Back to Journals » Journal of Pain Research » Volume 15
Effect of Transauricular Vagus Nerve Stimulation on Rebound Pain After Ropivacaine Single Injection Femoral Nerve Block for Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Trial
Authors Zhou Q, Yu L, Yin C, Zhang Q, Tai Y , Zhu L, Dong J, Wang Q
Received 13 April 2022
Accepted for publication 5 July 2022
Published 14 July 2022 Volume 2022:15 Pages 1949—1958
DOI https://doi.org/10.2147/JPR.S370589
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Andrea Tinnirello
Qi Zhou,1 Lili Yu,2 Chunping Yin,1 Qi Zhang,3 Yanlei Tai,1 Lian Zhu,4 Jiangtao Dong,5 Qiujun Wang1
1Department of Anesthesiology, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 2Department of Anesthesiology, Cangzhou Central Hospital, Cangzhou, Hebei, People’s Republic of China; 3Department of Anesthesiology, Hebei Children’s Hospital Affiliated to Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 4Department of Orthopaedics, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 5Department of Joint Surgery, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China
Correspondence: Qiujun Wang, Department of Anesthesiology, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China, Email [email protected]
Purpose: The aim of this study was to investigate whether transauricular vagus nerve stimulation (taVNS) could reduce the incidence of rebound pain in patients undergoing anterior cruciate ligament reconstruction (ACLR) under general anesthesia combined with preoperative femoral nerve block.
Methods: In total, 78 patients were enrolled in this prospective, randomized, double-blind, and sham-controlled study. Patients were randomly assigned to 2 groups (n=39): Group taVNS received taVNS (1h /1time, 6times) within the first 12 h after surgery; Group SS received sham stimulation (SS) in the same manner. Pain scores at 0, 4, 8, 12, 24, 48 h after surgery were assessed with Numeric Pain Rating Scale (NRS). The incidence, duration and onset of rebound pain were recorded. In addition, additional analgesic requirements and side effects in the first 48 h postoperatively, as well as sleep disturbance on the night of surgery, were examined.
Results: The incidence and duration of rebound pain were lower in the taVNS group than in the SS group (P=0.025 and P=0.015, respectively). Pain scores at 8 h and 12 h postoperatively were significantly lower in the taVNS group compared with the SS group (P< 0.05). The number of times to press the patient-controlled analgesia (PCA) pump and the number of patients requiring additional analgesic were significantly lower in the taVNS group than in the SS group until 12 h after surgery (P=0.021 and P=0.004, respectively). The number of patients with sleep disturbance in the taVNS group was lower than that in the SS group (P=0.030).
Conclusion: The taVNS exerts beneficial effect on rebound pain after femoral nerve block in patients undergoing ACLR, which reduces the incidence and duration of rebound pain, the need for postoperative additional analgesic, and the number of complications.
