Back to Journals » OncoTargets and Therapy » Volume 13
Effectiveness and Safety of Apatinib in Patients with Advanced or Metastatic Adenocarcinoma of Stomach or Gastroesophageal Junction: A Prospective Observation Study
Authors Shen B, Jiang H, Wang L, Qian J, Shu Y, Chen P , Mao G, Liu B, Zhang X, Liu C, Wu J, Li X, Cai W, Shen W, Wang Q, He J, Hua D, Zhang Z, Zhang Y, Feng J
Received 24 September 2019
Accepted for publication 10 April 2020
Published 20 May 2020 Volume 2020:13 Pages 4457—4464
DOI https://doi.org/10.2147/OTT.S232287
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Bo Shen,1,* Hua Jiang,2,* Lin Wang,3,* Jun Qian,4,* Yongqian Shu,5 Ping Chen,6 Guoxin Mao,7 Baorui Liu,8 Xizhi Zhang,9 Chaoying Liu,10 Jun Wu,11 Xiaoqin Li,12 Wei Cai,13 Wenxiang Shen,14 Qiong Wang,15 Jingdong He,16 Dong Hua,17 Ziwen Zhang,18 Youcheng Zhang,19 Jifeng Feng1
1Department of Oncology, Jiangsu Cancer Hospital and Jiangsu Institute of Cancer Research and The Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, People’s Republic of China; 2Department of Oncology, Changzhou No. 2 People’s Hospital, Changzhou, People’s Republic of China; 3Department of Oncology, Bayi Hospital of People’s Liberation Army, Nanjing, People’s Republic of China; 4Department of Oncology, Suzhou Municipal Hospital, Suzhou, People’s Republic of China; 5Department of Oncology, Jiangsu Province Hospital, Nanjing, People’s Republic of China; 6Department of Oncology, Yancheng City No. 1 People’s Hospital, Yancheng, People’s Republic of China; 7Department of Oncology, Affiliated Hospital of Nantong University, Nantong, People’s Republic of China; 8Department of Oncology, Nanjing Drum Tower Hospital, Nanjing, People’s Republic of China; 9Department of Oncology, Northern Jiangsu People’s Hospital, Yangzhou, People’s Republic of China; 10Department of Oncology, Wuxi People’s Hospital, Wuxi, People’s Republic of China; 11Department of Oncology, The First People’s Hospital of Changzhou, Changzhou, People’s Republic of China; 12Department of Oncology, Affiliated Hospital of Jiangsu University, Zhenjiang, People’s Republic of China; 13Department of Oncology, The First People’s Hospital of Wujiang, Wujiang, People’s Republic of China; 14Department of Oncology, The First People’s Hospital of Kunshan, Kunshan, People’s Republic of China; 15Department of Oncology, Jiangyin People’s Hospital, Jiangyin, People’s Republic of China; 16Department of Oncology, Huai’an First People’s Hospital, Huai’an, People’s Republic of China; 17Department of Oncology, Affiliated Hospital of Jiangnan University and Fourth People’s Hospital of Wuxi, Wuxi, People’s Republic of China; 18Department of Oncology, Changshu No. 2 People’s Hospital, Changshu, People’s Republic of China; 19Department of Oncology, Jiangning People’s Hospital, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jifeng Feng Nanjing 210000, People’s Republic of China
Tel +8625-83283598
Fax +8625-83283597
Email [email protected]
Background: Apatinib showed promising efficacy in the treatment of advanced or metastatic gastric cancer (mGC) in previous clinical studies. However, the real-world data are limited, and this study aimed to assess the effectiveness and safety of apatinib for the treatment of advanced or mGC in this setting.
Methods: In this prospective observational study, progression-free survival (PFS), overall survival (OS), overall response rate (ORR), disease control rate (DCR) and treatment-related adverse events (AEs) were recorded and evaluated. Univariate and multivariate analyses were conducted to explore potential biomarkers which might be related to the effectiveness.
