Back to Journals » OncoTargets and Therapy » Volume 13
Effective Treatment with PD-1 Antibody, Chidamide, Etoposide, and Thalidomide (PCET) for Relapsed/Refractory Natural Killer/T-Cell Lymphoma: A Report of Three Cases
Authors Du L , Zhang L, Li L, Li X, Yan J, Wang X, Fu X, Sun Z, Zhang X, Li Z , Wu J, Yu H , Chang Y, Zhou Z, Nan F, Wu X, Tian L, Zhang M
Received 14 May 2020
Accepted for publication 6 July 2020
Published 27 July 2020 Volume 2020:13 Pages 7189—7197
DOI https://doi.org/10.2147/OTT.S262039
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Lijun Du, Lei Zhang, Ling Li, Xin Li, Jiaqin Yan, Xinhua Wang, Xiaorui Fu, Zhenchang Sun, Xudong Zhang, Zhaoming Li, Jingjing Wu, Hui Yu, Yu Chang, Zhiyuan Zhou, Feifei Nan, Xiaolong Wu, Li Tian, Mingzhi Zhang
Department of Oncology, Zhengzhou University First Affiliated Hospital, Lymphoma Diagnosis and Treatment Center of Henan Province, Zhengzhou, Henan, People’s Republic of China
Correspondence: Mingzhi Zhang
Department of Oncology, Zhengzhou University First Affiliated Hospital, Lymphoma Diagnosis and Treatment Center of Henan Province, &Ngr;o. 1 Jianshe East Road, Zhengzhou, Henan, People’s Republic of China
Tel +86 13838565629
Fax +86 37166295563
Email [email protected]
Abstract: Extranodal natural killer (NK)/T-cell lymphoma, nasal type (ENKTL) is a specific subtype of peripheral T cell lymphoma (PTCL) with a poor prognosis. To date, there exist no standard therapeutic regimens for relapsed/refractory (R/R) ENKTL. More potent treatment strategies are urgently needed to improve the survival of these patients with R/R ENKTL. Herein, we present three R/R ENKTL patients who failed prior therapies (L-asparaginase containing chemotherapy, radiotherapy or biological-cell therapy, etc.) benefited from the combination regimen comprised of anti-programmed-death-1 (PD-1) antibody toripalimab, chidamide, etoposide, and thalidomide. They received the treatment regimen continuously until the disease progression occurs. As of data collection, two patients achieved complete remission (CR) after 4, 6 cycles of treatment, respectively, and another patient was evaluated as partial remission (PR) after 2 cycles. Treatment-related adverse events (AEs) mainly presented grade 2∼ 3 leukocytopenia and anemia, which were controllable. It follows that PD-1 antibody, chidamide, etoposide, and thalidomide (PCET) regimen may be a promising choice for patients with R/R ENKTL and warrants further investigation.
Keywords: NK/T-cell lymphoma, relapsed/refractory, PD-1, chidamide, targeted treatment
Introduction
ENKTL is prevalent in regions of Asian and Central American countries, and rare in North American and European countries.1,2 It accounts for approximately 11–15% of all lymphomas in China.3,4 ENKTL is a highly malignant disease that progresses rapidly. Relapsed/refractory cases of ENKTL characterizes the underlying trend of recurrence, and there are short of highly effective treatment modalities. Thus, more potent treatment options are needed to be explored to improve the prognosis and prolong the survival of these patients with R/R ENKTL. Recently, a few studies have reported that administering anti-PD-1 antibody alone to patients with R/R ENKTL showed encouraging effects. Yet few PD-1 antibody combination regimens used to patients with ENKTL have been established.
In this report, we evaluate the efficacy and safety of the PCET regimen in three patients with R/R ENKTL who were resistant to multi-line treatments.
Patients
Treatment and Response Assessments
A total of three patients with R/R ENKTL were treated with the PCET regimen. All patients had received at least two prior chemotherapy regimens including an L-asparaginase (or pegaspargase) containing regimen. PD-1 antibody toripalimab (200mg intravenously guttae, every 3 weeks), chidamide (20 mg taken orally twice a week), etoposide (100mg intravenously guttae, day1–3, every 3 weeks), and thalidomide (150mg taken orally daily) were administered to all patients every 3 weeks until the disease progression occurs. All patients were fully informed about the possible toxicities of the treatment regimen and gave written informed consent.