Keywords: rebound pain, transauricular vagus nerve stimulation, femoral nerve block, anterior cruciate ligament reconstruction
Introduction
Peripheral nerve blocks (PNBs) are widely used in orthopedic surgery to reduce perioperative pain and opioid consumption.1 Recently, however, an increasing number of anesthesiologists and surgeons are concerned about the rebound pain associated with PNB.2 Rebound pain is defined as “quantifiable difference in pain scores when the block is working versus the increase in acute pain encountered during the first few hours after the effects of PNB resolve”, characterized by persistent burning pain.2–4 The incidence of rebound pain is approximately 50% in patient received PNB.5 Risk factors for rebound pain include younger age, female gender, higher preoperative pain score, and bone surgery.5,6 In addition, rebound pain is associated with adverse consequences, including impaired quality of recovery, increased opioid consumption, side effects of opioid overdose, and sleep disturbance.7
The pathophysiology of rebound pain has not been fully understood and no effective precautions and treatments are available currently.8 Continuous PNB and perineural dexamethasone in single injection PNB have been suggested to prevent rebound pain.3,9,10 However, there are many concerns with these strategies because persistent PNB hinders rehabilitation and the perineural use of dexamethasone is off-label. Therefore, it is necessary to understand the pathogenesis of rebound pain and provide new insights for prevention and treatment. The rebound pain may be caused by multiple pathogenic mechanisms, such as surgical mechanical injury, proinflammation and neurotoxicity of local anesthetics retention and amplification of nociceptive signal memory when PNB wear off, or a combination of these.8,11 Animal studies demonstrated that the proinflammation and neurotoxicity of ropivacaine induced transient thermal hyperalgesia response in rats with sciatic nerve blockade, which might be one of the potential pathophysiologic mechanisms in rebound pain.12,13 This thermal hyperalgesia may be cause by abnormal spontaneous C-fiber hyperactivity and nociceptor hyperexcitability.14 The pain signals from C-fiber and nociceptor affect neuronal cell bodies or postganglionic axon of nociceptive neurons that output to neural circuits in the dorsal horn of the spinal cord, which in turn transmit the inputs to the brain through ascending neuronal pathways.15,16
As a novel neurostimulation modality, vagus nerve stimulation (VNS) has been successfully used in various diseases, including epilepsy and migraine.17,18 Several evidences suggest that VNS may relieve acute or inflammatory pain.19 Animal studies have shown that VNS reduces thermal stimulation-induced excitability of peripheral C fibers, resulting in analgesia in capsaicin-treated rats.20,21 In humans, researchers found that 1h of VNS raised pain thresholds for mechanical and heat pain.22,23 The transauricular VNS (taVNS), which stimulates the auricular branch of the VN in a completely non-invasive manner, can achieve the same effect as VNS.24 Based on the results of previous studies, transauricular vagus nerve stimulation (taVNS) may suppress peripheral mechanical pain from surgical injury and thermal hyperalgesia from local anesthetics. Currently, taVNS has not been studied in the prevention of rebound pain after femoral nerve block. To obtain more information on this topic, we conduct this study to investigate the effect of taVNS on rebound pain in patients undergoing anterior cruciate ligament reconstruction (ACLR) under general anesthesia combined with preoperative femoral nerve block.
Methods
This randomized double-blind clinical study was conducted in the Third Hospital of Hebei Medical University, approved by our Institutional Ethical Board (NO.2021-004-1), and registered in Chinese Clinical Trial Registry (ChiCTR2100044905). This study was conducted in accordance with the Declaration of Helsinki (October 2013) and clinical practice guidelines. Informed consent was obtained from all subjects involved in the study.
Participants
This study consecutively recruited patients who underwent ACLR from January 2022 to March 2022. The main inclusion criteria were age 18–65 years, American Society of Anesthesiologists (ASA) physical status I–III. The exclusion criteria were obesity (BMI>35kg/m2), allergy to local anesthetics, severe cardiopulmonary disease, any chronic pain, systemic steroid and chronic opioid use, psychiatric disorders, communication issues, an inability to use patient-controlled analgesia (PCA) pump, and a failed femoral nerve block (no sensory block or NRS score >3 in postanesthesia care unit [PACU]).
Randomization and Blinding
The recruited patients were divided into two groups by using a random number table: the taVNS group or the sham stimulation (SS) group. In the taVNS group, the custom-developed stimulating electrode and electrode clip were placed on the cymba concha and the earlobe of the left ear. In the SS group, electrode clips were placed on the ear lobe and the tail of the helix of the left ear (Figure 1). The cymba conchae of the ear contains auricular vagal projections, whereas the earlobe and the tail of the helix of the ear do not have vagal innervation. Stimulation pulses (30 Hz frequency, 300 μs pulse width) were generated by a commercial transcutaneous electrical nerve stimulation unit (TENS 7000, Roscoe Medical, Ohio, USA), and the amplitude was increased to the maximum amount that the subject could tolerate without pain. The stimulation phase continued for 1h followed by 1h pause. The stimulation started immediately after surgery and continued until the 12th h after surgery (1h/1time, 6times). The stimulation was administrated by a nurse not involved in the study. The electrodes were placed by the nurse and covered with opaque earmuffs. A blinded observer recorded study data. Patients, anesthesiologists, and investigators were blinded to study group assignment.