Results: A total of 321 mGC patients from 47 centers in China were enrolled between July 1, 2015, and March 1, 2018. Thirty-two patients achieved partial response, 155 patients achieved stable disease, and 115 patients had progressive disease, and no CR was achieved, illustrating an ORR of 10.60% and a DCR of 61.92%. The median PFS and OS were 4.0 and 8.2 months, respectively. Multivariate Cox analysis showed that the potential biomarkers associated with longer PFS were combination regimens plus taxel/docetaxel, and apatinib initial dosage ≥ 500mg, occurrence of AEs of leukopenia, and hand-foot syndrome. Main AEs were proteinuria (17.1%), hypertension (15.9%), and handfoot syndrome (8.7%).
Conclusion: The present prospective observational study showed favorable effectiveness and safety of apatinib in real-world patients with advanced or metastatic GC in China. (A prospective, multi-center, non-intervention study of apatinib in the treatment of advanced gastric cancer-Trial Registry Number: ChiCTR-OPN-15006601).
Keywords: apatinib, gastric cancer, non-intervention, VEGFR2 inhibitor
Introduction
According to global cancer statistics 2018,1 gastric cancer (GC) is ranked as the fifth leading cancer (6% of all cancers) and the third most common cause of cancer-related deaths (8% of all cancer deaths) worldwide.1 The highest incidence of GC is in Asia. There are about 679,000 new cases and 498,000 GC-related deaths in China annually, which costitutes almost 42% of the GC cases worldwide, which is a heavy burden to public health.2 The incidence and mortality of GC have substantially declined over the past half-century; nevertheless, prognosis for patients with metastatic disease remains poor, with a 5-year relative survival of around 5%.1 Until now, surgery remains the only curative approach for localized GC. Palliative chemotherapy is commonly used to prolong survival, ameliorate symptoms, and improve the quality of life of patients present with advanced disease at diagnosis, metastatic, or relapse after a prior curative surgery.3
More evidences have suggested that angiogenesis is essential for malignant tumor growth, and metastasis, VEGF and its receptors (VEGFRs) play a key role in tumor angiogenesis.4–6 Apatinib, a novel antiangiogenic agent that strongly inhibits VEGFR2, has demonstrated promising efficacy with acceptable toxicities across a broad range of malignancies6–9 and was licensed by National Medical Products Administration of China as the third-line treatment of advanced gastric cancer in China.6,10 Previous Phase II and III clinical trials have evaluated its efficacy and safety in patients with chemotherapy-refractory advanced or metastatic gastric carcinoma when compared with placebo.10,11 Results showed that apatinib significantly improved progression-free survival (PFS) and overall survival (OS) with an acceptable safety profile in heavily pretreated patients with advanced or metastatic gastric cancer refractory to two or more lines of prior chemotherapy.10,11 However, trial populations may not well present real-world patient populations and may have better outcomes than patients who not meet the inclusion criteria of clinical trials. Optimization of disease management should be based on clinical trial and real-world experience. The effectiveness and safety of apatinib in a real-world setting are still unclear. Herein, an observational study was implemented to investigate the effectiveness of apatinib in the treatment of patients with advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction in a real-world clinical setting.
Methods
Patient Enrollment
Patients who received apatinib during the period from July 1, 2015, to March 1, 2018, were included in this study from 47 centers in China. The inclusion criteria were as follows: (1) age ≥ 18 years; (2) patients who had cytologically confirmed advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction with measurable lesions (spiral CT scan ≥ 10 mm); (3) patients with therapeutic indications, and no contraindication to chemotherapy. Exclusion criteria were as follows: (1) patients with pregnancy or lactation; (2) patients with contraindications for apatinib; (3) patietns were unsuitable to this study according to the investigators' judgements. All patients provided written informed consent before apatinib therapy. This study was approved by the ethical committee of the Jiangsu Cancer Hospital.
Treatment
Apatinib was initiated from an oral dosage of 500 mg once daily, 28 days for a cycle, which could be adjusted according to patients’ actual performance status ranging from 850 mg to 250 mg once daily. Treatment interruptions, dose reductions, and supportive care were allowed for the management of adverse events (AEs). Apatinib combined with chemotherapy or monotherapy was decided by the physicians based on patients’ situations. The follow-up time was 1 year.