Image inspection such as an enhanced magnetic resonance imaging (MRI) scan, an enhanced computed tomography (CT) scan, or positron emission tomography-computed tomography (PET-CT) should be performed every two cycles of treatment to assess responses according to the Revised Response Criteria for Malignant Lymphoma. Treatment-related AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Case Presentation
Case 1
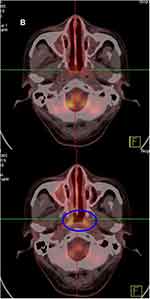
A 54-year-old male had a 1-month history of left nasal congestion when he visited the hospital initially. A nasal endoscopic examination suspected malignant tumor in the left middle nasal passage. He was diagnosed with NK/T-cell lymphoma, nasal type by biopsy pathology. The immunohistochemistry staining indicated CD20 (-), CD3ε (+), CD2 (-), CD5 (-), CD4 (-), CD8 (-), CD56 (+), EBER (+), Granzyme B (+), TIA-1 (+), and Ki-67 (70–80%). Then he experienced involved-field (56Gy/28F) and prevention area radiotherapy (50.4Gy/28F) in November 2018. PET-CT was performed one month after radiotherapy, revealing left nasal mucosa thickened that was considered the residual lymphoma, and bone marrow examination showed that NK tumor cells accounted for approximately 7.12% of all mature nucleated cells, which meant modified Ann Arbor stage Ⅳ. Subsequently, chemotherapy with P-GemOx (gemcitabine, oxaliplatin, and pegaspargase) regimen was performed for 3 cycles. A PET-CT scan after he transferred to our hospital in May 2019 showed that the nasopharynx and oropharynx mucosa was slightly thickened and hypermetabolic. No tumor cells were observed in bone marrow biopsy this time. Further chemotherapy with DDGP (gemcitabine, cisplatin, dexamethasone, and pegaspargase) regimen was given 3 cycles to him. After cessation of chemotherapy, he developed grade Ⅳ myelosuppression, which improved after administered granulocyte-macrophage colony-stimulating factor (GM-CSF). In July 2019, the findings of PET-CT scan revealed that the metabolic activity of the nasal cavity and nasopharynx was higher than that before together with the right ethmoid sinus and maxillary sinus mucosa thickening, which was considered progression of the disease. Given chemoresistance, The PCET regimen was administered to him after informing him of his condition. A nasopharyngeal MRI after 2 cycles showed PR, while PET-CT showed that the CR was achieved (Figure 1) after another 2 cycles. By January 2020, he has received 7 cycles of PCET regimen and remained in the CR state.
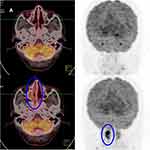 |
Figure 1 Continued. |
Case 2
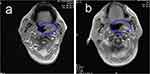
A 51-year-old man visited our hospital due to the progressive aggravation of redness and swelling in the lacrimal sac area of the right eye in January 2018. The biopsy of a mass in the right nasal cavity and sinuses was performed in February 2018. Histopathological analysis of the biopsy sample showed CD20 (-), CD3ε (+), CD21 (-), CD38 (+), CD56 (+), TIA-1 (+), Granzyme B (+), CD30 (+), Ki-67 (70% +), and EBER (+). The bone marrow examination showed no neoplastic cells. He was confirmed as extranodal NK/T-cell lymphoma, nasal type, modified Ann Arbor stage Ⅱ. From March 2018, he underwent local intensity-modulated radiation therapy (IMRT) for 25 times followed by 3 continuous cycles of VIPD (etoposide, Ifosfamide, cisplatin, dexamethasone) chemotherapy. Complete remission occurred after the last cycle of treatment. In June 2019, a routine MRI scan indicated the abnormal signals on the left nasopharyngeal sidewall and the left behind the tongue (Figure 2A), which were new lesions. The PET-CT scan also showed the involved field was larger than that before. A biopsy was performed again, and the Immunohistochemical staining demonstrated CD3ε (+), CD20 (-), CD21 (-), CD56 (+), TIA-1 (+), Granzyme B (+), CD30 (+), Ki-67 (50%+), and EBER (+), which indicated it remained ENKTL and suggested progression of the disease. Then PCET was utilized on him. His symptoms were gone after the first cycle carried out, and the MRI scan after 2 cycles showed that the abnormal signal on the left nasopharyngeal wall and the left rear of the tongue (Figure 2B) was significantly reduced compared with that in June 2019. His serum ferritin levels fell from 3385.6 U/L to 661.8 U/L (30–400 U/L). As of January 2020, 4 cycles of PCET regimen have been administered to him, and the second MRI scan showed stable disease.