 |
Figure 1 Stimulation sites in the taVNS group (A) and the SS group (B). |
Anesthesia and Perioperative Management
All patients received a standardized single injection femoral nerve block (20 mL of 0.5% ropivacaine) under ultrasound guidance by an experienced anesthesiologist prior to induction of anesthesia. Invasive blood pressure, pulse oxygen saturation, electrocardiogram, bispectral index, and end-tidal CO2 (ETCO2) were continuously monitored in the two groups during the surgery. Anaesthesia was induced intravenously with sufentanil (Yichang Renfu Pharmaceutical Co., Ltd., Hubei China), midazolam (Jiangsu Enhua Pharmaceutical Group Co., Ltd., China), propofol (Guangdong Jiabo Pharmaceutical Co., Ltd., Guangdong, China), and rocuronium (Fuan Pharmaceutical Group Qingyutang Pharmaceutical Co., Ltd., Chongqing, China), followed by placement of a laryngeal mask airway. Anesthesia was maintained with intravenous infusion of propofol (50 to 150 μg kg–1·min–1) and remifentanil (0.1 to 0.5 μg kg–1 min–1; Yichang Renfu Pharmaceutical Co., Ltd., Hubei, China). The ACLR was performed by one experienced surgeon. Palonosetron Hydrochloride (Nanjing Simcere TECO Pharmaceutical Co., Ltd., Jiangsu, China) 0.25 mg was administrated at 30 min before the end of surgery to prevent postoperative nausea and vomiting (PONV). All patients were transferred to PACU and escorted back to the ward by anesthesia nurses after recovery.
Intravenous PCA, including 1.5μg/kg sufentanil and 8mg ondansetron hydrochloride (Jiangsu Aosaikang Pharmaceutical Co., Ltd., Jiangsu, China), was started at the end of surgery. The pump was set to 2mL/h basal flow, 0.5mL bolus dose and 15 min lock-out time. Patients were instructed to press PCA pump when experiencing pain. In the event of insufficient analgesia, the patient received intramuscular ketorolac 30 mg (up to 90 mg/day) and tramadol 100 mg (up to 200 mg/day).
Outcome Measures
The primary outcomes were the incidence, duration and onset time of rebound pain. Pain score was assessed using a numerical rating scale score (NRS, 0–10). Rebound pain was defined as a change from mild pain (NRS pain score < 3) before block resolution to severe pain (NRS pain score ≥7) within 24 hours of femoral nerve block performance. The onset of rebound pain was measured from the time of performing femoral nerve block.
Our secondary outcomes included NRS scores at 0, 4, 8, 12, 24, and 48 h after ACLR, opioid-related and taVNS-related side effects (PONV, pruritus, lightheadedness, ear irritation, and tinnitus), the number of times to press the PCA, the number of patients requiring additional analgesic, and sleep disturbance on the night of surgery. Sleep disturbance was diagnosed in patients with decreased total sleep time, increased number of awakenings, and difficulty sleeping compared with usual. The follow-up period was 48h.
Statistical Analysis
In previous studies, the incidence of rebound pain after peripheral nerve block was 49.6%-82.9%.5,10 Thus, we assumed a 66% incidence of rebound pain in this study. We planned to detect 50% reduction in the incidence of rebound pain in the taVNS group compared with the SS group. 35 subjects per group would provide 90% power to detect this difference with a significance of 0.05 (two-tailed) (PASS 15.0, CNSS, UT, USA). Considering a 10% of loss to follow up, the sample size was determined to be 78 in this clinical study.