At the end of the second and third treatment cycles, efficacy and safety were evaluated. Later, the efficacy and safety profile was evaluated every two treatment cycles. Patients achieved CR, PR, and SD and continued to receive apatinib until disease progression. Patients who experienced PD or untolerable toxicities stopped apatinib treatment. For patients who experienced PD, survival data were followed-up until the completion of the study. For patients who stopped treatment for other reasons, tumor response and safety profile were evaluated every 8 weeks until disease progression and completion of the study.
Efficacy and Safety Assessment
Patients who had finished at least one cycle of apatinib therapy and whose efficacy was evaluated were included for effectiveness assessment. All patients who received at least one apatinib treatment were included in the safety population for analysis. The efficacy of apatinib was evaluated, including PFS, OS, objective response rate (ORR) and disease control rate (DCR). Tumor responses were categorized as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria. All AEs were reviewed and determined according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTC AEs, version 4.0) and classified as degree 0–4.
Statistical Analysis
Quantitative variables are presented as median (range) or number of patients (percentage). Survival analysis was conducted by the Kaplan–Meier method and was compared by the log-rank test. Exploratory univariate analyses were performed with the log-rank test using the following variables: age, gender, ECOG score, line of apatinib, combinational treatment, initial dosage, dose adjustment and number of metastatic sites. Responses and AEs were both aggregated in the form of frequency counts and percentages. All the statistical analyses were performed using SAS 9.2 software (SAS Institute, Cary, NC). P < 0.05 was regarded as statistically significant.
Statement of Ethics
The lead unit of the study was Jiangsu Cancer Hospital, and the ethical committee of the Jiangsu Cancer Hospital approved the study. Other hospitals chose to follow the ethical results approved by the Jiangsu Cancer Hospital Ethics Committee. The ethical approval number is 2015NL-31. All experimental procedures were performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Results
Patients and Characteristics
A total of 321 patients were enrolled from July 1, 2015, to March 1, 2018. Most patients were male (n=208, 64.80%), with an average age of 63 (IQR 56–70) years and had an ECOG PS of 0 (n=88, 27.41%) or 1 (n=213, 66.36%). The baseline demographics and clinical pathological characteristics of patients at the initiation of apatinib are shown in Table 1. Until the time of data cutoff (March 1, 2018), the median follow-up time was 10.57 (IQR 7.29–12.64) months. Twenty patients had disease progression, 225 patients died, 45 patients were lost to follow-up, and 22 patients completed the study.
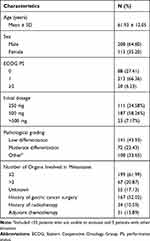 |
Table 1 Patients’ Baseline Characteristics |
Dose Adjustment
Apatinib was initiated from the dosage of 250 mg in 111 (34.58%) patients, 500 mg in 187 (58.26%) patients, and >500 mg in the remaining 23 (7.17%) patients. Initial dosage of apatinib was increased from 250 to 500 mg, 500 to 750 mg, 250 to 425 mg and 500 to 750 mg in 30, 8, 1 and 1 patients because of well tolerance and decreased from 500 to 250 mg, 750 to 500 mg, 850 to 500 mg, 425 to 250 mg, 675 to 500 mg, 750 to 250 mg, 850 to 425 mg and 850 to 750 mg in 47, 9, 2, 1, 1, 1, 1 and 1 patients because of adverse events.
