group versus the placebo group. An additional outcome measure was to evaluate a change in mean joint pain or discomfort as determined via CBPI questionnaire (Q#1–4) in the treatment group versus the placebo group. The treatment response end points were at 1 week (by in-home owner survey) and at the week 6 clinic visit utilizing the eleven-question-validated CBPI questionnaire. Each of the first ten questions on the CBPI questionnaire includes a zero to ten ordinal scale, with zero equating to no pain (or does not interfere) and ten equating to extreme pain (or completely interferes). The final CBPI question asks the owner to rate the dog’s overall quality of life (QOL) using a five-category Likert-type scale (poor/fair/good/very good/excellent), which was then converted to a numeric value (1–5) for statistical comparison. End points were then compared to placebo assessments. Additional outcome measures were the change in mean joint pain and mobility utilizing a Veterinary Canine Scoring Assessment (VCSA) and the change in mean lameness while walking (w) and trotting (t) utilizing a second VCSA. The joint pain and mobility VCSA consisted of a five-point ordinal scale assessment via palpation or manipulation of the most apparent affected joint or joints (Table 1), and the lameness VCSA consisted of a seven-point ordinal scale assessment while either walking or trotting (Table 2).
  | Table 1 Veterinary Canine Scoring Assessment (VCSA) for joint pain and mobility |
  | Table 2 Veterinary Canine Scoring Assessment (VCSA) for lameness when walking and trotting |
Assessment of serum CTX-II
A secondary objective of this study was to evaluate the change in mean serum CTX-II levels in the treatment group versus placebo at 6 weeks. Blood samples were collected in serum tubes at baseline and at the week 6 clinic visit. In brief, the blood samples were allowed to clot at room temperature for 15–30 minutes, followed by centrifugation at 1,000–2,000× g for ~10 minutes. Following transfer of the supernatant serum to a new tube, samples were stored frozen (-20°C) until analysis. Serum concentrations of CTX-II were measured via enzyme-linked immunosorbent assay using a commercial immunoassay (Serum Pre-Clinical CartiLaps® [CTX-II] EIA; Immunodiagnostic Systems, Inc., Gaithersburg, MD, USA) according to manufacturer’s instructions using a SpectraMax Plus 384 microplate reader (Molecular Devices LLC, Sunnyvale, CA, USA). Samples were run in duplicate when assayed.
Adverse events and safety
A final objective of this study was to evaluate safety, tolerability, and any adverse reactions associated with supplementation with EM. Blood samples were collected at baseline and at the week 6 clinic visit to evaluate treatment safety via clinical chemistry (total protein, albumin, globulin, albumin/globulin ratio, blood urea nitrogen, creatinine, blood urea nitrogen/creatinine ratio, glucose, alanine aminotransferase, and alkaline phosphatase) and hematology (platelet count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin count, red blood cell count, and white blood cell count with differential [lymphocytes, monocytes, neutrophils, eosinophils, and basophils]). Clinical chemistry testing was performed by a commercial veterinary testing laboratory (Antech Diagnostics, Chesterfield, MO, USA). The owner-assessment diaries were also reviewed, and any discomfort or other adverse events (AEs) were recorded and reported in accordance with the study protocol. AEs and serious AEs were assessed by the clinical investigator at each study visit and followed until resolution, as necessary. Serious AEs were required to be reported to the study monitor immediately.