Categorical variables were analyzed by χ2 test or Fisher’s exact test and presented as numbers and percentages. Shapiro–Wilk test was used to test the normality of the distribution of continuous variable. Continuous variables were tested with Student’s t-test for normal distribution or Mann–Whitney U-test for skewed distribution and reported as mean ± standard deviation or median and interquartile ranges (IQR). NRS scores at different times in the two groups were analyzed by mixed model longitudinal data analysis followed by Bonferroni multiple comparison correction. A P value < 0.05 was considered statistically significant. We used SPSS software version 22.0 for all analyses (IBM, NY, USA). The box-plot of NRS scores was drawn using GraphPad Prism 8.3.0 (GraphPad, CA, USA).
Results
In total, 83 patients were screened from January 2022 to March 2022 in this study. Among them, 2 patients did not meet the inclusion criteria, 3 patients refused to participate this study. Finally, 78 patients were randomly assigned to the taVNS group (n=39) and the SS group (n=39) (Figure 2). Demographic, intraoperative characteristics, and preoperative pain score were similar between the two groups (Table 1).
 |
Table 1 Baseline Characteristics |
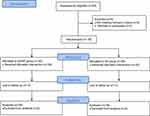 |
Figure 2 Study flowchart. |
As shown in Figure 3, the NRS scores in the taVNS group at 8 h and 12 h after surgery were significantly lower than those in the SS group. However, at other time points, no significant differences were observed between the two groups. The incidence of rebound pain was significantly lower in the taVNS group (7 of 39 patients [17.9%]) than in the SS group (16 of 39 patients [41%]) (P=0.025). The duration of rebound pain was 1.7±0.6 h in the taVNS group and 2.4±0.5 h in the SS group (P=0.015). Rebound pain occurred at 11 h (IQR: 10.8–11.9 h) after femoral nerve block in the taVNS group and 11.9 h (IQR: 11–12.1 h) in the SS group (P=0.189). The number of times to press PCA and the number of patients requiring additional analgesic within 12 h after surgery in taVNS group were significantly lower than those in the SS group (P=0.021 and P=0.004, respectively). However, there were no significant differences in the above indicators between the two groups at other time intervals (Table 2).
 |
Table 2 Rebound Pain and Postoperative Data |
The incidence of PONV (25.6% vs 33.3%) and pruritus (0% vs 2.6%) were similar between the taVNS and the SS group. During the night of the surgery, 12 patients (30.8%) in the taVNS group experienced sleep disturbance compared with 21 patients (53.8%) in the SS group (P=0.030). taVNS was well tolerated and did not cause adverse reactions such as lightheadedness, ear irritation, and tinnitus (Table 3).
 |
Table 3 Postoperative Adverse Events Up to 48h After Surgery |
Discussion
In the present study, postoperative taVNS significantly reduced the incidence and duration of rebound pain, the number of patients requiring additional analgesic, and the number of times to press PCA within 12 h postoperatively. In addition, taVNS significantly improved sleep disturbance due to postoperative pain on the night of surgery.
Recently, several studies have reported the incidence of rebound pain after peripheral nerve block in patients undergoing orthopedic surgery.5,9,10 Although there are no accepted diagnostic criteria for rebound pain, a number of studies reported that rebound pain is an acute postoperative pain (NRS≥7) following resolution of nerve blocks.25,26 Patients with significant pain (NRS≥3) in PACU were excluded from this study because these patients might experience failed block. Based on this, our study demonstrated that the incidence of rebound pain was 41% after single-injection femoral nerve block in control patients undergoing ACLR. Barry et al showed that 49.6% of patients experienced rebound pain within 24 h of block performance, which was consistent with our study.5 Rebound pain ensues following resolution of a nerve block. The resolution time for single-injection femoral nerve block with ropivacaine is approximately 12 h.27 In this study, the median time to onset of rebound pain was 11 h postoperatively, which was in accordance with the time of resolution of the nerve block. Rebound pain is a transient phenomenon, lasting about 2.4 h in the SS group. Henningsen et al showed that patients would experience excruciating rebound pain about 2h that did not respond to intravenous opioids administration.26
There are currently no effective preventive measures and treatments for rebound pain. Although the use of opioids can relieve pain, there is no evidence that it is effective for rebound pain. In addition, there is growing evidence of opioid-induced side effects, such as PONV, respiratory depression, and hyperalgesia.28 Woo et al and An et al suggested that perineural dexamethasone to nerve block reduced the incidence of rebound pain after block resolution, which might be related to the prolongation of nerve block duration and anti-inflammatory effect.10,13 However, the use of dexamethasone as an adjunctive anesthetic for peripheral nerve blocks is off-label, and its long-term safety has not been evaluated. Higher perineural dexamethasone doses may cause risks of wound infections and neurotoxicity.6,29 In addition, Williams et al reported that an additional 33 hours of nerve block duration reduced the rebound pain score by 1 unit (NRS), which might be associated with the reduction in nociceptive input and central sensitization.3,30 Administering of continuous PNB on discharged patients is more technically challenging that creates sizable workload disadvantages, and hinders rehabilitation. Therefore, it is important to take other steps to prevent rebound pain.
