Back to Journals » International Journal of General Medicine » Volume 15
Effective Doses of Nalbuphine Combined with Propofol in Painless Hysteroscopy
Authors Zhong W , Chen C, Tang W
Received 26 March 2022
Accepted for publication 31 May 2022
Published 11 June 2022 Volume 2022:15 Pages 5609—5614
DOI https://doi.org/10.2147/IJGM.S367449
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Weiwei Zhong,* Chen Chen,* Weixiang Tang
Department of Anesthesiology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weixiang Tang, Department of Anesthesiology, The First Affiliated Hospital of Anhui Medical University, No. 218 Jixi Road, Hefei, 230022, People’s Republic of China, Tel +86-551-62922384, Email [email protected]
Purpose: Nalbuphine is becoming a common analgesic used in hysteroscopic operations. The aim of this study was to identify the median effective dose (ED50) and 95% effective dose (ED95) of nalbuphine combined with propofol in painless hysteroscopy.
Patients and Methods: Twenty-five patients aged 18– 60 years with an American Society of Anesthesiologists classification of I–II who were scheduled for painless hysteroscopy were recruited. The initial dose of nalbuphine was set at 0.15 mg/kg and varied by 0.01 mg/kg according to the Dixon sequential method. The ED50/ED95 of nalbuphine combined with propofol for hysteroscopy was calculated by the probit method.
Results: The ED50 of nalbuphine was 0.122 (95% confidence interval (CI) 0.092– 0.137) mg/kg, and the ED95 of nalbuphine was 0.153 (95% CI 0.138– 0.361) mg/kg.
Conclusion: The ED50/ED95 values of nalbuphine combined with propofol in painless hysteroscopy are 0.122 mg/kg and 0.153 mg/kg, respectively. Nalbuphine at 0.153 mg/kg combined with propofol is effective and safe for painless hysteroscopy.
Keywords: nalbuphine, propofol, hysteroscopy, effective dose, ED50, ED95
Introduction
Hysteroscopy is considered the “gold standard” for the diagnosis and treatment of intrauterine pathologies. Because of its minimally invasive technique and visualization of the entire uterine cavity, hysteroscopy is widely used in gynecology.1 However, most patients cannot tolerate the intense pain and discomfort from hysteroscopy without sedation and analgesia.2 Therefore, hysteroscopy under intravenous anesthesia is becoming increasingly popular. The most commonly used sedation anesthetic for endoscopy is propofol. However, propofol has weak analgesic effect as well as cardiovascular adverse effects, thus, its combination with opioids can reduce the dose of propofol and alleviate the cardiovascular response.3
Nalbuphine is an agonist‑antagonist opioid that acts on both the κ-receptor (agonist) and μ-receptor (antagonist). Due to its pharmacological properties, nalbuphine has a unique analgesic effect on visceral pain. Meanwhile, nalbuphine causes a lower incidence of side effects (eg, respiratory depression, nausea and vomiting, pruritus, or sedation) compared to opioid μ receptor agonists.4,5 Therefore, nalbuphine is becoming popular in the analgesia of hysteroscopic operations.
However, the minimum effective dose of nalbuphine when administered in combination with propofol has not yet been defined. The aim of this study was to identify the median effective dose (ED50) and 95% effective dose (ED95) of nalbuphine when combined with propofol for painless hysteroscopy.
Methods
This study was approved by the Ethical Committee of The First Affiliated Hospital of Anhui Medical University, Hefei, China (Approval No. PJ2021-01-20) and registered in the Chinese Clinical Trial Registry (www.chictr.org.cn; registration number ChiCTR2100042494). Informed consent forms were signed by all participants.
Participants
From January to March 2021, a total of 25 patients, American Society of Anesthesiologists I–II (ASA), aged between 18 and 60, who planned to undergo painless hysteroscopy and therapeutic surgery at our institution were selected. Patients were excluded if they had a history of allergy to propofol or opioids, hepatic or renal insufficiency, cardiovascular or neurological disease, escalation to tracheal intubation and general anesthesia, a procedure lasting >30 min, or an inability to communicate.