Statistical analysis
As this is the first efficacy trial in dogs, historical data were not available to serve as a basis for sample size determination. However, the hypothesis for this study is that the treatment group will be superior to that of the placebo group in improving suboptimal joint function. A 15% absolute change in the mean primary treatment response (joint function score will decrease by an average of at least 15% in the treatment group versus the placebo group) was used for sample size determination. Based upon this, it was estimated that a sample size of 40 dogs (20 treatment and 20 placebo) would need to be enrolled to provide the study with a statistical power of 80% to detect a treatment effect difference between the treatment group and the placebo group, assuming a rate of response of 20% in the treatment group, a rate of response of 5% in the placebo group, and a withdrawal rate of 5%. Since the enrollment for the study was 51 dogs, this should be sufficient to provide adequate safety and comparative effectiveness information. Descriptive statistics were calculated, including mean age and weight, and comparisons of these demographic data from the eight clinical sites were made with a Kruskal–Wallis test for multiple independent samples at baseline to validate randomization. Within-group comparisons, using the Kruskal–Wallis test for multiple independent samples, were also made within clinical sites to rule out any site bias. Post-baseline statistical analyses were performed as repeated measures analysis of variance. The items found to have statistical significance with repeated measures analysis of variance were then compared using a Wilcoxon test for dependent samples. In all cases, statistical significance was accepted at P<0.05. Analysis of the primary end point and all secondary end points was conducted on the intent-to-treat population (ie, including all randomized subjects with at least one efficacy assessment after randomization). The last observation carried forward approach was used for subjects that made at least one follow-up visit but that did not complete the study (lost to follow-up) to minimize missing data points for statistical transformations. SYSTAT software (Version 13) was used for all statistical analyses.34
Results
Subject recruitment began in August 2014 at eight veterinary clinics in the Saint Louis, Missouri metropolitan area, and the final evaluation was completed in August 2015. A total of 51 dogs between the ages of 3 years and 14 years with suboptimal joint function were enrolled in the study and underwent randomization. Of these subjects, 12% (6/51) were from site 1, 20% (10/51) were from site 2, 12% (6/51) were from site 3, 25% (13/51) were from site 4, 10% (5/51) were from site 5, 8% (4/51) were from site 6, 10% (5/51) were from site 7, 4% (2/51) were from site 8, 21 (41%) were female, 30 (59%) were male, and all were either spayed or neutered. The treatment joints consisted of stifle/knee (24), hip (21), shoulder (3), and elbow (10). Of the 24 subjects in which the stifle/knee was the affected joint, six (25%) had bilateral incidence. Of the 21 subjects in which the hip was the affected joint, eleven (52%) had bilateral incidence. Complete subject demographics, subdivided by treatment group, are reported in Table 3. Forty-six dogs completed the 6-week study per the protocol. The dropout rate (9.8%; three placebo and two treatment) was moderately higher than the estimated dropout rate (5%) used in the sample size determination; however, enrollment well exceeded the sample size of 40 dogs. From each group, two dogs were withdrawn due to lack of efficacy, and one dog was withdrawn from the placebo group due to not meeting the inclusion criteria. Compliance with the study treatment regimen was good in both treatment groups.
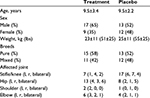  | Table 3 Subject demographics Note: Except where indicated otherwise, values are reported as mean ± standard deviation (n=51). Abbreviations: l, left, r right. |
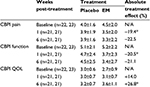
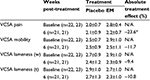
Subject demographic data were initially evaluated to ensure randomization within each site (not shown). Additionally, subject data were evaluated between sites to exclude site bias (not shown). During the randomization evaluation, data from site 1 exhibited some abnormalities, and upon further investigation, randomization could not be guaranteed so data from these dogs (6) were excluded from further evaluation. As there were no abnormalities within the remaining sites, the data (45) were pooled for all subsequent analyses. A clinical comparison of valid subjects (excluding noncompliance) was carried out to obtain mean baseline values for each of the CBPI outcome measures (Table 4). Statistical analysis of the primary outcome measure (CBPI function) revealed that supplementation with EM produced a significant treatment response versus placebo at 1 week (20.5% improvement, P=0.028), but fell short of significance at 6 weeks post-treatment (21.1% improvement, P=0.155), despite a sizeable treatment effect. Similarly, supplementation with EM produced a significant treatment response versus placebo at 1 week for CBPI pain (19.4% improvement, P=0.010), but fell just short of significance at 6 weeks (22.5% improvement, P=0.098), again despite a sizeable treatment effect. Supplementation with EM was not significant versus placebo at 1 week for CBPI QOL (14.0% improvement, P=0.155), but produced a significant treatment response by the end of the 6-week study (26.8% improvement, P=0.033). A clinical comparison of valid subjects (excluding noncompliance) was also carried out to obtain mean baseline values for each of the VCSA outcome measures (Table 5). Statistical analysis of the secondary outcome measures (VCSA pain, mobility, lameness while walking, and lameness while trotting) revealed that supplementation with EM produced a significant treatment response versus placebo at 6 weeks for VCSA pain (23.6% improvement, P=0.012), but fell short of significance for VCSA mobility (11.7% improvement, P=0.141), VCSA lameness (w, 9.4% improvement, P=0.329), and VCSA lameness (t, 10.8% improvement, P=0.358).