Recent studies indicated that taVNS alleviated hand osteoarthritis pain, oesophageal pain and musculoskeletal pain in humans.31–33 In the present study, our results showed that taVNS alleviated postoperative pain at 8 and 12 h after ACLR and reduced the incidence of rebound pain in patients receiving femoral nerve block compared with SS. There was no difference in NRS scores between the two groups at 4 h postoperatively, probably because most patients (87.2%) did not experience severe pain (NRS<3) under the effect of femoral nerve at this time. taVNS might have little effect on suppressing mild pain. Patients in the taVNS group received taVNS treatment (1h/1 time; 6 times) within the first 12 hours after surgery. Based on the duration of treatment, it was not surprising that there was no difference in NRS scores between 24 h and 48 h postoperatively. In addition, taVNS reduced the patient’s need for analgesic within 12 hours postoperatively.
The favorable effects on rebound pain may be related to the biological effects of taVNS. First, animal studies have shown that VNS could suppress neuronal discharge in spinal segments and spinothalamic tract that transmit nociceptive information form somatic to brain.34,35 Human studies reported that taVNS increased the mechanical, pressure, and thermal pain threshold.22,36 Furthermore, Aranow et al reported that a total of 20 min-taVNS reduced the plasma levels of substance P, which regulates pain perception by altering cellular signaling pathways, in patients with systemic lupus erythematosus.32,37 taVNS may influence central and peripheral perception of rebound pain. Second, it is well known that acute pain is a cardinal feature of inflammation.38 Both ACLR and local anesthetic ropivacaine could induce inflammatory response.39 taVNS activates the solitary nucleus tract and locus coeruleus to release acetylcholine and norepinephrine, thereby suppressing inflammatory responses.40 A recent study reported that the stimulation of locus coeruleus by VNS activated sympathetic nerve in knee joint that ultimately released norepinephrine to suppress joint inflammation.41 Another clinical study proved that taVNS inhibited postoperative acute inflammation response.42 Therefore, taVNS may reduce local and systemic inflammation induced by ACLR and local anesthetic. Huang et al suggested that lower plasma inflammatory cytokines might be associated with delayed onset of rebound pain.43 However, in this study, rebound pain was observed at approximately 11 h in both the taVNS and SS groups. taVNS reduced the incidence of rebound pain, but failed to delay the onset of rebound pain. Therefore, the relationship between taVNS, rebound pain, and inflammatory responses remains to be studied.
Rebound pain occurs around 12 h after surgery, which affects the need for additional analgesia within 24 h after surgery and the quality of sleep at the night of surgery.10 Therefore, we observed these side effects within 24 hours after surgery. Our results proved that taVNS significantly reduced the consumption of additional analgesics within 12 h postoperatively and improved sleep disturbance on the night of surgery. Notably, taVNS increases the proportion of the delta frequency bands in the EEG during sleep, thereby enhancing deep sleep.44 This effect may be attributed to long-term taVNS (four week).45 All patients who experienced rebound pain reported sleep disturbance. Among patients without rebound pain, 15.6% (5/32) patients in the taVNS group and 19.2% (5/23) patients in the SS group reported sleep disturbance (P=0.562). Thus, the improvement in sleep disturbance may be attributed to the inhibition of pain by taVNS.
