Back to Journals » Journal of Asthma and Allergy » Volume 13
Effectiveness of Low-Dose Itraconazole in Fungal-Associated Severe Asthma: A Retrospective Study
Authors Lin CY , Huang YC , Huang HY, Chung FT, Lo YL, Lin SM, Wang CH , Kuo HP
Received 10 August 2020
Accepted for publication 18 September 2020
Published 8 October 2020 Volume 2020:13 Pages 453—461
DOI https://doi.org/10.2147/JAA.S276289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Amrita Dosanjh
Chun-Yu Lin,1,2 Yu-Chen Huang,1,2 Hung-Yu Huang,1,2 Fu-Tsai Chung,1,2 Yu-Lun Lo,1,2 Shu-Min Lin,1,2 Chun-Hua Wang,1,2 Han-Pin Kuo3
1Department of Thoracic Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan; 2College of Medicine, Chang Gung University, Taoyuan, Taiwan; 3College of Medicine, Taipei Medical University, Taipei, Taiwan
Correspondence: Chun-Hua Wang
Department of Thoracic Medicine, Chang Gung Memorial Hospital at Linkou, 5 Fu-Hsing Street, Kweishan, Taoyuan 33305, Taiwan
Tel +8863-3281200 ext.8467
Fax +8863-3287787
Email [email protected]
Background: The efficacy of antifungal therapy in fungal-associated severe asthma remains controversial.
Objective: We aimed to evaluate the differences in the clinical presentation and response to antifungal therapy between severe asthmatics with fungal sensitization and positive fungal isolates.
Methods: This retrospective study included 73 patients with severe asthma from January 2004 to December 2017. We examined the presentation, medication, exacerbations, pulmonary function, serum IgE, blood eosinophils, and sputum culture results. Follow-up care was provided to each patient for minimum 3 years.
Results: We classified the patients into four groups: group 1, neither fungal sensitization nor fungal isolates in the sputum (n=16); group 2, positive fungal sensitization (n=16); group 3, positive fungal isolates (n=31); and group 4, concomitant positive fungal sensitization and positive fungal isolates (n=10). There were four participants in group 2, 15 in group 3, and 6 in group 4 had received itraconazole therapy for 3 months. Patients in group 3 presented with lower serum IgE level than those in groups 2 and 4. Antifungal therapy significantly improved ACT score during the first year in groups 3 (from 18 [15– 22] to 24 [23– 24], p=0.0004) and resulted in a long-lasting ACT improvement till the third year in group 3 (from 18 [15– 22] to 24 [22– 24], p=0.0013).
Conclusion: Antifungal therapy could effectively control the symptoms in patients with severe asthma with positive fungal isolates, contrary to those with merely fungal sensitization; therefore, highlighting the need for a more precise treatment strategy in future for fungal-associated severe asthma.
Keywords: fungal-associated asthma, severe asthma, fungal sensitization, antifungal therapy, itraconazole
Introduction
Severe asthma is prevalent in nearly 3.6% of asthmatics.1 However, the annual healthcare cost of each patient with severe asthma in UK is nearly twice of those with mild to moderate asthma.2 In Taiwan, the prevalence of severe asthma is around 6% and is also a burden in our health insurance care.3 Fungal sensitization is observed in some cases of asthma; several evidences have demonstrated the close correlation between fungal sensitization and the severity of asthma.4 Furthermore, sensitization to fungal allergens would characteristically increase the risk of severe asthma exacerbations, consequently requiring multiple hospital admissions and increased medical cost.5,6
Severe asthma with fungal sensitization (SAFS) was first reported in 2006 by Denning et al.7 The definition of SAFS includes severe asthma, fungal sensitization demonstrated by a positive skin prick test response or specific IgE to at least one of 7 fungi and no evidence of allergic bronchopulmonary aspergillosis (ABPA).6 Sensitization to fungal allergens was associated with reduced lung function,8 frequent exacerbations, greater corticosteroid requirement,9 increased risk of life-threatening asthma.4 Additionally, isolation of filamentous fungi in sputum indicated poorer forced expiratory volume in one second (FEV1).10 However, the phenotype of fungi associated with severe asthma remained unclear.