Viable serum samples from both baseline and week 6 were obtained from 26 (14 treatment and 12 placebo) of the 42 dogs that completed the study. A clinical comparison of these valid subjects was carried out to obtain mean baseline values for the cartilage degradation biomarker, CTX-II. Statistical analysis of serum CTX-II levels revealed that supplementation with EM produced a significant treatment response versus placebo at 6 weeks (47.9% improvement, P=0.018; Figure 1; placebo, baseline: 5.0±6.9 pg/mL and week 6: 6.9±7.5 pg/mL; EM, baseline: 5.0±11.6 pg/mL and week 6: 4.5±9.0 pg/mL). The intra-assay coefficient of variation was 5.13.
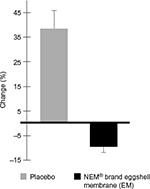  | Figure 1 Percentage of change from baseline in serum CTX-II levels after 6 weeks in EM-supplemented and placebo groups. Abbreviation: CTX-II, cross-linked telopeptide of type II collagen. |
There were 15 AEs reported during the study. These were composed of three instances of loose stool, two instances of diarrhea, seven instances of vomiting, two instances of rash, and one instance of constipation. Eight AEs occurred in five placebo group subjects and seven AEs occurred in four treatment group subjects. All of the AEs were deemed unrelated to the study treatment. There were no serious AEs reported during the study. There were no treatment-related abnormalities in any of the clinical chemistry parameters evaluated in the study. Subject dog owners reported that the treatment was well tolerated by their pets.
Discussion
Joint and connective tissue disorders are extremely common in dogs and can have a significant impact on the QOL for those that suffer from the debilitating conditions. This trial was designed to evaluate the efficacy, safety, and tolerability of EM ESM as a natural treatment alternative for dogs suffering from suboptimal joint function. Results of this study demonstrate that EM is both effective and safe for treating pain (CBPI and VCSA) and immobility (CBPI) associated with suboptimal joint function and effectively improves the QOL (CBPI) of the dogs receiving the supplement.
Subject dogs experienced a relatively rapid (1 week) response as rated by CBPI pain with a mean improvement of 19.4%. By the end of the 6-week follow-up period, the mean response for joint pain improved to 23.6% as judged by veterinarians (VCSA). By comparison, Moreau et al30 found no benefit in dogs with OA even after 8 weeks of treatment with a supplement containing glucosamine (GluN) hydrochloride and CS. McCarthy et al32 found a similar treatment response from a Glu/CS supplement to that found from EM presented here; however, this benefit was only seen after >9 weeks of supplementation. Importantly, both studies utilized veterinarian assessments nearly identical to our VCSA. The rapid onset of the treatment effect from EM is on par with that found for meloxicam and carprofen evaluated in these same studies. A brief responder analysis of the pain-related data from the current study provides a number of clinically relevant highlights. Approximately one-half (48%) of the EM-treated subjects experienced a ≥33% improvement in both VCSA pain and CBPI pain by 6 weeks (not shown). Importantly, the owner assessments of pain were corroborated by the veterinarian assessments of pain, and these results align well with results from previous responder analyses in clinical studies of EM that were conducted in humans.20–22
Subject dogs also experienced a relatively rapid (1 week) response as rated by CBPI function with a mean improvement of 20.5%. By the end of the 6-week follow-up period, the mean response for CBPI function improved to 21.1%. The sizeable improvements noted by the dog owners could not be fully corroborated by the veterinarian assessments of mobility and lameness (w and t), which improved by an average of 11.7%, 9.4%, and 10.8%, respectively. This disagreement may be a consequence of the difference in precision between the two instruments. That is, the CBPI questionnaire utilizes a ten-point scale, whereas the VCSA questionnaires utilize a five-point scale (mobility) or seven-point scale (lameness). Therefore, a more substantial change in joint function is required to result in a change in VCSA scoring. It is also possible that the disagreement in instruments arises from the inherent design of the questionnaires. That is, the VCSA questionnaires are more specific in their assessment of joint function, whereas the CBPI questionnaire evaluates joint function more broadly and generally.
