Back to Journals » International Journal of General Medicine » Volume 15
Effectiveness and Tolerability of Anlotinib Plus PD-1 Inhibitors for Patients with Previously Treated Metastatic Soft-Tissue Sarcoma
Authors Sun X, Xu J, Xie L, Guo W
Received 20 June 2022
Accepted for publication 25 August 2022
Published 28 September 2022 Volume 2022:15 Pages 7581—7591
DOI https://doi.org/10.2147/IJGM.S379269
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xin Sun, Jie Xu, Lu Xie, Wei Guo
Department of Musculoskeletal Tumor Center, Peking University People’s Hospital, Beijing, 100044, People’s Republic of China
Correspondence: Wei Guo, Department of Musculoskeletal Tumor Center, Peking University People’s Hospital, No. 11, Xizhimen South Street, Xicheng District, Beijing, 100044, People’s Republic of China, Tel +86 13810548607, Email [email protected]
Objective: This study was to investigate the effectiveness and tolerability of anlotinib plus PD-1 inhibitors for patients with previously treated metastatic soft tissue sarcoma (STS).
Methods: Patients with previously treated metastatic STS who were administered with anlotinib plus PD-1 inhibitors in clinical practice were included for analysis retrospectively. All the common subtypes of advanced STS were appropriate for analysis. Efficacy of the regimen was assessed according to the change of target lesion radiologically, and all the patients were followed up regularly. Safety profile during the combination administration was recorded and documented specifically. Clinical significance according to different STS subtypes was analyzed accordingly.
Results: From September 2018 to January 2022, a total of 32 patients with previously treated metastatic STS who received anlotinib plus PD-1 blockades were screened for the analysis in this study. The best overall response during the combination administration indicated that partial response was observed in 11 patients, stable disease was noted in 16 patients and progressive disease was found in 6 patients, yielding an objective response rate (ORR) of 34.4% (95% CI: 18.6– 53.2%) and a disease control rate (DCR) of 84.4% (95% CI: 67.2– 94.7%). Furthermore, the median PFS of 32 patients with metastatic STS was 7.6 months (95% CI: 3.31– 11.89) and the median OS was 14.9 months (95% CI: 8.36– 21.44). Besides, adverse reactions related to the treatment during anlotinib plus PD-1 inhibitors administration were observed in 29 patients (90.6%), of whom, a total of 13 patients (40.6%) were deemed as grade 3– 4 adverse reactions and no grade 5 adverse reaction was found. Specifically, the most common adverse reactions were fatigue, hypertension, hand-foot syndrome, diarrhea and dermal toxicity.
Conclusion: Anlotinib plus PD-1 inhibitors demonstrated durable and promising efficacy and tolerable safety for patients with metastatic STS in real world. Further prospective clinical trials were warranted to validate the feasibility of anlotinib plus PD-1 blockades clinically.
Keywords: soft tissue sarcoma, anlotinib, PD-1 inhibitors, effectiveness, tolerability
Introduction
Soft tissue sarcoma (STS) was consisted of a heterogeneous group of rare malignant solid tumors originated from the mesenchymal tissue that accounted for approximately 1% of all adult malignancies,1 which included almost 100 different entities that were associated with different morbidity and mortality.2 It was estimated that there were approximately 24,000 new cases in China annually, and the incidence was profoundly higher in adolescents.3,4 The most common subtypes of STS included undifferentiated sarcoma (US), liposarcoma (LPS), synovial sarcoma (SS) and leiomyosarcoma (LMS), which were associated with distinct molecular and behavioral characteristics.5,6 The NCCN guidelines of STS exhibited that surgical resection was the only curable therapeutic option for STS. However, the other treatment regimens for STS were comparatively scarce with dismal prognosis.7 The median overall survival (OS) was approximately two years for advanced LMS but less than one year for most other advanced STS, resulting in a 5-year OS rate of around 10% for patients with STS currently. Therapeutic options for metastatic STS remained disappointing own to the rarity and heterogeneity of STS. It was reported that single-agent chemotherapy (dacarbazine, doxorubicin, epirubicin or ifosfamide) or anthracycline-based combination regimens were the standard of care as first-line therapy for patients with metastatic STS over the past decades,8 which yielded an objective response rate (ORR) of almost 20% and a median progression-free survival (PFS) of 5 months and median OS of approximately 16 months.9 Other available therapeutic options for patients with metastatic STS who progressed after the first-line therapy included pazopanib or single-agent chemotherapy. However, each of these agents demonstrated modest efficacy and prognosis.10 As a result, patients with metastatic STS were associated with a worse prognosis currently.