Efficacy
A total of 302 patients were included in the final analysis. At the time of data cutoff, 32 patients (10.60%) achieved PR, 155 patients (51.32%) had SD, and 115 patients (38.08%) had PD; CR was not achieved in all patients. This resulted in an ORR of 10.60% and a DCR of 61.92% (Table 2). For survival outcome, the median PFS was 4.0 months (95% CI: 3.5–4.4) and the median OS was 8.2 months (95% CI: 7.8–8.7). The Kaplan–Meier analysis of PFS is shown in Figure 1.
 |
Table 2 The Efficacy of Apatinib in Patients with Advanced Gastric Carcinoma |
 |
Figure 1 Kaplan–Meier curves for progression-free survival (A) and overall survival (B) in patients. |
As shown in Table 3, univariate analysis indicated that there was a significant association of mPFS with the line of therapy (P=0.001), apatinib initial dosage (P=0.001), number of metastatic sites (P=0.009) and PS score (P=0.001). In detail, the mPFS of patients treated as first line, second line, third line, and above the third line was 5.9 months, 4.1 months, 3.4 months, and 3.4 months. The mPFS of patients with ≤2 metastatic sites was longer than those with >2 metastatic sites (5.0 vs 4.0 months, P = 0.0087). Patients with PS of 0 or 1 got longer mPFS (5.7 vs 4.3 vs 3.0 months, P < 0.001). Initial dosage ≥500 mg got longer mPFS (3.4 vs.4.5 vs.5.0 months, P <0.001).
 |
Table 3 Prognostic Factors Associated with Apatinib Treatment |
Univariate analysis of mOS showed similar results, and line of therapy, apatinib initial dosage, number of metastatic sites and PS score were also significantly associated with OS. In detail, the mOS of patients treated as first line, second line, third line, and above the third line was 12.9 months, 8.5 months, 7.6 months, and 5.9 months. The mOS of patients with ≤2 metastatic sites was longer than those with >2 metastatic sites (9.1 vs 6.6 months, P=0.0006). Patients with PS of 0 or 1 got longer mOS (8.7 vs 8.2 vs 6.6 months, P=0.0316). Initial dosage ≥500 mg got longer mOS (7.7 vs.9.5 vs.11.8 months, P=0.0059).
In the multivariate Cox analysis which included 6 variables (Figure 2), combination regimens of apatinib plus taxel/docetaxel, and apatinib initial dosage ≥500 mg, occurrence of proteinuria or leukopenia remained significantly associated with longer mPFS. The factors of occurrence of hand-foot syndrome and apatinib treatment as first-line treatment showed an obvious trend in association with longer mPFS.
 |
Figure 2 Multivariate Cox regression analyses for PFS. |
We also analyzed whether hypertension, hand-foot syndrome, proteinuria, and leukopenia were associated with PFS and OS; the results showed a significant association (all P <0.05), especially, these four AEs occurred in the first 4 weeks vs more than 4 weeks was strongly correlated with better clinical outcomes (all P <0.01).
Safety
At the end of follow-up, among 321 patients enrolled, a total of 313 patients were included in the safety analysis set (Table 4), of which 239 patients (76.36%) reported AEs. Generally, toxicities were well tolerated, 4.3% of the patients reported grade 3 AEs, and no patient reported grade 4 AEs. The main AEs were leukopenia (18.69%), proteinuria (17.13%), and hypertension (15.89%). The most common grade 3 AEs were proteinuria (n=8, 2.49%), hypertension (n=7, 2.18%), and liver damage (n=2, 0.62%). In most cases, hypertension was mild and controllable by oral antihypertension agents. Proteinuria, hypertension, hand-foot syndrome, and leukopenia were the four most common AEs and occurred mostly within 4 weeks (cycle 1) after initiation of apatinib therapy.
 |
Table 4 Analysis of Adverse Events |
Discussion
Apatinib, a novel small-molecule tyrosine kinase inhibitor targeting VEGFR2, has shown promoting effect in patients with chemotherapy-refractory mGC in a phase II and III trial.10,11 However, the real‑world data on this drug are limited. In this observational study in a real-world setting, when patients were treated with apatinib, an ORR of 10.60% and a DCR of 61.92% were achieved, median PFS was 4.0 months (95% CI: 3.5–4.4 months), and median OS was 8.2 months (95% CI: 7.8–8.7 months).