Case 3
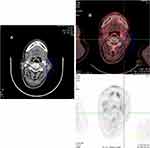
Patient 3 is a 53-year-old male came to our hospital due to a mass on his left neck in March 2012. Biopsy of the enlarged lymph node in the left neck was performed, and the pathological findings suggested NK/T-cell lymphoma. The tumor cells were CD3ε (+), CD20 (-), CD79a (-), CD10 (-), EMA (-), ALK (-), CD43 (+), Ki-67 (80%+), Vimentin (+), CD30 (+), CD56 (+), TIA-1 (+), Granzyme B (+), and EBER (+). No neoplastic cells were present in the bone marrow examination. He was diagnosed with ENKTL, modified Ann Arbor stage III based on the radiological findings and laboratory tests. During the next more than 1 year, he underwent multi-line chemotherapies including 2 cycles of gemcitabine, cisplatin, pegaspargase, dexamethasone, and thalidomide, 2 cycles of DDGP, 2 cycles of methotrexate, mitoxantrone, and dexamethasone, 2 cycles of SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) and 2 cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), cervical radioactive therapy, and twice half-matched NK cells infusions, none of which made him complete remission. A few months later, he had a symptom of left nasal congestion. An enhanced CT scan revealed that oropharyngeal, laryngopharyngeal mucosa thickened which were new lesions. Subsequently, he received second-line chemotherapy, 4 cycles of DICE (dexamethasone, ifosfamide, cisplatin, and etoposide), and nasal radiotherapy, and remained stable disease until he came to our hospital again in July 2019 because of a fever over 39 °C. The biopsy of the original cervical lymph node was performed again. Pathological findings confirmed still ENKTL. The neoplastic cells were CD2 (+), CD7 (+), CD56 (+), CD3ε (+), CD43 (+), Granzyme B (+), TIA-1 (+), CD20 (-), CD79a (-), AE1/AE3 (-), Ki-67 (40%+), and EBER (+). An enhanced CT scan showed that lymphadenopathy of the left neck remained (Figure 3A). Ultimately, he received the PCET regimen from August 2019. After one cycle of treatment, he had no longer a fever, and the symptom of nasal congestion was relieved. And he was evaluated PR by a routine enhanced CT scan after 2 cycles of treatment. His lactate dehydrogenase (LDH) levels rose from 269 U/L to 313 U/L after 1 cycle and went back to normal 2 cycles later. There was no significant change in the disease in the next several cycles, while complete remission occurred after the sixth cycle completed. The PET-CT scan displayed no hypermetabolic foci in the primary lymphadenopathy of the left neck (Figure 3B). Currently, he has received 8 cycles of PCET regimen.
Results
The EBV DNA and β2-microglobulin (β2-MG) levels in these three cases were always within the normal range during the treatment period with PCET regimen. The LDH levels of case 1 and case 2 were always normal.
The overall response rate (ORR) was 100%, with a complete response (CR) occurring in two (66.7%) patients and a PR observed in one (33.3%) patients. They all underwent grade 2~3 anemia and leukopenia.
Discussion
Currently, radiotherapy combined with or without chemotherapy is recommended for ENKTL patients who are diagnosed with stage Ⅰ-Ⅱ,5,6 and patients in stage IIIIV are treated with systemic chemotherapy.7 The L-asparaginase or pegaspargase containing regimen is a preferred chemotherapy option. However, the optimal therapies for relapsed/refractory patients remain undefined. For multi-therapy failure and CD30 or CD38 positive expression cases, CD30 antibody brentuximab vedotin alone8 or plus bendamustine9 or CD38 antibody daratumumab10 may be a promising salvage strategy. There however were a few reported cases, and these agents or regimens needed more data to verify the effect in ENKTL. In a retrospective analysis of 82 adults (≥18 years) ENKTL patients undergoing allogeneic hematopoietic cell transplantation (allo-HCT) between 2000 and 2014 from the observational database of the Center for International Blood and Marrow Transplant Research (CIBMTR), the 3-year progression-free (PFS) and overall survival (OS) were 28% and 34%, respectively.11 Efforts should continue to improve the current treatment regimens and discover more effective agents for relapsed/refractory ENKTL patients.