Clinical Protocol
All patients fasted from solids for 8 h and from liquids for 2 h. Once the patient entered the operating room, peripheral capillary oxygen saturation (SpO2), noninvasive blood pressure and electrocardiography were monitored regularly. All patients inhaled oxygen with a mask (5 L/min). The Dixon sequential method was used in this study. Nalbuphine (Ren Fu Rui Jing Pharmaceutical, Henan, China; lot no. 01J09021) was injected intravenously before the operation, and the initial dose was 0.15 mg/kg. At 3 min after the onset, propofol was injected slowly for at least 1 min. Hysteroscopy was performed when the patient lost consciousness. The initial dose of intravenous propofol was 2 mg/kg, and 0.5 mg/kg of propofol was added if hysteroscopy failed (defined as poor cervical dilation and hysteroscopic placement, or Ramsay Sedation Scale (RSS) score < 5, any physical movement, and frowning by the patient within 5 min). In this case, the dosage of nalbuphine for the next patient was increased by one dose gradient. Conversely, the dose gradient for the next patient was decreased by one dose gradient if the examination was completed successfully. The difference between the two adjacent doses was 0.01 mg/kg. The experiment was completed when seven crossovers were achieved. The RSS score was measured on a 6-point scale-level, with one representing the least amount of sedation and six representing the most.
Adverse hemodynamic events were defined as hypotension (blood pressure < 20% of baseline), and sinus bradycardia (heart rate (HR) < 50 beats/min). Respiratory depression was considered to be significant when SpO2 was below 90% or the respiratory rate was <6 breaths/minute. Hypotension and bradycardia were treated with ephedrine (5–10 mg) and atropine (0.3–0.5 mg), respectively. Assisted ventilation with oxygen via a facial mask was applied when respiratory depression occurred.
Observation indices included the following: the dose of nalbuphine, the initial dose of propofol, the total dose of propofol, the examination time (the time of hysteroscopy), and the anesthesia recovery time (the time from the last administration of propofol to the patient’s recovery). Pain intensity at the point of recovery was evaluated with the visual analog scale (VAS) pain score (0 = none, 10 = most severe). In addition, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), HR and SpO2 were recorded before induction (T1), 1 min after induction (T2) and at the time of recovery (T3). Common side effects, including drowsiness, nausea and vomiting, were also recorded.
Statistical Analysis
All statistical analyses were performed by SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Data are reported as the means ± standard deviation. Normally distributed continuous variables at different time points were compared with paired t tests. A p value <0.05 was considered to be statistically significant. The ED50/ED95 and 95% confidence interval (CI) of nalbuphine when combined with propofol were calculated by the probit method (probability unit regression).
Results
Overall, 25 patients were enrolled and completed the experiment. The flow diagram of the study is shown in Figure 1. The demographic characteristics of the patients are presented in Table 1. The perioperative profiles of the patients are shown in Table 2. The postoperative VAS score was 1.56 ±1.12. Among all patients, only two developed transient respiratory depression, and no nausea or vomiting was observed (Table 2). All vital signs were stable during the operation. The MAP and HR at T2 were significantly decreased compared with those at T1 (MAP 107.52 ± 11.17 vs 86.44 ± 8.99; HR 83.96 ± 10.71 vs 77.76 ± 9.33, P<0.05), but the reduction was less than 20% of the baseline value. Moreover, the HR at T3 was statistically lower than the HR at T1 (83.96 ± 10.71 vs 76.32 ± 11.61) (Table 3).
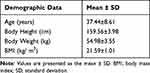 |
Table 1 Demographic Data |
 |
Table 2 Perioperative Profiles of the Patients |
 |
Table 3 Hemodynamic Parameters at Different Time Points |
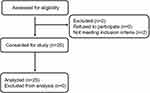 |
Figure 1 Flow diagram of the study. |
The ED50 of nalbuphine determined by the up-and-down sequential allocation method was 0.122 (95% CI 0.092–0.137) mg/kg, and the ED95 of nalbuphine was 0.153 (95% CI 0.138–0.361) mg/kg. The sequential doses of nalbuphine coadministered with propofol for painless hysteroscopy are shown in Figure 2.