Antifungal therapy continues to be a controversial therapeutic modality in the SAFS patients. Denning et al demonstrated that orally administering itraconazole for 32 weeks improved the quality of life of severe asthmatics with fungal sensitization.11 However, Agbetile et al reported that administering voriconazole for 3 months did not reduce the exacerbation or improve the quality of life of the patients; however, it improved the clinical response with regard to the positive sputum fungal culture.12
This study aimed to evaluate the clinical characteristics, inflammatory presentation in severe asthma associated with fungal sensitization, and attempted to identify the individuals who would benefit from antifungal therapy.
Methods
Subjects
This retrospective, observational study was conducted from January 2004 to December 2017 at the Linkou Chang Gung Memorial Hospital, Taiwan. Asthma diagnosis was considering a clear clinical history of asthma and evidence of variable airflow obstruction and its reversibility (>12% and 200 mL increase in FEV1) after inhaling bronchodilators, or evidence of hyperresponsiveness on methacholine challenge with PC20<8 mg/mL, as recommended.13 Patients were included if they were ≥18 years of age and were receiving step 5 Global Initiative for Asthma (GINA) treatment, with high doses of inhaled corticosteroids (>500 μg equivalent of fluticasone per day) and long-acting beta agonists. Subjects were excluded if they either presented with invasive fungal infection, allergic bronchopulmonary aspergillosis (ABPA), and cystic fibrosis. We recorded the following demographic characteristics: age, gender, smoking status and body mass index (BMI), comorbidity, serum IgE level, eosinophil counts in exacerbation, eosinophil cationic protein (ECP), sputum fungus culture, pulmonary function, medication, asthma control test score (ACT) and exacerbation. All the patients were followed up for at least 3 years.
The patients were categorized according to their fungal sensitization status and sputum fungal isolate results: group 1, neither fungal sensitization nor fungal isolates from sputum; group 2, positive fungal sensitization; group 3, positive fungal isolates; and group 4, concomitant fungal sensitization and positive fungal isolates. The antifungal prescriptions were decided by clinical physicians. The patients in groups 2, 3, and 4 were further divided into the observe and itraconazole group (itraconazole 100 mg twice daily for 3 months). Changes in pulmonary function, ACT score, and exacerbations after antifungal therapy were subsequently evaluated.
The study was approved by the institutional review board of Chang Gung Memorial Hospital (approval no. CGMH 101–4591B). Informed consent was waived due to the retrospective nature of this study, along with no modifications in patient management. All personal information was encrypted in the database, and patient data accessed was de-identified. There was no breach of privacy.