Noteworthily, small molecule anti-angiogenesis tyrosine kinase inhibitor (TKI) demonstrated promising efficacy in metastatic STS. Pazopanib exhibited potential therapeutic significance for patients with previously treated metastatic STS subtype except LPS according to the phase III clinical trial,11 which suggested that pazopanib significantly improved median PFS (4.6 months vs 1.6 months for placebo, P<0.001). Therefore, pazopanib was recommended as a potential option for patients with metastatic STS. Similarly, as a novel oral multi-target TKI with the inhibition of VEGFR1-3, FGFR1~4, PDGFRα~β, c-Kit and Ret,12 anlotinib exhibited convincing PFS (median PFS: 6.27 months vs 1.47 months, P<0.001) and tolerable safety profile for patients with refractory metastatic STS according to ALTER0203 trial.13 As a result, anlotinib was licensed by Chinese NMPA for patients with previously treated metastatic STS since 2019. However, it should be noted that the ORR of anlotinib monotherapy for patients with metastatic STS was disappointing (ORR = 13%),14 which highlighted that new combination strategies were needed to be explored clinically.
Furthermore, immunotherapy represented by PD-1 blockades revolutionized the therapeutic landscape of various tumors and unprecedented long-term survivorship was observed in immunotherapy recently.15 As a result, previous studies have found the potential therapeutic activity of PD-1 inhibitors for STS. Pembrolizumab single agent was investigated for the therapeutic activity for patients with previously treated STS in the SARC28 phase II clinical trial,16 yielding an ORR of 18% among of all the STS subtypes. Additionally, nivolumab monotherapy demonstrated limited ORR (5%) for patients with previously treated STS according to a multicenter Phase II clinical trial.8 However, the ORR was more promising when nivolumab was used in combination with ipilimumab (an ORR of 16%), which highlighted the necessity of appropriate combination therapeutic strategies in STS as well.
Interestingly, the combination of PD-1/PD-L1 blockades with antiangiogenic targeted drugs might play a promising synergistic action in cancer treatment recently.17 Unfortunately, the available therapeutic evidence of anlotinib plus PD-1 blockades for patients with metastatic STS remained scanty. As a result, this study was to investigate the effectiveness and tolerability of anlotinib plus PD-1 blockades for patients with previously treated metastatic STS retrospectively.
Patients and Methods
Design of This Study and Eligibility Criteria
Given that anlotinib was approved by Chinese NMPA for patients with previously treated metastatic STS since 2019, and PD-1 inhibitors were licensed in China since 2018. A certain number of patients with previously treated metastatic STS were treated with anlotinib plus PD-1 blockades in clinical practice. Consequently, our study was designed as a retrospective study and patients with metastatic STS who received anlotinib plus PD-1 blockades (any PD-1 inhibitors that were licensed in China) in the Department of Musculoskeletal Tumor Center of Peking University People’s Hospital from September 2018 to January 2022 were screened for eligibility retrospectively. In order to produce more rigorous data, necessary eligibility criteria were adopted accordingly. The main inclusion criteria manifested as (1) histologically confirmed soft tissue sarcoma with distant metastasis; (2) aged of 18 years or older; (3) ECOG performance status of 0–2 score; (4) patients with metastatic STS who had progressed after the previous systemic treatments were treated with anlotinib plus PD-1 blockade regimens clinically; (5) patients had at least one measurable targeted lesion according to RECIST 1.1 criteria. Furthermore, the key exclusion criteria were (1) patients were concomitant with serious diseases or another cancer that might compromise the survival of the subjects; (2) patients were diagnosed with clinically active or symptomatic brain metastases. However, patients with stable brain metastases were permitted to be included in this study; (3) patients were diagnosed of gastrointestinal stromal tumor, osteosarcoma and Ewing’s sarcoma (these subtypes might show limited efficacy to anlotinib plus PD-1 blockade therapy); (4) Since PD-1 inhibitors were administered, those with active or uncontrolled autoimmune disease were also excluded; (5) patients’ demographic and therapeutic characteristics were deficient substantially were also unsuitable to be included.