In recent years, immune checkpoint inhibitors, especially PD-1 inhibitors, have shined in anti-tumor treatment. In terms of lymphoma, the PD-1 antibody has shown significant efficacy on R/R classic Hodgkin’s lymphoma.12 Kwong et al13 reported promising results of PD-1 antibody pembrolizumab in R/R ENKTL patients failing L-asparaginase, which showed 100% (7/7) of overall response rate (ORR) and 71.4% (5/7) of CR rate. Our center previously treated several patients with R/R ENKTL with pembrolizumab alone, in which the ORR was 57.1% (4/7) including two CR and two PR.14 A case report presented a 37-year-old female with R/R ENKTL, who achieved complete clinical remission after 11 cycles of treatment with PD-1 antibody pembrolizumab.15 Another case report reported that low-dose nivolumab exerted to make one of three patients failing L-asparaginase achieve CR and long-term survival.16 Our center detected higher PD-L1 expression on NK/T and NK cell lymphoma cell lines than normal NK cells and rhinitis tissue.17 PD-L1 expression was found to be related to the Epstein-Barr virus (EBV). PD-L1 was upregulated through the EBV-encoded latent membrane protein (LMP)-1 pathway.18,19 Conjugation of PD-1 and its ligand (PD-L1) leads to the TCR (T-cell receptor)-mediated proliferation of T cells be inhibited, namely “T-cell exhaustion” that results in immune escape of tumor cells.20 The reduction can be reversed by the PD-1 blockade.
Chidamide, a novel benzamide class of histone deacetylase (HDAC) inhibitor selectively blocking HDAC1, 2, 3 and 10,21 has been approved for the treatment of R/R PTCL by the China Food and Drug Administration (CFDA).22 A few in vitro experiments demonstrated chidamide induced tumor cells growth arrest and apoptosis through various pathways in NK/T-cell lymphoma cell lines and human leukemia cells.23,24 There are two ex vivo assays provided evidence that chidamide in the appropriate dose enhanced CD8+ CTL and NK cell-mediated cytotoxicity.21,25 Furthermore, chidamide could enhance the anti-tumor activity of PD-1 (+) cells including T cells and B cells.26 A study indicated that there was a significantly high-intensity expression of HDAC1, and HDAC2, in patients with ENKTL. Also, the expression degree of HDAC2 was closely correlated with the proliferation index (Ki-67) in patients with ENKTL.27 A multicenter real-world study compared chidamide monotherapy and chidamide combined with chemotherapies in various subtypes of PTCL, in which the CR rate of chidamide monotherapy and combined with chemotherapy were 6.06% (2/33) and 10% (4/40), respectively.28 Taking chidamide alone for a period was found to be prone to resistance, and terminating chidamide suddenly may lead to recurrence. Overall, it is difficult to eliminate the tumor of patients with chidamide monotherapy.