This study had several limitations. First, we observed the incidence, duration and onset of rebound pain, but failed to measure the rebound pain score. To date, there is no widely accepted method to quantify rebound pain. Williams et al calculated the rebound pain score by subtracting lowest pain score in 12 h before nerve block resolution from the highest pain score during the first 12 h after the nerve block resolution.6 However, many investigators believe that it is difficult to pinpoint the exact point of nerve block resolution.25 Second, pain scores were assessed every 4 h for the first 12 h after surgery, which might obscure the trend of rebound pain. Nevertheless, patients were asked to record the duration and the time of onset of rebound pain. Third, the optimal stimulation parameters and duration of taVNS for pain relief have not been determined. We set parameters based on previous studies and the characteristics of rebound pain.32 Finally, long-term observations may be important to assess the relationship between taVNS and rebound pain. Further studies may focus on the conversion of acute rebound pain to chronic pain.
Conclusions
In conclusion, our study showed that taVNS reduced the incidence and duration of rebound pain and improved postoperative pain-related complications such as analgesic consumption and sleep disturbance. Further studies need to observe the long-term effect of taVNS on rebound pain and to explore the mechanism of this intervention.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Zhidi Xu for critical help with the study design, data analysis, and statistics of this manuscript.
Funding
This work was supported by Innovation guide Project Science and Technology Winter Olympics special project [grant numbers 19977790D]; Hebei Provincial government funded the specialty capacity building and specialty leader training program.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Joshi G, Gandhi K, Shah N, et al. Peripheral nerve blocks in the management of postoperative pain: challenges and opportunities. J Clin Anesth. 2016;35:524–529. doi:10.1016/j.jclinane.2016.08.041
2. Lavand’homme P. Rebound pain after regional anesthesia in the ambulatory patient. Curr Opin Anaesthesiol. 2018;31:679–684. doi:10.1097/aco.0000000000000651
3. Williams BA, Bottegal MT, Kentor ML, et al. Rebound pain scores as a function of femoral nerve block duration after anterior cruciate ligament reconstruction: retrospective analysis of a prospective, randomized clinical trial. Reg Anesth Pain Med. 2007;32:186–192. doi:10.1016/j.rapm.2006.10.011
4. Williams BA. Forecast for perineural analgesia procedures for ambulatory surgery of the knee, foot, and ankle: applying patient-centered paradigm shifts. Int Anesthesiol Clin. 2012;50:126. doi:10.1097/AIA.0b013e31821a00d0
5. Barry GS, Bailey JG, Sardinha J, et al. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br J Anaesth. 2021;126:862–871. doi:10.1016/j.bja.2020.10.035
6. Williams BA, Ibinson JW, Mangione MP, et al. Research priorities regarding multimodal peripheral nerve blocks for postoperative analgesia and anesthesia based on hospital quality data extracted from over 1300 cases (2011–2014). Pain Med. 2015;16:7–12. doi:10.1111/pme.12609
7. Sunderland S, Yarnold CH, Head SJ, et al. Regional versus general anesthesia and the incidence of unplanned health care resource utilization for postoperative pain after wrist fracture surgery: results from a retrospective quality improvement project. Reg Anesth Pain Med. 2016;41:22–27. doi:10.1097/AAP.0000000000000325
8. Nobre LV, Cunha GP, Sousa PC, et al. Peripheral nerve block and rebound pain: literature review. Rev Bras Anestesiol. 2020;69:587–593.