The study profile of this work is illustrated in Figure 1, which indicated that a total of 32 patients with metastatic STS were suitable to be included in our study finally. The protocol of this study was approved by the Ethics Committee of Peking University People’s Hospital. Written informed consent was obtained by the subjects included according to the recommendations of the Declaration of Helsinki.
 |
Figure 1 Research flow chart of this retrospective study. |
Therapeutic Regimens
Patients with previously treated metastatic STS who failed the previous systemic treatments were administered with anlotinib plus PD-1 blockades in clinical practice. Anlotinib was given orally at an initial dosage of 12mg or 10mg per day, which was used continuously for 14 days and stopped for 7 days, every 21 days was deemed as one therapeutic cycle. All the PD-1 blockades were those approved in China mainland and available for Chinese patients in clinical practice, which was consisted of camrelizumab (Jiangsu Hengrui Pharmaceutical Co., LTD), sintilimab (Innovent biopharmaceutical (Suzhou) Co., LTD) and pembrolizumab (Merck (China) Co., LTD). The three PD-1 inhibitors were intravenously administered of 200mg on day 1, every 21 days was one therapeutic cycle. The combination regimens might be discontinued when disease progression or intolerable adverse reactions occurred. Additionally, dosage adjustment of anlotinib to either 10mg or 8mg once daily was permitted based on the tolerability during the treatment.
Schedule of Efficacy Assessment and Document of Safety Profile
The response of the combination regimen was assessed based on RECIST version 1.1.18 Radiological images of the target lesions using computed tomography (CT) or magnetic resonance imaging (MRI) were performed for each subject before and during the combination administration. The therapeutic activity was presented by the change of target lesions, which was evaluated using CT or MRI scans every two cycles or depended on the actual situation when it was necessary for the patients. Baseline demographic characteristics and status of disease progression of each patient were collected through the electronic medical record system when the patients underwent hospitalization. Additionally, follow-up of the patients was mainly carried out using mobile phone with the communication with the relatives monthly.
Furthermore, Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 criteria were used to evaluate the available adverse reactions of the patients. All the adverse reactions during the treatment of anlotinib plus PD-1 inhibitors were collected specifically to present the safety profile among patients with metastatic STS.
Retrospective study adopted the endpoints such as progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR) and safety profile of the combination regimen.
Statistical Analysis
The statistical data presented in this study were analyzed by SPSS software. Specifically, ORR was determined by the proportion of complete response (CR) and partial response (PR) among the 32 patients. DCR was calculated by the proportion of CR and PR and stable disease (SD) among the 32 patients. All the data related in the analysis of this study were described as median (range) and number of patients (percentage), respectively. Furthermore, PFS and OS were defined according to the previous study.19 Association between clinical outcomes (ORR and PFS) and various STS subtypes was analyzed using chi-square test and Log rank test, respectively. Regarding the survival analysis, patients with no disease progression or death events at the date of data cut-off were deemed as censored data. A number of patients (percentage) were used in the safety analysis to estimate the incidence of the adverse reactions during the combination administration. P<0.05 was considered suggestive.
Results
Baseline and Demographic Characteristics
From September 2018 to January 2022, a total of 32 patients were included in this study. The baseline and demographic characteristics are shown in Table 1. All the 32 patients with STS were the common sarcomas in clinical practice. The median age of the 32 patients was 48 years with the range from 18 to 68 years. Male patients accounted for 62.5% among the subjects included. The ECOG performance status of 0–1 score was the predominant performance status with the proportion of 75%. A total of 27 patients had received surgical treatment previously. Interestingly, 10 patients were treated with first-line systemic chemotherapy, 12 patients received second-line treatment, 6 patients were treated with third-line therapy and 4 patients underwent subsequent-line systemic treatment. Noteworthily, the most common STS subtypes in this study were synovial sarcoma (SS, 25.0%), alveolar soft-part sarcoma (ASPS, 21.9%), leiomyosarcoma (LMS, 15.6%) and liposarcoma (LPS, 12.5%). Besides, the other 8 subtypes were consisted of epithelioid sarcoma (n = 2), clear cell sarcoma (n = 2), undifferentiated sarcoma (n = 1), embryonal sarcoma (n = 1), angiosarcoma (n = 1) and spindle cell lipoma (n = 1). Additionally, initial dosage of anlotinib with 12mg and 10mg was observed in 21 and 11 patients, respectively. Regarding the PD-1 blockades, three PD-1 blockades were administered in this study, which suggested that sintilimab, camrelizumab and pembrolizumab were administered in 14, 13 and 5 patients, respectively.