Etoposide in front-line combination chemotherapy of various histological subtypes of malignant lymphomas was observed the promising response rate decades ago.29 Many etoposide containing regimens have demonstrated significant effect and been recommended to be used in ENKTL patients, such as DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin), VIPD (etoposide, ifosfamide, cisplatin, and dexamethasone), ESHAP (etoposide, steroid, high-dose cytarabine, and cisplatin), VIDL (etoposide, ifosfamide, dexamethasone, and L-asparaginase) and SMILE regimen, etc.7 Patients with ENKTL are sometimes complicated by hemophagocytic syndrome (aka hemophagocytic lymphohistiocytosis, HLH) that is a deadly serious condition with a worse prognosis.30,31 Etoposide could selectively eliminate pathologically activated T cells of HLH patients.32
The extensive vascularity in lymph node samples or bone marrow of patients with hematological malignancies has been found.33,34 Thalidomide showed the ability of angiogenesis inhibition in animal corneal models,35,36 and showed a specific effect in multiple myeloma (MM) treatment by inducing MM cell lines apoptosis or G1 growth arrest.37 Besides, thalidomide also has immunoregulatory properties. It enhances T cell proliferation through co-stimulation effect and increases CD8 + T cell toxicity,38 and enhances T helper cell type 2 (Th2) response via inducing cytokine (IL-4 and IL-5) production and suppressing interferon-γ (IFN-γ) production.39 Thalidomide selectively inhibits tumor necrosis factor-α (TNF-α) production of human monocytes, which is a protective but also detrimental cytokine of the inflammatory response.40 Thalidomide could suppress TNF-dependent NF-κB activation,41 and thus thalidomide inhibits etoposide-induced NF-κB activation and enhances etoposide-induced tumor cells apoptosis and inhibition of tumor cells proliferation when thalidomide combined with etoposide in vitro and in vivo.42 The combination of etoposide and thalidomide slowed tumor growth and increased mouse survival in vivo.42
Tumor immunotherapy, for example, PD-1 inhibitors, has two special features. First, tumor immunotherapy acts on the immune system and encourages building a cellular immune response rather than acting directly on the tumor, so that it often takes some time to show a clinical response, ie a delayed treatment effect. Second, PD-1 inhibitors and other immunotherapy methods make patients with malignant tumor durable responses. A study reported that most of 129 non-small cell lung cancer (NSCLC) patients treated with PD-1 antibody nivolumab showed clinical responses in 8–16 weeks.43 As of the time of data analysis, responses were ongoing in 9 patients in this study.43 However, ENKTL is a highly aggressive disease, and R/R ENKTL develops even more rapidly. While etoposide, a cytotoxic agent, can prevent the disease from advancing rapidly, in addition to further anti-tumor activity combined with chidamide. Thalidomide regulates immune function and reduces tumor-mediated inflammation together with blocking vasotrophic approaches of the tumor. Additionally, the bone marrow hematopoiesis of R/R ENKTL patients who previously underwent multiple chemotherapy and radiotherapy has been suppressed, and they can no longer withstand high-intensity chemotherapy. And the PCET regimen produces less bone marrow suppression than chemotherapy. Taken together, these four agents take advantages from each other, and characterize multiple anti-tumor effects including immunotherapy, chemotherapy, and tumor microenvironment regulation.
Diarrhea, fatigue, pruritus, and rash were frequent in PD-1 inhibitors treatment, and more serious AEs were immune-mediated effects such as hypothyroidism, hyperthyroidism, and pneumonitis.44 The combination of PD-1 inhibitors and chemotherapy could lead to AST or ALT elevation.44 Fortunately, none of the above mentioned AEs were identified in them, and they underwent just anemia grade 2~3 and leukopenia grade 2~3 during the PCET treatment. The result of these three patients indicated the PCET regimen produced good efficacy and was safe.
Conclusion
This report showed that the PCET regimen was effective and safe for R/R ENKTL patients who were resistant to multi-line treatments, and it needs more evidence to evaluate and confirm the activity and safety in patients with R/R ENKTL.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of Zhengzhou University First Affiliated Hospital. All patients provided written informed consent and allowed us to analyze and report their data in public.