In a phase II trial, apatinib therapy for the chemotherapy-refractory advanced metastatic gastric cancer with two treatment regimens (850 mg once daily and 425 mg twice daily) led to a median PFS of 3.67 months (95% CI, 2.17–6.80 months) and 3.20 months (95% CI, 2.37–4.53 months) and OS of 4.83 months (95% CI, 4.03–5.97 months) and 4.27 months (95% CI, 3.83–4.77 months).10 And in a Phase III trial, apatinib also significantly prolonged median PFS (2.6 months; 95% CI, 2.0–2.9 months) and OS (6.5 months; 95% CI, 4.8–7.6 months) in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction.11 Although in another global multicenter phase III study (ANGEL study) sponsored by LSK, a US company with overseas ownership of apatinib, the primary study endpoint OS was not reached, the secondary study endpoint mPFS achieved was 2.8 months (95% CI, 2.8–1.8 months). In ANGEL study, all patients were from Europe, America, Korea, and Japan; there were Chinese patients.12 The baseline characteristics of the patients enrolled in these two studies were different, which could also affect the OS. Therefore, the results of the ANGEL study could not dismiss the efficacy of apatinib in Chinese patients. And in a real-world study performed by Zhang et al., apatinib as the third or more line treatment for gastric cancer achieved an mPFS of 2.65 months (95% CI 1.66–3.54 months), an mOS of 5.8 months (95% CI 4.77–6.83 months), an ORR of 5.56%, and a DCR of 58.33%.16 Compared with these studies,10,11,16 in our study, patients seemed to benefit more with 10.60% ORR, 4.0 months mPFS, and 8.2 months mOS. In this study, 52.6% of patients received apatinib as first- and second-line therapy. While in the apatinib phase II and phase III studies, all the patients received apatinib as third therapy. It might mean that patients who received apatinib as early as first- or second-line therapy could benefit more for response and survival. The subgroup analysis also supports this hypothesis; the mPFS and mOS were 5.9 and 12.9 months in first-line treatment, 4.1 and 8.5 months in second-line treatment, while in third and above treatment, the mPFS and mOS were 3.4 and 5.9 months.
The univariate analysis also showed that the survival benefit was associated with combination regimen, apatinib initial dosage, numbers of metastatic sites, ECOG scores, and specific AEs related to apatinib. In this study, 37.75% of patients received combined therapy. Among these patients, 10.3% of patients received apatinib plus taxol/docetaxel regimen with mPFS of 5.5 months and mOS of 11.7 months and 25.2% received apatinib plus other chemotherapy with mPFS of 4.8 months and mOS of 8.8 months. The prolonged mPFS and mOS in apatinib combined with taxol/docetaxel regimen might explain the fact that apatinib significantly increased the intracellular accumulation of substrate drugs by reversing the multidrug resistance.17 The dosage of apatinib used in this real-world setting was more flexible, 34.6% of patients initiated apatinib with 250 mg, 58.2% of patients initiated with 500 mg, and 7.2% patients initiated with >500 mg. During the treatment period, many patients experienced dose increase and dose decrease according to physician’s discretion. The subgroup analysis showed that initial dosage >500 mg got longer mPFS compared with 500 mg and 250 mg (5.0 vs.4.5 vs.3.4 months) and mOS (11.8 vs.9.5 vs.7.7 months). Patients with ECOG score of 0–1 and less metastasis also achieved longer mPFS and mOS. The specific AEs of hypertension, hand-foot syndrome, proteinuria, and leukopenia, which especially occurred in the first 4 weeks vs more than 4 weeks, were associated with better clinical outcomes, which was consistent with previous studies.13–15
The multivariate Cox analysis showed that combination regimens of apatinib plus taxel/docetaxel, and apatinib initial dosage ≥ 500 mg, occurrence of leukopenia or proteinuria remained significantly associated with longer mPFS, which mean that patients who received combination therapy of apatinib plus taxel/docetaxel, received apatinib initial dosage ≥ 500 mg, and occurred leukopenia or proteinuria especially in the first 4 weeks might benefit longer mPFS.