Discussion
Hysteroscopy is widely used for the diagnosis and treatment of uterine diseases. Although the duration of the operation is short, most patients cannot tolerate the pain of cervical dilatation and endometrial curettage.1 Propofol alone or combined with opioid μ receptor agonists, such as fentanyl and sufentanil, are current the anesthetic methods used for painless hysteroscopy.6,7 However, propofol has a weak analgesic effect and injection pain,8 while opioid μ receptor agonists are associated with an increased risk of respiratory depression, nausea and vomiting,9 which are unexpected for both patients and anesthesiologists. Therefore, the anesthetic strategies still need to be improved.
Kappa (k)-opioid receptors are a subtype of opioid receptors, that are particularly effective for visceral pain. Compared with μ receptor agonists, k-agonists do not induce addiction, respiratory depression or gastrointestinal transit inhibition.10 The uterus is dominated by sympathetic and parasympathetic nerves from the spinal cord. Nalbuphine is a classic opioid receptor agonist–antagonist with a high concentration in the spinal cord. It has a rapid onset, reduced propofol injection pain, hemodynamic stability and fewer adverse reactions,11–13 which might be suitable for intrauterine operation.
However, there are few official reports regarding nalbuphine use in painless hysteroscopy. Therefore, verifying the ED50/ED95 of nalbuphine combined with propofol can provide a reference for future clinical use. The Dixon sequential method is commonly used to determine the effective dose of drugs and was used in this study. The initial dose of nalbuphine was defined according to the results of Li et al, who achieved an ED50/ED95 of nalbuphine during painless gastroscopy of 0.078 mg/kg and 0.162 mg/kg, respectively.3 Therefore, in this study, the initial dose of nalbuphine was determined as 0.15 mg/kg. During the first examination, the patient had physical movement; thus, the first point of dosage (Figure 2) was counted as one crossover, and the dose of nalbuphine for the next patient was sequentially increased by 0.01 mg/kg. When seven crossovers occurred, the ED50 and ED95 values for nalbuphine coadministered with propofol anesthesia for hysteroscopy were calculated as 0.122 (95% CI 0.092–0.137) mg/kg and 0.153 (95% CI 0.138–0.361) mg/kg, respectively.
In this study, no nausea or vomiting was observed in any of the 25 patients. Only two patients had a short duration of respiratory depression, with SpO2 higher than 90%. The MAP and HR were decreased at T2 but were not lower than 20% of the baseline value. Since nalbuphine has little effect on hemodynamics and no effect on the cardiovascular system,14,15 the mild reduction in MAP and HR was mainly due to the effect of propofol. At the time of recovery (T3), patients were normally quiet and peaceful, with a lower HR compared with the baseline value. The mean VAS score was 1.56 ±1.12, which was considered slight pain. Both patients and gynecologists were satisfied with the operation procedure.
One limitation of our study is that no elderly patients were included, since the patients were recruited from 18 to 60 years old. It is necessary to explore the appropriate dose of nalbuphine for elderly patients when coadministrated with propofol for painless hysteroscopy. Additionally, this study was only conducted at a single center, and a multicenter study on the effect of nalbuphine for painless hysteroscopy should also be implemented in the future.
Conclusion
The ED50/ED95 of nalbuphine combined with propofol for patients in painless hysteroscopy were 0.122 mg/kg and 0.153 mg/kg, respectively. Therefore, to achieve safe and effective painless hysteroscopy in adults, nalbuphine at 0.153 mg/kg combined with propofol is recommended.
Abbreviations
ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; ED50, median effective dose; ED95, 95% effective dose; HR, heart rate; MAP, mean arterial pressure; RSS; Ramsay Sedation Scale; SBP, systolic blood pressure; SD; standard deviation; SpO2, peripheral capillary oxygen saturation; VAS, visual analogue scale.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
Ethical approval (Ethical Committee No. PJ2021-01-20) was provided by the ethics committee of the First Affiliated Hospital of Anhui Medical University in January 2021. All patients provided informed consent and all procedures were conducted according to the Declaration of Helsinki.