The effect a treatment has on overall QOL can be an important determinant of treatment efficacy and ultimately future treatment compliance. Although joint pain, immobility, and lameness certainly factor into QOL, there are also certain intangible qualities that affect this aspect of treatment, as well. Importantly, in the present study, dog owners reported a rapid (1 week) response in CBPI QOL with a mean improvement of 14.0%. By the end of the 6-week follow-up period, the mean response for CBPI QOL improved to 26.8%. The magnitude of this improvement would be expected to be clinically meaningful in the QOL of dogs suffering from suboptimal joint function.
Symptom relief is certainly a critical component of any arthritis treatment. However, the further capacity to reduce inflammation within the joint and preserve articular cartilage integrity – to be disease modifying – is lacking in currently available treatments. We report here for the first time the chondroprotective effects in dogs, as evidenced by the substantial reduction (47.9%) in the change in serum CTX-II levels after 6 weeks of supplementation with EM versus placebo. The capacity of EM to impact CTX-II so profoundly was first shown in a rat model of OA;35 however, the current study is the first evidence demonstrated in naturally occurring joint disease. Evidence from prior studies involving EM indicates that this chondroprotective effect likely arises from reduced joint inflammation18,19 coupled with reduced levels of various cartilage-degrading matrix metalloproteinases.35
The safety profile for EM is also of significance as there have been no reports of serious AEs associated with treatment in any of the clinical studies conducted to date. No side effects from consuming EM have thus far been identified, excluding the obvious egg allergy concern. This is of obvious importance in canine conditions such as hip dysplasia and OA that require long-term treatment.
The trial had a somewhat limited enrollment (51 subjects); however, there was a fairly low drop-out rate (9.8%) and good treatment compliance. The variability in the severity of the suboptimal joint function in the study dogs likely made it more difficult to detect treatment responses, and this was complicated by the wide variety of breeds (and concurrent sizes) of dogs enrolled in the study, as well. The owner assessment (CBPI), although validated for use in the treatment of OA with NSAIDs, appeared to effectively detect changes in joint pain and joint function in this study. The veterinarian assessments (VCSA), although based upon previous assessment designs,29–31 were modified to a significant degree for this study and appeared to be effective tools for clinician assessment. The addition of additional objective measures of joint function (eg, force plate analysis, gait analysis) could prove beneficial. Further research is warranted to validate the use of serum CTX-II as a diagnostic or prognostic biomarker for canine cartilage status.
With so many dogs suffering from suboptimal joint function, it is important for dog owners to have treatment options that are both safe and effective. The reporting of the results from this eight-center randomized controlled trial demonstrates that EM ESM is a viable therapeutic option for the management of the pain and loss of function associated with suboptimal joint function in dogs. Supplementation with EM, 6 mg/lb (~13.5 mg/kg) taken once daily, significantly reduced joint pain and improved joint function rapidly (CBPI 1 week) and demonstrated a lasting improvement in joint pain (VCSA 6 weeks) leading to an improved QOL (CBPI 6 weeks). Moreover, a profound chondroprotective effect was demonstrated following 6 weeks of supplementation with EM. There were also clinically meaningful results from a brief responder analysis, demonstrating that a significant proportion of treated dogs will benefit substantially from EM supplementation.