The most common AEs were leukopenia, proteinuria, hypertension, and hand-foot syndrome which occurred in 18.69%, 17.13%, 15.89%, and 8.72% of patients, respectively, and the most common AEs over grade 3 were proteinuria (2.49%), hypertension (2.18%), and liver damage (0.62%). The safety profile of apatinib was almost consistent in our study with previous clinical trials, except that the incidence of specific AEs such as hand-foot syndrome was a little lower.10,11 Moreover, dose upregulation and combination with taxol or docetaxel-based chemotherapy did not increase the incidence and severity of AEs, which meant a well-tolerance of patients. The present study observed the effectiveness and safety of apatinib in mGC patients in a real-world setting. The major limitation of this study is its relative small sample size and potential bias of the observational design.
In conclusion, the present prospective observational study in 47 centers in China demonstrated that apatinib has a favorable effectiveness and safety profile in patients with advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. Patients with combination regimens of apatinib plus taxel/docetaxel, and apatinib initial dosage ≥ 500 mg, occurrence of leukopenia or proteinuria especially in the first 4 weeks might benefit more. Further well-designed studies with larger numbers will be needed and the specific application strategies need further exploration.
Acknowledgments
The authors thank all the patients who participated in the clinical study. The study was funded by the cancer research foundation of the Chinese Society of Clinical Oncology (Y-HR2015-137 and Y-HR2016-025).
Author Contributions
All authors contributed to data analysis and drafting or revising the article, approved the final version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. doi:10.3322/caac.21492
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol. 2015;33(16):1760–1769. doi:10.1200/JCO.2014.60.1799
4. Fontanella C, Ongaro E, Bolzonello S, Guardascione M, Fasola G, Aprile G. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med. 2014;2(12):123. doi:10.3978/j.issn.2305-5839.2014.08.14
5. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi:10.1038/nm0603-669
6. Roviello G, Ravelli A, Polom K, et al. Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett. 2016;372(2):187–191. doi:10.1016/j.canlet.2016.01.014
7. Wu F, Zhang S, Xiong A, et al. A phase II clinical trial of apatinib in pretreated advanced non-squamous non-small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e831–e842. doi:10.1016/j.cllc.2018.06.002
8. Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135(8):1961–1969. doi:10.1002/ijc.28829
9. Zhen L, Jiali C, Yong F, Han X, Hongming P, Weidong H. The efficacy and safety of apatinib treatment for patients with unresectable or relapsed liver cancer: a retrospective study. J Cancer. 2018;9(16):2773–2777. doi:10.7150/jca.26376
10. Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31(26):3219–3225. doi:10.1200/JCO.2013.48.8585
11. Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–1454. doi:10.1200/JCO.2015.63.5995
12. Kang Y-K, Kang WK, Di Bartolomeo M, et al. LBA43 randomized phase III ANGEL study of rivoceranib (apatinib) + best supportive care (BSC) vs placebo + BSC in patients with advanced/metastatic gastric cancer who failed ≥2 prior chemotherapy regimens. Ann Oncol. 2019;30(Supplement_5):v877–v878. doi:10.1093/annonc/mdz394.034
13. Poprach A, Pavlik T, Melichar B, et al.; Czech Renal Cancer Cooperative G. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann Oncol. 2012;23(12):3137–3143. doi:10.1093/annonc/mds145
14. Osterlund P, Soveri LM, Isoniemi H, Poussa T, Alanko T, Bono P. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br J Cancer. 2011;104(4):599–604. doi:10.1038/bjc.2011.2
15. George S, Reichardt P, Lechner T, Li S, Cohen DP, Demetri GD. Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with sunitinib. Ann Oncol. 2012;23(12):3180–3187. doi:10.1093/annonc/mds179
16. Zhang Y, Han C, Li J, et al. Efficacy and safety for Apatinib treatment in advanced gastric cancer: a real world study. Sci Rep. 2017;7(1):13208. doi:10.1038/s41598-017-13192-8
17. Tong XZ, Wang F, Liang S, et al. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem Pharmacol. 2012;83(5):586–597. doi:10.1016/j.bcp.2011.12.007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