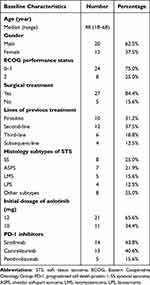 |
Table 1 Baseline Characteristics of the 32 Patients with Metastatic STS |
Efficacy of the Combination Regimen
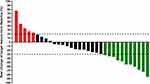
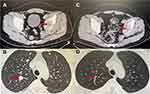
All the patients included in this study were available for response outcomes of anlotinib plus PD-1 blockades. The optimal response of the 32 patients with metastatic STS during anlotinib plus PD-1 blockades administration was assessed based on the efficacy assessment criteria of RECIST1.1, indicating that no complete response (CR) was found, partial response (PR) was observed in 11 patients, stable disease (SD) was noted in 16 patients and progressive disease (PD) was detected in 5 patients, yielding an ORR of 34.4% (95% confidence interval (CI): 18.6–53.2%) and a DCR of 84.4% (95% CI: 67.2–94.7%). Additionally, waterfall plot was used in this study to present the changes in the target lesions of the 32 patients with metastatic STS after anlotinib plus PD-1 blockade administration, which is illustrated in Figure 2. Obviously, the target lesions of considerable patients were shrunk significantly and a total of 11 patients achieved PR response after the treatment of anlotinib plus PD-1 blockades administration. Furthermore, the target lesions of 23 patients shrank to some extent during the combination treatment. Additionally, radiological results of the CT scans before and after anlotinib plus sintilimab administration of one female patient are illustrated in Figure 3. The target lesions reduced significantly after the administration of anlotinib plus sintilimab, which suggested that this patient benefited significantly from anlotinib plus PD-1 inhibitor combination therapy.
Prognosis of the Combination Regimen
The data cut-off date of this study was May 15, 2022, resulting in a median follow-up duration among the 32 patients with metastatic STS of 12.7 months (range: 0.7–34.8 months). It should be noted that 3 patients were still in the treatment and 29 patients discontinued the treatment at the date of data cut-off. Regarding the PFS analysis, a total of 24 progression or death events were noted at the date of data cut-off, producing the PFS data maturity of 75.0%. Accordingly, the PFS survival curve is shown in Figure 4, the median PFS of the 32 patients with metastatic STS who received anlotinib plus PD-1 blockades was 7.6 months (95% CI: 3.31–11.89). Additionally, the 12-month and 24-month PFS rate was 36.7% (95% CI: 20.4–53.1%) and 15.4% (95% CI: 3.6–34.9%), respectively.
 |
Figure 4 Progression-free survival and overall survival of the 32 patients with metastatic STS who received anlotinib plus PD-1 blockades administration. |
Furthermore, OS was also analyzed in this study, a total of 21 death events were observed at the date of data cut-off, yielding the OS data maturity of 65.6%. Similarly, the OS survival curve was illustrated in Figure 4, the median OS of the 32 patients with metastatic STS who received anlotinib plus PD-1 inhibitors was 14.9 months (95% CI: 8.36–21.44). Furthermore, the 24-month and 30-month OS rate was 31.7% (95% CI: 14.7–50.2%) and 25.3% (95% CI: 9.6–44.7%), respectively.