Definitions and Classifications
Definition of severe asthma was based on the international ERS/ATS guidelines11 and the Global Initiative for Asthma (GINA) 2019 criteria (www.ginasthma.com). Acute exacerbations (AE) was defined as an event that was clinically diagnosed by a physician and required systemic steroid prescription for acute onset of increasing cough, worsening dyspnea, and chest tightness as described previously.14 We recorded the AE baseline frequency in 3 years before evaluating fungal sensitization, along with emergency department (ED) visits and hospitalization due to asthma AE. Allergen-specific IgE levels were determined using a commercial assay for IgE (ImmunoCAP; Phadia). SAFS diagnosis was performed to evaluate the positive reaction in the specific IgE antibody test against specific fungi (one or more positive results against eight types of fungi), according to the diagnostic criteria proposed by Agarwal et al.15
Measurements of Lung Function
Pulmonary function test including forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio was performed with a spirometer according to the ATS and the ERS criteria.16
Fungal Culture and Identification
If the physician suspected the patients were fungal associated with severe asthma, the sputum plugs were collected prior to this study and plated directly onto fungal-specific culture media and incubated at 37°C for approximately 7 days.8 The fungi were classified according to the macroscopic and microscopic morphology. The fungal species were subsequently isolated and confirmed via sequencing and using PCR conditions as previously described.10
Statistical Analysis
Categorical variables were described using counts (percentages). Parametric data were expressed as means ± standard deviation (SD). Among the group comparisons, one-way analysis of variance (ANOVA) followed by Dunnett’s test, where appropriate, was used to determine the statistical significance of the difference between means. The follow-up parametric data were compared using the paired Student’s t-test. Non-parametric data were determined by chi-squared tests. All analyses were two-sided, and p<0.05 was considered statistically significant. Statistical analyses were performed using Prism version 5 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Patients Baseline Characteristics
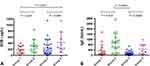
Data from all 73 adult patients diagnosed with severe asthmatics were analyzed, of which 16 were in group 1, 16 in group 2, 31 in group 3, and 10 in group 4. Table 1 summarizes the clinical characteristics of all patients. The four groups were not significantly different considering the following parameters: age, gender, BMI, smoking status, non-fungal sensitization status, ECP level, comorbidities (including sinusitis, GERD and bronchiectasis), add-on therapies including systemic steroids, anti-IgE therapy of omalizumab and long-acting muscarinic antagonist (LAMA), ACT scores, and acute exacerbations. In patients with fungal sensitization (groups 2 and 4), 100% had sensitization to non-fungal allergen and only half of those without fungal sensitization (groups 1 and 3) had sensitization to non-fungal allergen (p=0.0003). Compared to groups 1, group 4 had greater eosinophil count in acute exacerbation (178 [69–303] vs 264 [215–657], p=0.023, Table 1 and Figure 1A). Compared to group 3, groups 2 and 4 had significantly higher IgE levels (82 [22–230] vs 809 [264–1296], p<0.0001; 82 [22–230] vs 275 [122–748], p=0.0008, respectively; Table 1 and Figure 1B).
 |
Table 1 Clinical Characteristics of the Four Groups of Severe Asthma According to Fungal Sensitization Status and Fungal Isolates from Sputum |
Follow-Up Status
Median follow-up duration was 6.1 years [2.9–7.4]. Four patients in group 2, 15 in group 3, and 6 in group 4 had received itraconazole 100 mg twice daily for 3 months. Baseline FEV1 and FVC values were relatively similar for the four groups, following which groups 2–4 were further divided into the observe and itraconazole groups (Table 2). There was no significant change in the baseline and follow-up FEV1 and FVC values in the first year between groups 1, 2 and group 4, along with the observe subgroups of groups (Table 2 and Figure 2). Patients in group 3 who underwent itraconazole therapy showed significantly FVC improvement in the first year (from 2.0 [1.2–2.4] to 2.1 [1.7–2.5], Δ0.37±0.36, p=0.0014, Table 2 and Figure 2). The follow-up ACT score in the first year was comparable between groups 1, 2 and 4, along with the observe subgroups of group 3 (Table 2 and Figure 2). Patients in group 3 who underwent itraconazole therapy demonstrated a greater improvement in the ACT scores at the first-year follow-up than the baseline ACT (from 18 [15–22] to 24 [23–24], Δ5.7±4.5, p=0.0004, Table 2 and Figure 2).
The FEV1 and FVC follow-up values in the third year were similar for all the groups (Table 2 and Figure 3). The follow-up ACT score at the third year of itraconazole subgroup in the group 3 improved persistently when compared to the baseline ACT score (from 18 [15–22] to 24 [22–24], Δ6.8±5.5, p=0.001, Table 2 and Figure 3). With regard to exacerbations, the itraconazole subgroup of group 3 also showed significant decline in AE than the baseline AE (from 2.0 [0–7.0] to 1.0 [0–2.0], Δ-2.6±3.5, p=0.017, Table 2).