Acknowledgments
The sponsor would like to thank all of the participating veterinarians, Pamela Von Behrens, DVM, Mark Lux, DVM, Mathew Mahn, DVM, DACVS, James Scheussler, DVM, DABVP, Edward Migneco, DVM, DABVP, Lawrence Zeis, DVM, Doug Pernikoff, DVM, Wayne Boillat, DVM, Kent Thornberry, DVM, Meggan Bayer, DVM, Gwen Bilyk, DVM, Dale Diesel, DVM, Missouri State University supervising veterinarian Michael Stafford, DVM, and are especially grateful to all of the participant dog owners. The authors would also like to thank United Pet Group for providing the bottled tablets used in the study.
Disclosure
KJR and MB are employed by the sponsor. KJK is a paid consultant for the sponsor. The other authors report no conflicts of interest in this work.
References
Coopman F, Verhoeven G, Saunders J, Duchateau L, van Bree H. Prevalence of hip dysplasia, elbow dysplasia and humeral head osteochondrosis in dog breeds in Belgium. Vet Rec. 2008;163(22):654–658. | ||
Rettenmaier JL, Keller GG, Lattimer JC, Corley EA, Ellersjeck MR. Prevalence of hip dysplasia in a veterinary teaching hospital population. Vet Radiol Ultrasound. 2002;43(4):313–318. | ||
Stanin D, Pavlak M, Vrbanac Z, Potočnjak D. Prevalence of hip dysplasia in dogs according to official radiographic screening in Croatia. Veterinarski Arhiv. 2011;81(2):235–248. | ||
Simon S, Ganesh MR, Ayyappan S, et al. Incidence of canine hip dysplasia: a survey of 272 cases. Vet World. 2010;3(5):219–220. | ||
Marshall WG, Bockstahler BA, Hulse DA, Carmichael S. A review of osteoarthritis and obesity: current understanding of the relationship and benefit of obesity treatment and prevention in the dog. Vet Comp Orthop Traumatol. 2009;22(5):339–345. | ||
Rialland P, Bichot S, Moreau M, et al. Clinical validity of outcome pain measures in naturally occurring canine osteoarthritis. BMC Vet Res. 2012;8:162. | ||
Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. | ||
Oestergaard S, Chouinard L, Doyle N, et al. The utility of measuring C-terminal telopeptides of collagen type II (CTX-II) in serum and synovial fluid samples for estimation of articular cartilage status in experimental models of destructive joint diseases. Osteoarthritis Cartilage. 2006;14(7):670–679. | ||
Valdes AM, Meulenbelt I, Chassaing E, et al. Large scale meta-analysis of urinary C-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type II collagen and their role in prevalence, incidence and progression of osteoarthritis. Osteoarthritis Cartilage. 2014;22(5):683–689. | ||
van Spil WE, DeGroot J, Lems WF, Oostveen JCM, Lafeber FPJG. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage. 2010;18(5):605–612. | ||
Patraa D, Sandell LJ. Recent advances in biomarkers in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):465–470. | ||
Dam EB, Byrjalsen I, Karsdal MA, Qvist P, Christiansen C. Increased urinary excretion of C-telopeptides of type II collagen (CTX-II) predicts cartilage loss over 21 months by MRI. Osteoarthritis Cartilage. 2009;17(3):384–389. | ||
Hurlbeck C, Einspanier R, Pfeil I, Bondzio A. Evaluation of biomarkers for osteoarthritis caused by fragmented medial coronoid process in dogs. Res Vet Sci. 2014;96(3):429–435. | ||
Henrotin Y, Sanchez C, Balligand M. Pharmaceutical and nutraceutical management of canine osteoarthritis: present and future perspectives. Vet J. 2005;170(1):113–123. | ||
Wong M, Hendrix MJC, von der Mark K, Little C, Stern R. Collagen in the egg shell membranes of the hen. Dev Biol. 1984;104(1):28–36. | ||
Baker JR, Balch DA. A study of the organic material of hen’s-egg shell. Biochem J. 1962;82:352–361. | ||
Long FD, Adams RG, DeVore DP, Inventors. Preparation of hyaluronic acid from eggshell membrane. 6946551. 2005 Sep 20. | ||