Exploratory Analysis Regarding the Clinical Outcomes According to Subtypes of STS
Given that STS was consisted of various subtypes, as shown in Table 1, SS, ASPS, LMS, LPS and other subtypes were included in this study, the potential association between clinical outcomes (ORR and PFS) and subtypes of STS was investigated subsequently. Therefore, the ORR according to subtypes of STS is illustrated in Figure 5 separately. Interestingly, it should be noted that patients with ASPS conferred a trend for higher ORR (ORR = 57.1%, 95% CI: 18.4–90.1%) than that of other subtypes, even the difference was not statistically significant (P=0.237).
Additionally, the correlation between PFS and subtypes of STS and baseline characteristics were also identified, which is exhibited in Table 2. Noteworthily, regarding the analysis between PFS and baseline characteristics, ECOG performance status score and initial dosage of anlotinib were dramatically associated with PFS among the 32 patients with metastatic STS, highlighting that patients with ECOG performance status of 0–1 score conferred a longer PFS than that of patients with 2 score (median PFS: 9.2 vs 5.1 months, P=0.031), patients with 12mg of anlotinib were associated with a better PFS than that of patients of 10mg (median PFS: 7.6 vs 5.1 months, P=0.028). Additionally, in terms of the association between PFS and subtypes of STS, patients with ASPS conferred a longer PFS than that of the rest subtypes of STS (median PFS: 13.6 months vs 6.3 months, P=0.019). Consequently, ECOG performance status score, initial dosage of anlotinib and ASPS were included in the Cox multivariate analysis for adjustment of confounding factors, which is also shown in Table 2. Still and all, all the three variables were also statistically significant after multivariate analysis, suggesting that ASPS subtype of STS was an independent factor to predict PFS of anlotinib plus PD-1 blockades among patients with previously treated metastatic STS.
 |
Table 2 Association Analysis Between PFS and Subtypes of STS and Baseline Characteristic Subgroups in Univariate Analysis and Multivariate Analysis |
Safety Profile of the Combination Regimen
Furthermore, the maximum grade adverse reactions among the 32 patients with metastatic STS occurred during the administration of anlotinib plus PD-1 blockades were recorded and presented in detail. Additionally, adverse reactions related to the therapy during anlotinib plus PD-1 blockades were found in 29 patients (90.6%), of whom, a total of 13 patients (40.6%) were observed of grade 3–4 adverse reactions and no grade 5 adverse reaction or no new adverse signal was detected during the treatment.
Specifically, as exhibited in Table 3, the common adverse reactions detected in this study were fatigue (62.5%), hypertension (53.1%), hand-foot syndrome (43.8%), diarrhea (34.4%), dermal toxicity (31.3%), nausea and vomiting (25.0%), proteinuria (18.8%), abnormal liver function (15.6%), pneumonia (12.5%) and hemorrhage (9.4%). Additionally, the serious adverse reactions (grade 3–4) manifested as hypertension (15.6%), fatigue (9.4%), hand-foot syndrome (6.2%), abnormal liver function (6.2%), diarrhea (3.1%) and dermal toxicity (3.1%). In a word, the safety profile of the 32 patients with metastatic STS who received anlotinib combined with PD-1 inhibitors was tolerable and acceptable.
 |
Table 3 Safety Profile of the 32 Patients with Metastatic STS Who Received Anlotinib Plus PD-1 Blockades |
Discussion
Our retrospective study provided real-world evidence regarding the feasibility and tolerability of the combination of anlotinib plus PD-1 inhibitors for patients with previously treated metastatic STS retrospectively.