Acknowledgments
We would like to thank the Lymphoma Diagnosis and Treatment Cancer Center of Henan Province for providing technical assistance. We also thank all the patients and their families for allowing us to analyze and report their data in public.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Haverkos BM, Pan Z, Gru AA, et al. Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT): an update on epidemiology, clinical presentation, and natural history in North American and European cases. Curr Hematol Malig Rep. 2016;11(6):514–527. doi:10.1007/s11899-016-0355-9
2. Shi Y. Current status and progress of lymphoma management in China. Int J Hematol. 2018;107(4):405–412. doi:10.1007/s12185-018-2404-8
3. Yang QP, Zhang WY, Yu JB, et al. Subtype distribution of lymphomas in Southwest China: analysis of 6382 cases using WHO classification in a single institution. Diagn Pathol. 2011;6:77. doi:10.1186/1746-1596-6-77
4. Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138(3):429–434. doi:10.1309/AJCP7YLTQPUSDQ5C
5. Huang MJ, Jiang Y, Liu WP, et al. Early or up-front radiotherapy improved survival of localized extranodal NK/T-cell lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat Oncol Biol Phys. 2008;70(1):166–174. doi:10.1016/j.ijrobp.2007.05.073
6. Wang ZY, Li YX, Wang WH, et al. Primary radiotherapy showed favorable outcome in treating extranodal nasal-type NK/T-cell lymphoma in children and adolescents. Blood. 2009;114(23):4771–4776. doi:10.1182/blood-2009-07-235853
7. Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood. 2018;131(23):2528–2540. doi:10.1182/blood-2017-12-791418
8. Kim HK, Moon SM, Moon JH, Park JE, Byeon S, Kim WS. Complete remission in CD30-positive refractory extranodal NK/T-cell lymphoma with brentuximab vedotin. Blood Res. 2015;50(4):254–256. doi:10.5045/br.2015.50.4.254
9. Poon LM, Kwong YL. Complete remission of refractory disseminated NK/T cell lymphoma with brentuximab vedotin and bendamustine. Ann Hematol. 2016;95(5):847–849. doi:10.1007/s00277-016-2627-9
10. Hari P, Raj RV, Olteanu H. Targeting CD38 in refractory extranodal natural killer Cell-T-Cell Lymphoma. N Engl J Med. 2016;375(15):1501–1502. doi:10.1056/NEJMc1605684
11. Kanate AS, DiGilio A, Ahn KW, et al. Allogeneic haematopoietic cell transplantation for extranodal natural killer/T-cell lymphoma, nasal type: a CIBMTR analysis. Br J Haematol. 2018;182(6):916–920. doi:10.1111/bjh.14879
12. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. doi:10.1056/NEJMoa1411087
13. Kwong YL, Chan T, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437–2442. doi:10.1182/blood-2016-12-756841
14. Li X, Cheng Y, Zhang M, et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11(1):15. doi:10.1186/s13045-018-0559-7
15. Lai J, Xu P, Jiang X, Zhou S, Liu A. Successful treatment with anti-programmed-death-1 antibody in a relapsed natural killer/T-cell lymphoma patient with multi-line resistance: a case report. BMC Cancer. 2017;17(1):507. doi:10.1186/s12885-017-3501-4
16. Chan T, Li J, Loong F, Khong PL, Tse E, Kwong YL. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Ann Hematol. 2018;97(1):193–196. doi:10.1007/s00277-017-3127-2
17. Han L, Liu F, Li R, et al. Role of programmed death ligands in effective T-cell interactions in extranodal natural killer/T-cell lymphoma. Oncol Lett. 2014;8(4):1461–1469. doi:10.3892/ol.2014.2356
18. Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–3473. doi:10.1158/1078-0432.CCR-13-0855
19. Bi XW, Wang H, Zhang WW, et al. PD-L1 is upregulated by EBV-driven LMP1 through NF-kappaB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol. 2016;9(1):109. doi:10.1186/s13045-016-0341-7
20. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi:10.1084/jem.192.7.1027
21. Ning ZQ, Li ZB, Newman MJ, et al. Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol. 2012;69(4):901–909. doi:10.1007/s00280-011-1766-x
22. Shi Y, Dong M, Hong X, et al. Results from a multicenter, open-label, pivotal Phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26(8):1766–1771. doi:10.1093/annonc/mdv237
23. Zhou J, Zhang C, Sui X, et al. Histone deacetylase inhibitor chidamide induces growth inhibition and apoptosis in NK/T lymphoma cells through ATM-Chk2-p53-p21 signalling pathway. Invest New Drugs. 2018;36(4):571–580. doi:10.1007/s10637-017-0552-y