Fungal Sensitization Species and Fungal Isolated Species
Among the 73 severe asthmatics, 26 presented with fungal sensitization, of which had multiple fungal sensitization. Penicillium chrysogenum was the most common sensitization species found (15 of 26, 58%), followed by Aspergillus fumigatus (14 of 26, 54%) and Cladosporium herbarum (6 of 26, 23%, Table 3). Forty-one patients had positive fungal isolates from sputa, 29 had multiple fungal isolates, while the remaining 12 had a single fungal isolate. Penicillium spp. was also the most common identified species (18 of 41, 44%), followed by Cladosporium spp. (17 of 41, 41%) and Aspergillus spp. (13 of 41, 32%, Table 3). Seven patients presented with an undifferentiated mold.
 |
Table 3 Fungal Sensitization Species and Fungal Isolated Species in Severe Asthmatics |
Discussion
This is possibly the first study to investigate the effectiveness of antifungal therapy and the inflammatory characteristics in severe asthmatics with fungal sensitivity and fungal isolation from sputum. Our study demonstrated that low dose of antifungal therapy could effectively improve lung function and acute exacerbation in patients with severe asthma with positive fungal isolates, contrary to those with merely fungal sensitization. Compared to patients with fungal sensitization, those presenting with only positive fungal isolates demonstrated relatively lower serum IgE levels. Moreover, the ACT score increased significantly after antifungal therapy among the severe asthmatic patients with positive fungal isolates, but remained constant in those with fungal sensitization. Taken together, the stratification of fungal colonization or fungal sensitivity is very important for a more precise treatment strategy in future for fungus-associated severe asthma.
Asthmatics with fungal exposure and sensitization, along with the isolation of fungal filaments from the sputum typically demonstrated lower post-bronchodilator FEV1.8,10,17,18 Patients with fungal sensitization may also present with frequent exacerbations, greater corticosteroids requirement, need for ICU admission, and mechanical ventilation.4,9 However, in our current study, the pulmonary functions are comparable in each group. Additionally, acute exacerbation events, ED visit, and hospitalization were similar in each group.
Fungi are linked to Th2 cell-related airway inflammation and may participate in the pathogenesis, with increased serum eosinophil counts, IgE and IL-33 levels.4,8,9,19,20 Fungal colonization is considered an important part of fungal sensitization and persistent airway inflammation.7,21 Recent study from Saglani group22 demonstrated that pediatric SAFS was associated with higher IgE level, maintaining oral steroids and higher IL-33 levels. They indicated that fungal sensitivity associated with severe asthma may be related to IL-33-mediated type 2 inflammation. Thus, the fungal sensitization groups presented with relatively higher IgE level, compatible with previous study.22 However, no study has evaluated the blood eosinophil count and serum IgE level in the patients presenting with fungal isolation without fungal sensitization. Asthmatics with both fungal sensitization and fungal isolates showed the highest eosinophil counts at exacerbations. However, to our surprise, severe asthmatics with positive fungal isolates and without fungal sensitization demonstrated relatively low serum IgE levels. This finding implicated that fungal sensitization and colonization may be involved in different inflammatory processes.