It seemed that there was no widely accepted standard regimen in second-line treatment for previously treated STS clinically for decades, which suggested that the different subtypes of STS might confer different sensitivity.20 Consequently, when the patients progressed after the first-line therapy, the regimens for metastatic STS were determined by histology.21 As a result, we observed that gemcitabine demonstrated potential activity for LMS and angiosarcoma and dacarbazine exhibited therapeutic significance for solitary fibrous tumors and trabectedin showed promising efficacy for liposarcomas.22 Noteworthily, although the development of targeted drugs was scanty own to the heterogeneity of STS and the absence of driver mutation, some previous work had explored that the angiogenetic pathway might be one of the promising targets clinically.23
Patients included in the present study were the metastatic STS who had received at least first-line systemic chemotherapy treatment, similar as the baseline characteristics of ALTER0203 clinical trial, which suggested that the combination administration of anlotinib plus PD-1 blockades was reasonable and ethical, given that anlotinib had the indication for patients with previously treated metastatic STS in China. To our knowledge, the ORR and DCR of anlotinib monotherapy for patients with previously treated metastatic STS was 13% and 74%, respectively.14 The ORR and DCR of pembrolizumab single agent for patients with previously treated STS were 18% and 56%, respectively.16 In our study, ORR and DCR of the 32 patients with metastatic STS who received anlotinib combined with PD-1 blockades improved to 37.5% and 84.4%, respectively, which yielded an acceptable efficacy numerically. It seemed that combination of anlotinib plus PD-1 blockades played a synergistic action for patients with metastatic STS clinically. Additionally, it should be noted that the PFS in the present study was 7.6 months, which was dramatically better than that of anlotinib monotherapy for patients with previously treated STS in a phase II clinical trial (median PFS = 5.6 months).14 Furthermore, we also observed another real-world data regarding anlotinib in the treatment of patients with unresectable locally advanced or metastatic STS, yielding a median PFS of 6.1 months.13 Collectively, anlotinib plus PD-1 blockades might produce superior PFS benefit for patients with metastatic STS in clinical practice. Interestingly, one exploratory study initiated by Yuan et al investigated the efficacy and safety of anlotinib plus PD-1 inhibitors in patients with advanced refractory solid tumors.24 A total of 26 patients with metastatic lung cancer, gallbladder and STS who received anlotinib combined with sintilimab, toripalimab, camrelizumab, nivolumab and pembrolizumab were included for analysis. Among the 26 patients, only 4 patients were STS, the ORR, DCR and median PFS of the 4 patients with STS who received anlotinib plus PD-1 inhibitors were 25%, 100% and 5.64 months, respectively, which was consistent with the clinical outcomes in our study even the sample size in this study for STS was really small. Additionally, another previous study initiated by Wang et al launched a retrospective study to investigate the efficacy of apatinib (another antiangiogenic TKI, similar to anlotinib in China) and camrelizumab (PD-1 blockade) in patients with advanced clear cell sarcoma.25 A total of 12 patients with advanced clear cell sarcoma who received apatinib or camrelizumab were screened retrospectively. Unfortunately, the efficacy of camrelizumab monotherapy for clear cell sarcoma was limited, which might be different from that in our study. We speculated that the discrepancy might be the heterogeneity of subtypes of STS, indicating that more prospective trials were needed to be launched to confirm the consistency of the efficacy of anlotinib plus PD-1 inhibitors among different subtypes of STS in real world.
Interestingly, given that the follow-up duration was long enough (range: 0.7–34.8 months), a relatively high OS data maturity (65.6%) was obtained and relatively long OS data was observed with the median OS of the combination regimen of 14.9 months. It seemed that the OS data was longer than that of the previous study numerically. As we know, the previous studies indicated that anlotinib monotherapy or PD-1 blockade single agent might yield a median OS of around 12 months for patients with previously treated metastatic STS.8,14,16 We speculated that the superior OS in our study of patients with metastatic STS who received anlotinib plus PD-1 blockades might be attributed to fact that considerable antiangiogenic TKIs and immunotherapy drugs (PD-1 or PD-L1 inhibitors) were licensed in China since 2018, which might contribute to the survival benefit for the patients with previously treated metastatic STS in subsequent therapy consecutively.
Amazingly, although the sample size was small in this study, we still performed the subgroup analysis for ORR and PFS in efficacy analysis. In terms of ORR, patients with ASPS conferred a trend of superior ORR (57.1% vs 34.4%) when compared with that of other subtypes, even the difference was not statistically significant. Furthermore, patients with ASPS still conferred a significantly longer PFS than that of the other subtypes of STS (median PFS: 13.6 vs 7.6 months, P=0.019). It seemed that the benefit of anlotinib plus PD-1 blockade regimens for patients with ASPS was more profound than the other subtypes. However, we thought this conclusion should be interpreted with caution. On one hand, the sample was small and only 7 patients with ASPS were included for subtype analysis. On the other hand, the previous study had indicated that patients with ASPS trended to have a better prognosis when they were treated with anlotinib monotherapy.14 Collectively, whether patients with ASPS might benefit from anlotinib plus PD-1 inhibitor treatment should be confirmed in prospective clinical trials subsequently.