Benson KF, Ruff KJ, Jensen GS. Effects of natural eggshell membrane (NEM) on cytokine production in cultures of peripheral blood mononuclear cells: increased suppression of tumor necrosis factor-α levels after in vitro digestion. J Med Food. 2012;15(4):360–368. | ||
Ruff KJ, DeVore DP. Reduction of pro-inflammatory cytokines in rats following 7-day oral supplementation with a proprietary eggshell membrane-derived product. Mod Res Inflamm. 2014;3(1):19–25. | ||
Ruff KJ, DeVore DP, Leu MD, Robinson MA. Eggshell membrane: a possible new natural therapeutic for joint and connective tissue disorders. Results from two open-label human clinical studies. Clin Interv Aging. 2009;4:235–240. | ||
Ruff KJ, Winkler A, Jackson RW, DeVore DP, Ritz BW. Eggshell membrane in the treatment of pain and stiffness from osteoarthritis of the knee: a randomized, multicenter, double-blind, placebo-controlled clinical study. Clin Rheumatol. 2009;28(8):907–914. | ||
Danesch U, Seybold M, Rittinghausen R, Treibel W, Bitterlich N. NEM® brand eggshell membrane effective in the treatment of pain associated with knee and hip osteoarthritis: results from a six-center, open-label German Clinical Study. J Arthritis. 2014;3(3):136. | ||
Bauer KL, Dierenfeld ES, Hartup BK. Evaluation of a nutraceutical joint supplement in cranes. Proc North Am Crane Workshop. 2014;12:27–32. | ||
Dierenfeld ES, Baum D, Hampe L, Jensen J, Atwell C, Wedekind K. Evaluation of a nutraceutical joint supplement in camels. Am Holistic Vet Med Assoc J. 2014;39:59–66. | ||
Wedekind KJ, Coverdale JA, Hampton TR, et al. Efficacy of an equine joint supplement, and the synergistic effect of its active ingredients (chelated trace minerals and natural eggshell membrane), as demonstrated in equine, swine, and an osteoarthritis rat model. Open Access Anim Physiol. 2015;7:13–27. | ||
Brown DC, Boston RC, Coyne JC, Farrar JT. Development and psychometric testing of an instrument designed to measure chronic pain in dogs with osteoarthritis. Am J Vet Res. 2007;68(6):631–637. | ||
Brown DC, Boston RC, Coyne JC, Farrar JT. Ability of the Canine Brief Pain Inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc. 2008;233(8):1278–1283. | ||
Brown DC, Boston RC, Farrar JT. Comparison of force plate gait analysis and owner assessment of pain using the canine brief pain inventory in dogs with osteoarthritis. J Vet Intern Med. 2013;27(1):22–30. | ||
Brown DC, Bell M, Rhodes L. Power of treatment success definitions when the Canine Brief Pain Inventory is used to evaluate carprofen treatment for the control of pain and inflammation in dogs with osteoarthritis. Am J Vet Res. 2013;74(12):1467–1473. | ||
Moreau M, Dupuis J, Bonneau NH, Desnoyers M. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet Rec. 2003;152(11):323–329. | ||
Innes JF, Fuller CJ, Grover ER, Kelly AI, Burn JF. Randomised, double-blind, placebo-controlled parallel group study of P54FP for the treatment of dogs with osteoarthritis. Vet Rec. 2003;152(15):457–460. | ||
McCarthy G, O’Donovan J, Jones B, McAllister H, Seed M, Mooney C. Randomised double-blind, positive-controlled trial to assess the efficacy of glucosamine/chondroitin sulfate for the treatment of dogs with osteoarthritis. Vet J. 2007;174(1):54–61. | ||
U.S. Food and Drug Administration [webpage on the Internet]. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf. Accessed January 3, 2014. | ||
Systat Software, Inc [homepage on the Internet]. Available from: www.systat.com. Accessed September 14, 2013. | ||
Sim BY, Bak JW, Lee HJ, et al. Effects of natural eggshell membrane (NEM) on monosodium iodoacetate-induced arthritis in rats. J Nutr Health. 2015;48(4):310–318. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.