There were two large, randomized control trials focusing on antifungal therapy for asthmatics with fungal sensitization. Denning et al enrolled 58 asthmatics who were being treated with the British Thoracic Society (BTS) step 4 treatment and demonstrated that 200 mg of itraconazole, twice daily, for 32 weeks improves the quality of life and decreases serum IgE level.11 Alternatively, 3 months therapy with 200 mg of voriconazole twice daily failed to improve the exacerbations and quality of life for 59 moderate-to-severe asthmatics.12 However, with regard to sputum culture in the same study, there was a significantly greater number of responders in the voriconazole group than in the placebo group, despite the incomplete sputum clearance. It is challenging to deduce a meaningful comparison due to the small number of subjects with definitive sputum data.12 Here, we evaluated the treatment response in severe asthmatics who were considered as uncontrolled under the GINA step 5 therapy and classified according to the fungal sensitization status and fungal isolates from sputum. Antifungal therapy most significantly improved ACT in those with positive fungal isolates, but had no remarkable benefits in those with simply fungal sensitization. In patients with fungal sensitization without fungal isolation, antifungal therapy alone may not be able to eliminate the stimulation from environment fungi. Unavoidable environmental exposure may also explain the modest response of the antifungal therapy during the first year in patients with both fungal sensitization and positive fungal isolates. Systemic steroid administration may produce desirable outcomes for asthmatics with fungal sensitization with a predominant Th2 inflammation presenting as high IgE level. Nevertheless, long-term steroid exposure had several complications and increased medical costs.2,23 Moreover, Fraczek et al demonstrated that patients receiving steroid therapy had significantly higher levels of fungus load in their bronchoalveolar lavage, independent of the antifungal therapy.24 Increased fungal load deteriorated the airway inflammation, subsequently resulting in a vicious cycle. Considering the numerous inflammatory processes between fungal sensitization, fungal colonization status, and persistent stimulation from environmental fungi, a combination of antifungal treatment and antibody therapies targeting the Th2 inflammation pathway, such as the anti‐IgE or anti‐IL5 antibodies, may improve fungal sensitization in patients.
Alternaria alternata is associated with SAFS22 that we observed in fungal sensitized people; however, this was not observed in fungal colonized people. The possibility is that Alternaria species are mesophilic, growing at an optimum temperature of 18°C to 22°C. They are not thermotolerant fungi and may be unable to grow in body temperature. They rarely cause infection but can be encountered as allergens.21
Our study had several inherent limitations. First, this was a retrospective study and we only included severe asthmatics who were categorized as uncontrolled under the GINA step 5 therapy, which may have resulted in selection biases and diminished generalizability. Nevertheless, these patients may have the highest medical costs and require a more precise treatment strategy. Second, the dose of itraconazole, 100mg twice daily, is relatively low dose and we did not measure the serum drug levels. We choose this dose because Asian people usually had relatively smaller body size and we used itraconazole in managing fungal-associated asthma by reducing fungal load and not for invasive fungal infection. Most of our patients did not sequentially follow the sputum fungal culture, which led to the loss of the status of fungal colonization after antifungal therapy. There were no adverse effects recorded in our observation. The appropriate itraconazole dose, serum drug level and adverse effects need further investigation. Finally, pulmonary functions in the third year had no significant difference between groups. This may attribute to the fact that the follow-up duration was not long enough, although our study had the longest follow-up duration than the other studies on the subject. Owing to the retrospective nature of our data, our sample size was limited. Further larger prospective studies are warranted to validate our results.
Conclusion
Fungal isolates from the sputum in severe asthmatics may present different inflammatory processes to those with fungal sensitization. Antifungal therapy could effectively control the symptoms in severe asthmatics with positive fungal isolates, but not in those with fungal sensitization. There is a need to device a more precise treatment strategy to treat fungus-associated asthma.
Abbreviations
SAFS, Severe asthma with fungal sensitization; FEV1, forced expiratory volume in one second; GINA, Global Initiative for Asthma; ABPA, allergic bronchopulmonary aspergillosis; BMI, body mass index; ECP, eosinophil cationic protein; ACT, asthma control test; AE, acute exacerbations; ED, emergency department; FVC, forced vital capacity; SD, standard deviation; LAMA, long-acting muscarinic antagonist.