24. Gong K, Xie J, Yi H, Li W. CS055 (Chidamide/HBI-8000), a novel histone deacetylase inhibitor, induces G1 arrest, ROS-dependent apoptosis and differentiation in human leukaemia cells. Biochem J. 2012;443(3):735–746. doi:10.1042/BJ20111685
25. Yao Y, Zhou J, Wang L, et al. Increased PRAME-specific CTL killing of acute myeloid leukemia cells by either a novel histone deacetylase inhibitor chidamide alone or combined treatment with decitabine. PLoS One. 2013;8(8):e70522. doi:10.1371/journal.pone.0070522
26. Zhang W, Shen H, Zhang Y, et al. Circulating PD-1 (+) cells may participate in immune evasion in peripheral T-cell lymphoma and chidamide enhance antitumor activity of PD-1 (+) cells. Cancer Med. 2019;8(5):2104–2113. doi:10.1002/cam4.2097
27. Min SK, Koh YH, Park Y, et al. Expression of HAT1 and HDAC1, 2, 3 in diffuse large B-cell lymphomas, peripheral T-cell lymphomas, and NK/T-cell lymphomas. Korean J Pathol. 2012;46(2):142–150. doi:10.4132/KoreanJPathol.2012.46.2.142
28. Shi Y, Jia B, Xu W, et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol. 2017;10(1):69. doi:10.1186/s13045-017-0439-6
29. Sinkule JA. Etoposide: a semisynthetic epipodophyllotoxin. Chemistry, pharmacology, pharmacokinetics, adverse effects and use as an antineoplastic agent. Pharmacotherapy. 1984;4(2):61–73. doi:10.1002/j.1875-9114.1984.tb03318.x
30. Liu YZ, Bi LQ, Chang GL, Guo Y, Sun S. Clinical characteristics of extranodal NK/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis. Cancer Manag Res. 2019;11:997–1002. doi:10.2147/CMAR.S183784
31. Jin Z, Wang Y, Wang J, et al. Multivariate analysis of prognosis for patients with natural killer/T cell lymphoma-associated hemophagocytic lymphohistiocytosis. Hematology. 2018;23(4):228–234. doi:10.1080/10245332.2017.1385191
32. Johnson TS, Terrell CE, Millen SH, Katz JD, Hildeman DA, Jordan MB. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol. 2014;192(1):84–91. doi:10.4049/jimmunol.1302282
33. Vacca A, Di Loreto M, Ribatti D, et al. Bone marrow of patients with active multiple myeloma: angiogenesis and plasma cell adhesion molecules LFA-1, VLA-4, LAM-1, and CD44. Am J Hematol. 1995;50(1):9–14. doi:10.1002/ajh.2830500103
34. Ribatti D, Vacca A, Nico B, Fanelli M, Roncali L, Dammacco F. Angiogenesis spectrum in the stroma of B-cell non-Hodgkin’s lymphomas. An immunohistochemical and ultrastructural study. Eur J Haematol. 1996;56(1–2):45–53. doi:10.1111/j.1600-0609.1996.tb00293.x
35. D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91(9):4082–4085. doi:10.1073/pnas.91.9.4082
36. Kenyon BM, Browne F, D’Amato RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res. 1997;64(6):971–978. doi:10.1006/exer.1997.0292
37. Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98(1):210–216. doi:10.1182/blood.V98.1.210
38. Haslett PA, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med. 1998;187(11):1885–1892. doi:10.1084/jem.187.11.1885
39. McHugh SM, Rifkin IR, Deighton J, et al. The immunosuppressive drug thalidomide induces T helper cell type 2 (Th2) and concomitantly inhibits Th1 cytokine production in mitogen- and antigen-stimulated human peripheral blood mononuclear cell cultures. Clin Exp Immunol. 1995;99(2):160–167. doi:10.1111/j.1365-2249.1995.tb05527.x
40. Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med. 1991;173(3):699–703. doi:10.1084/jem.173.3.699
41. Majumdar S, Lamothe B, Aggarwal BB. Thalidomide suppresses NF-kappa B activation induced by TNF and H2O2, but not that activated by ceramide, lipopolysaccharides, or phorbol ester. J Immunol. 2002;168(6):2644–2651. doi:10.4049/jimmunol.168.6.2644
42. Hiramatsu T, Oho AUID, Yoshizawa J, et al. Thalidomide potentiates etoposide-induced apoptosis in murine neuroblastoma through suppression of NF-kappaB activation. Pediatr Surg Int. 2018;34(4):443–450. doi:10.1007/s00383-018-4234-4
43. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (Anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012. doi:10.1200/JCO.2014.58.3708
44. Arnaud-Coffin P, Maillet D, Gan HK, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer. 2019;145(3):639–648. doi:10.1002/ijc.32132
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.