Consent for Publication
The Authors agree to publication in the International Journal of General Medicine.
Acknowledgments
We would like to thank all participants who contributed their time to the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82001185).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vitale SG, Bruni S, Chiofalo B, Riemma G, Lasmar RB. Updates in office hysteroscopy: a practical decalogue to perform a correct procedure. Updates Surg. 2020;72(4):967–976. doi:10.1007/s13304-020-00713-w
2. Ahmad G, Saluja S, O’Flynn H, Sorrentino A, Leach D, Watson A. Pain relief for outpatient hysteroscopy. Cochrane Database Syst Rev. 2017;2017(10):10. doi:10.1002/14651858.CD007710.pub3
3. Li S, Wang Y, Chen X, Huang T, Li N. Effective doses of nalbuphine combined with propofol for painless gastroscopy in adults: a randomized controlled trial. Front Pharmacol. 2021;12. doi:10.3389/fphar.2021.673550.
4. Liu X, Hu J, Hu X, et al. Preemptive intravenous nalbuphine for the treatment of post-operative visceral pain: a multicenter, double-blind, placebo-controlled, randomized clinical trial. Pain Ther. 2021;10(2):1155–1169. doi:10.1007/s40122-021-00275-8
5. Deng C, Wang X, Zhu Q, Kang Y, Yang J, Wang H. Comparison of nalbuphine and sufentanil for colonoscopy: a randomized controlled trial. PLoS One. 2017;12(12):12. doi:10.1371/journal.pone.0188901
6. Yan Q, Su Y, Gao L, et al. Impact of CYP3A4*1G polymorphism on fentanyl analgesia assessed by analgesia nociception index in Chinese patients undergoing hysteroscopy. Chin Med J. 2018;131(22):2693–2698. doi:10.4103/0366-6999.243934
7. Yu J, Xiang B, Song Y, Chen H, Li Y, Liu C. ED50 of propofol in combination with low-dose sufentanil for intravenous anaesthesia in hysteroscopy. Basic Clin Pharmacol Toxicol. 2019;125(5):460–465. doi:10.1111/bcpt.13280
8. Desousa KA. Pain on propofol injection: causes and remedies. Indian J Pharmacol. 2016;48(6):617–623. doi:10.4103/0253-7613.194845
9. Machelska H, Celik M. Advances in achieving opioid analgesia without side effects. Front Pharmacol. 2018;9. doi:10.3389/fphar.2018.01388.
10. Rivière PJM. Peripheral kappa-opioid agonists for visceral pain. Br J Pharmacol. 2004;141(8):1331–1334. doi:10.1038/sj.bjp.0705763
11. Zeng Z, Lu J, Shu C, et al. A comparision of nalbuphine with morphine for analgesic effects and safety: meta-analysis of randomized controlled trials. Sci Rep. 2015;5. doi:10.1038/srep10927
12. Chen C, Tang W, Ye W, Zhong W, Li Y. ED50 of propofol combined with nalbuphine on the sedative effect in painless hysteroscopy. Pain Ther. 2021;10(2):1235–1243. doi:10.1007/s40122-021-00280-x
13. Wang J, Duan J, Xie C, Yu Y, Lu Y. Comparison between intravenous nalbuphine and lidocaine in reducing propofol-induced injection pain during gastroscopy: a randomized controlled trial. Pain Ther. 2020;9(2):563–571. doi:10.1007/s40122-020-00188-y
14. Chawda PM, Pareek MK, Mehta KD, Priti D, Chawda M. Effect of nalbuphine on haemodynamic response to orotracheal intubation. J Anaesthesiol Clin Pharmacol. 2010;26(4):458–460. doi:10.4103/0970-9185.74584
15. Zsigmond EK, Winnie AP, Raza MA, et al. Nalbuphine as an analgesic component in balanced anesthesia for cardiac surgery. Anesth Anal. 1987; 66:1155–1164.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