9. Fang J, Shi Y, Du F, et al. The effect of perineural dexamethasone on rebound pain after ropivacaine single-injection nerve block: a randomized controlled trial. BMC Anesthesiol. 2021;21:1–10. doi:10.1186/s12871-021-01267-z
10. Woo JH, Lee HJ, Oh HW, et al. Perineural dexamethasone reduces rebound pain after ropivacaine single injection interscalene block for arthroscopic shoulder surgery: a randomized controlled trial. Reg Anesth Pain Med. 2021;46:965–970. doi:10.1136/rapm-2021-102795
11. Verlinde M, Hollmann MW, Stevens MF, et al. Local anesthetic-induced neurotoxicity. Int J Mol Sci. 2016;17:339. doi:10.3390/ijms17030339
12. Kolarczyk LM, Williams BA. Transient heat hyperalgesia during resolution of ropivacaine sciatic nerve block in the rat. Reg Anesth Pain Med. 2011;36:220–224. doi:10.1097/AAP.0b013e3182176f5a
13. An K, Elkassabany NM, Liu J, Tang S-J. Dexamethasone as adjuvant to bupivacaine prolongs the duration of thermal antinociception and prevents bupivacaine-induced rebound hyperalgesia via regional mechanism in a mouse sciatic nerve block model. PLoS One. 2015;10:e0123459. doi:10.1371/journal.pone.0123459
14. Truini A. A review of neuropathic pain: from diagnostic tests to mechanisms. Pain Ther. 2017;6:5–9. doi:10.1007/s40122-017-0085-2
15. Tracey WD. Nociception. Curr Biol. 2017;27:R129–r133. doi:10.1016/j.cub.2017.01.037
16. Truini A, Garcia-Larrea L, Cruccu G. Reappraising neuropathic pain in humans–how symptoms help disclose mechanisms. Nat Rev Neurol. 2013;9:572–582. doi:10.1038/nrneurol.2013.180
17. Mertens A, Gadeyne S, Lescrauwaet E, et al. The potential of invasive and non-invasive vagus nerve stimulation to improve verbal memory performance in epilepsy patients. Sci Rep. 2021;12(1):1–3.
18. Hu B, Akerman S, Goadsby PJ. Characterization of opioidergic mechanisms related to the anti-migraine effect of vagus nerve stimulation. Neuropharmacology. 2021;195:108375. doi:10.1016/j.neuropharm.2020.108375
19. Multon S, Schoenen J. Pain control by vagus nerve stimulation: from animal to man … and back. Acta Neurol Belg. 2005;105:62.
20. Ren K, Zhuo M, Randich A, et al. Vagal afferent stimulation-produced effects on nociception in capsaicin-treated rats. J Neurophysiol. 1993;69:1530–1540. doi:10.1152/jn.1993.69.5.1530
21. Willis WD. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi:10.1007/s002210050637
22. Kirchner A, Stefan H, Bastian K, et al. Vagus nerve stimulation suppresses pain but has limited effects on neurogenic inflammation in humans. Eur J Pain. 2006;10:449–455. doi:10.1016/j.ejpain.2005.06.005
23. Busch V, Zeman F, Heckel A, et al. The effect of transcutaneous vagus nerve stimulation on pain perception–an experimental study. Brain Stimul. 2013;6:202–209. doi:10.1016/j.brs.2012.04.006
24. Kaniusas E, Kampusch S, Tittgemeyer M, et al. Current directions in the auricular vagus nerve stimulation I - a physiological perspective. Front Neurosci. 2019;13:854. doi:10.3389/fnins.2019.00854
25. Zhu T, Gao Y, Xu X, et al. Effect of ketamine added to ropivacaine in nerve block for postoperative pain management in patients undergoing anterior cruciate ligament reconstruction: a randomized trial. Clin Ther. 2020;42:882–891. doi:10.1016/j.clinthera.2020.03.004
26. Henningsen MJ, Sort R, Møller AM, et al. Peripheral nerve block in ankle fracture surgery: a qualitative study of patients’ experiences. Anaesthesia. 2018;73:49–58. doi:10.1111/anae.14088
27. Safa B, Flynn B, McHardy PG, et al. Comparison of the analgesic duration of 0.5% bupivacaine with 1:200,000 epinephrine versus 0.5% ropivacaine versus 1% ropivacaine for low-volume ultrasound-guided interscalene brachial plexus block: a randomized controlled trial. Anesth Analg. 2021;132:1129–1137. doi:10.1213/ane.0000000000005373
28. Ayoo K, Mikhaeil J, Huang A, et al. The opioid crisis in North America: facts and future lessons for Europe. Anaesthesiol Intensive Ther. 2020;52:139–147. doi:10.5114/ait.2020.94756
29. Williams BA, Hough KA, Tsui BY, et al. Neurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaine. Reg Anesth Pain Med. 2011;36:225–230. doi:10.1097/AAP.0b013e3182176f70