Furthermore, the safety profile of the 32 patients with metastatic STS who received anlotinib plus PD-1 blockades exhibited that 29 patients (90.6%) underwent any grade adverse reactions and 13 patients (40.6%) experienced grade 3–4 adverse reactions, which suggested that administration of anlotinib plus PD-1 regimen was acceptable and was consistent with the safety profile of previous study regarding anlotinib plus PD-1 blockade treatment for patients with refractory small cell lung cancer.26 Interestingly, it should be noted that the common adverse reactions such as hypertension, hand-foot syndrome, hemorrhage and proteinuria might be attributed to the administration of anlotinib, which was in concert with the specific adverse actions of anlotinib single agent for patients with STS.27 Similarly, the other adverse reactions such as dermal toxicity, abnormal liver function and pneumonia might be resulted from the administration of PD-1 inhibitors, which was consistent with the adverse reactions of PD-1 blockades single agent for patients with metastatic STS in the previous study.28 Noteworthily, fatigue was proved to be the most common adverse reactions in our study, which might be attributed to the antitumor therapy clinically and was in line with the previous study.29 Collectively, the overall adverse reactions of anlotinib plus PD-1 blockades for patients with previously treated metastatic STS were acceptable and manageable.
The major limitation of our study was that the sample size was relatively small as in a real-world study, only 32 patients were included. Feasibility and tolerability of anlotinib combined with PD-1 blockades were still needed to be confirmed in more patients. Furthermore, multiPD-1 blockades were administered in our study, which might yield heterogeneous and various efficacy to some extent. Besides, we failed to perform the PD-L1 expression test to identify the association between PD-L1 expression and the clinical outcomes of anlotinib plus PD-1 inhibitors. Still and all, our study was of potential significance to provide the real-world evidence for anlotinib combined with PD-1 blockades among patients with previously treated metastatic STS.
Conclusion
Anlotinib plus PD-1 inhibitors demonstrated durable and promising efficacy and tolerable safety for patients with metastatic STS in real world. Further prospective clinical trials were warranted to validate the feasibility and safety of anlotinib plus PD-1 blockades subsequently.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Endo M, Setsu N, Fujiwara T, et al. Diagnosis and management of subcutaneous soft tissue sarcoma. Curr Treat Options Oncol. 2019;20(7):54. doi:10.1007/s11864-019-0656-z
2. Mirili C, Paydas S, Guney IB, et al. Assessment of potential predictive value of peripheral blood inflammatory indexes in 26 cases with soft tissue sarcoma treated by pazopanib: a retrospective study. Cancer Manag Res. 2019;11:3445–3453. doi:10.2147/cmar.s191199
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Zheng B, Qu Y, Wang J, Shi Y, Yan W. Pathogenic and targetable genetic alterations in resected recurrent undifferentiated pleomorphic sarcomas identified by targeted next-generation sequencing. Cancer Genomics Proteomics. 2019;16(3):221–228. doi:10.21873/cgp.20127
5. Kim J, Kim JH, Kang HG, et al. Integrated molecular characterization of adult soft tissue sarcoma for therapeutic targets. BMC Med Genet. 2018;19(Suppl 1):216. doi:10.1186/s12881-018-0722-6
6. Yu X, Zhao Z, Shen H, et al. Clinical and genetic features of patients with juvenile amyotrophic lateral sclerosis with Fused in Sarcoma (FUS) mutation. Med Sci Monit. 2018;24:8750–8757. doi:10.12659/msm.913724
7. George S. Developments in systemic therapy for soft tissue and bone sarcomas. J Natl Compr Canc Netw. 2019;17(5.5):625–628. doi:10.6004/jnccn.2019.5020