Data Sharing Statement
The data sets analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgment
We thank all the investigators and members of the Department of Thoracic Care Medicine for their effort.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042
2. Barry LE, Sweeney J, O’Neill C, Price D, Heaney LG. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017;18(1):129. doi:10.1186/s12931-017-0614-x
3. Chen HC, Huang CD, Chang E, Kuo HP. Efficacy of omalizumab (Xolair (R)) in patients with moderate to severe predominately chronic oral steroid dependent asthma in Taiwan: a retrospective, population-based database cohort study. BMC Pulm Med. 2016;16(1):3. doi:10.1186/s12890-015-0156-2
4. Medrek SK, Kao CC, Yang DH, Hanania NA, Parulekar AD. Fungal sensitization is associated with increased risk of life-threatening asthma. J Allergy Clin Immunol Pract. 2017;5(4):1025–1031 e1022. doi:10.1016/j.jaip.2016.11.015
5. Agarwal R, Nath A, Aggarwal AN, Gupta D, Chakrabarti A. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with acute severe asthma in a respiratory intensive care unit in North India. Mycoses. 2010;53(2):138–143. doi:10.1111/j.1439-0507.2008.01680.x
6. O’Driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm Med. 2005;5(1):4. doi:10.1186/1471-2466-5-4
7. Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27(3):615–626.
8. Fairs A, Agbetile J, Hargadon B, et al. IgE sensitization to aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med. 2010;182(11):1362–1368. doi:10.1164/rccm.201001-0087OC
9. Goh KJ, Yii ACA, Lapperre TS, et al. Sensitization to aspergillus species is associated with frequent exacerbations in severe asthma. J Asthma Allergy. 2017;10:131–140. doi:10.2147/JAA.S130459
10. Agbetile J, Fairs A, Desai D, et al. Isolation of filamentous fungi from sputum in asthma is associated with reduced post-bronchodilator FEV1. Clin Exp Allergy. 2012;42(5):782–791. doi:10.1111/j.1365-2222.2012.03987.x
11. Denning DW, O’Driscoll BR, Powell G, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: the Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179(1):11–18. doi:10.1164/rccm.200805-737OC
12. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134(1):33–39. doi:10.1016/j.jaci.2013.09.050
13. Global Initiative for Asthma., National Heart Lung and Blood Institute. Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention.
14. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99.
15. Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43(8):850–873.
16. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
17. Hernberg S, Sripaiboonkij P, Quansah R, Jaakkola JJK, Jaakkola MS. Lung function is reduced among subjects with asthma exposed to mold odor. Chest. 2014;146(1):e28–e29. doi:10.1378/chest.14-0582
18. Menzies D, Holmes L, McCumesky G, Prys-Picard C, Niven R. Aspergillus sensitization is associated with airflow limitation and bronchiectasis in severe asthma. Allergy. 2011;66(5):679–685. doi:10.1111/j.1398-9995.2010.02542.x
19. Porter PC, Lim DJ, Maskatia ZK, et al. Airway surface mycosis in chronic TH2-associated airway disease. J Allergy Clin Immunol. 2014;134(2):325–331. doi:10.1016/j.jaci.2014.04.028
20. Masaki K, Fukunaga K, Matsusaka M, et al. Characteristics of severe asthma with fungal sensitization. Ann Allergy Asthma Immunol. 2017;119(3):253–257.
21. Rick EM, Woolnough K, Pashley CH, Wardlaw AJ. Allergic fungal airway disease. J Investig Allergol Clin Immunol. 2016;26(6):344–354. doi:10.18176/jiaci.0122
22. Castanhinha S, Sherburn R, Walker S, et al. Pediatric severe asthma with fungal sensitization is mediated by steroid-resistant IL-33. J Allergy Clin Immunol. 2015;136(2):312–322 e317. doi:10.1016/j.jaci.2015.01.016
23. Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–1495. doi:10.1016/j.jaci.2015.07.046
24. Fraczek MG, Chishimba L, Niven RM, et al. Corticosteroid treatment is associated with increased filamentous fungal burden in allergic fungal disease. J Allergy Clin Immunol. 2018;142(2):407–414. doi:10.1016/j.jaci.2017.09.039
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.