30. Waldman SD. Pain Management E-Book. Elsevier Health Sciences; 2011.
31. Farmer AD, Albusoda A, Amarasinghe G, et al. Transcutaneous vagus nerve stimulation prevents the development of, and reverses, established oesophageal pain hypersensitivity. Aliment Pharmacol Ther. 2020;52:988–996.
32. Aranow C, Atish-Fregoso Y, Lesser M, et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann Rheum Dis. 2021;80:203–208. doi:10.1136/annrheumdis-2020-217872
33. Badran BW, Dowdle LT, Mithoefer OJ, et al. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRI study and review. Brain Stimul. 2018;11:492–500. doi:10.1016/j.brs.2017.12.009
34. Thies R, Foreman RD. Inhibition and excitation of thoracic spinoreticular neurons by electrical stimulation of vagal afferent nerves. Exp Neurol. 1983;82:1–16. doi:10.1016/0014-4886(83)90238-8
35. Chandler MJ, Hobbs SF, Bolser DC, et al. Effects of vagal afferent stimulation on cervical spinothalamic tract neurons in monkeys. Pain. 1991;44:81–87. doi:10.1016/0304-3959(91)90152-n
36. Ness TJ, Fillingim RB, Randich A, et al. Low intensity vagal nerve stimulation lowers human thermal pain thresholds. Pain. 2000;86:81–85. doi:10.1016/s0304-3959(00)00237-2
37. Lisowska B, Siewruk K, Lisowski A, d’Acquisto F. Substance P and acute pain in patients undergoing orthopedic surgery. PLoS One. 2016;11:e0146400. doi:10.1371/journal.pone.0146400
38. Ji -R-R, Chamessian A, Zhang Y-Q. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–577. doi:10.1126/science.aaf8924
39. Wohlrab P, Boehme S, Kaun C, et al. Ropivacaine activates multiple proapoptotic and inflammatory signaling pathways that might subsume to trigger epidural-related maternal fever. Anesth Analg. 2020;130:321–331. doi:10.1213/ANE.0000000000004402
40. Murray K, Rude KM, Sladek J, et al. Divergence of neuroimmune circuits activated by afferent and efferent vagal nerve stimulation in the regulation of inflammation. J Physiol. 2021;599:2075–2084. doi:10.1113/JP281189
41. Bassi GS, Dias DPM, Franchin M, et al. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav Immun. 2017;64:330–343. doi:10.1016/j.bbi.2017.04.003
42. Salama M, Akan A, Mueller MR. Transcutaneous stimulation of auricular branch of the vagus nerve attenuates the acute inflammatory response after lung lobectomy. World J Surg. 2020;44:3167–3174. doi:10.1007/s00268-020-05543-w
43. Hwang J-T, Jang JS, Lee JJ, et al. Dexmedetomidine combined with interscalene brachial plexus block has a synergistic effect on relieving postoperative pain after arthroscopic rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2020;28:2343–2353. doi:10.1007/s00167-019-05799-3
44. Luo M, Li L, Zhang J, et al. Sleep electroencephalography power spectral response to transcutaneous auricular vagus nerve stimulation on insomnia rats. Heart Mind. 2019;3:55. doi:10.4103/hm.hm_51_19
45. Zhang S, He JK, Meng H, et al. Effects of transcutaneous auricular vagus nerve stimulation on brain functional connectivity of medial prefrontal cortex in patients with primary insomnia. Anat Rec. 2021;304:2426–2435. doi:10.1002/ar.24785
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