8. D’Angelo SP, Mahoney MR, Van Tine BA, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, Phase 2 trials. Lancet Oncol. 2018;19(3):416–426. doi:10.1016/s1470-2045(18)30006-8
9. Seddon B, Strauss SJ, Whelan J, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled Phase 3 trial. Lancet Oncol. 2017;18(10):1397–1410. doi:10.1016/s1470-2045(17)30622-8
10. Schöffski P, Cornillie J, Wozniak A, Li H, Hompes D. Soft tissue sarcoma: an update on systemic treatment options for patients with advanced disease. Oncol Res Treat. 2014;37(6):355–362. doi:10.1159/000362631
11. van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi:10.1016/s0140-6736(12)60651-5
12. Cheng Y, Wang Q, Li K, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled Phase 2 study. Br J Cancer. 2021;125(3):366–371. doi:10.1038/s41416-021-01356-3
13. Zhang RS, Liu J, Deng YT, Wu X, Jiang Y. The real-world clinical outcomes and treatment patterns of patients with unresectable locally advanced or metastatic soft tissue sarcoma treated with anlotinib in the post-ALTER0203 trial era. Cancer Med. 2022;11:2271–2283. doi:10.1002/cam4.4613
14. Chi Y, Fang Z, Hong X, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res. 2018;24(21):5233–5238. doi:10.1158/1078-0432.ccr-17-3766
15. Cable J, Greenbaum B, Pe’er D, et al. Frontiers in cancer immunotherapy-a symposium report. Ann N Y Acad Sci. 2021;1489(1):30–47. doi:10.1111/nyas.14526
16. Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493–1501. doi:10.1016/s1470-2045(17)30624-1
17. Song Y, Fu Y, Xie Q, et al. Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol. 2020;11:1956. doi:10.3389/fimmu.2020.01956
18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
19. Li XY, Rao Y, Sun B, Mao XM. Efficacy and safety of anlotinib combined with PD-1 blockades for patients with previously treated epithelial ovarian cancer: a retrospective study. Int J Gen Med. 2022;15:3977–3989. doi:10.2147/ijgm.s352536
20. Liu W, Jiang Q, Zhou Y. Advances of systemic treatment for adult soft-tissue sarcoma. Chin Clin Oncol. 2018;7(4):42. doi:10.21037/cco.2018.08.02
21. Eriksson M. Histology-driven chemotherapy of soft-tissue sarcoma. Ann Oncol. 2010;21 Suppl 7:vii270–276. doi:10.1093/annonc/mdq285
22. Skafida E, Kokkali S, Nikolaou M, Digklia A. Metastatic soft tissue sarcoma: current treatment landscape and future perspectives. Expert Rev Anticancer Ther. 2017;17(6):537–543. doi:10.1080/14737140.2017.1321989
23. Kyriazoglou A, Gkaralea LE, Kotsantis I, et al. Tyrosine kinase inhibitors in sarcoma treatment. Oncol Lett. 2022;23(6):183. doi:10.3892/ol.2022.13303
24. Yuan M, Zhu Z, Mao W, et al. Anlotinib combined with Anti-PD-1 antibodies therapy in patients with advanced refractory solid tumors: a single-center, observational, prospective study. Front Oncol. 2021;11:683502. doi:10.3389/fonc.2021.683502
25. Wang J, Gao S, Yang Y, et al. Clinical experience with apatinib and camrelizumab in advance clear cell sarcoma: a retrospective study. Cancer Manag Res. 2021;13:8999–9005. doi:10.2147/cmar.s337253
26. Hao YY, Qiao YP, Cheng JD. Clinical activity and safety of anlotinib combined with PD-1 blockades for patients with previously treated small cell lung cancer. Int J Gen Med. 2021;14:10483–10493. doi:10.2147/ijgm.s337316
27. Tian Z, Liu H, Zhang F, et al. Retrospective review of the activity and safety of apatinib and anlotinib in patients with advanced osteosarcoma and soft tissue sarcoma. Invest New Drugs. 2020;38(5):1559–1569. doi:10.1007/s10637-020-00912-7
28. Scheinberg T, Lomax A, Tattersall M, et al. PD-1 blockade using pembrolizumab in adolescent and young adult patients with advanced bone and soft tissue sarcoma. Cancer Rep. 2021;4(2):e1327. doi:10.1002/cnr2.1327
29. Mo J, Darke AK, Guthrie KA, et al. Association of fatigue and outcomes in advanced cancer: an analysis of four SWOG treatment trials. JCO Oncol Pract. 2021;17(8):e1246–e1257. doi:10.1200/op.20.01096
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.