Back to Journals » ClinicoEconomics and Outcomes Research » Volume 13
Effect of the Pandemic on Quality-of-Life Data Collection in Prostate Cancer Patients
Authors Shirsat A, Pashilkar S, Chavan A, Kalra D, Gota V, Joshi A, Nookala Krishnamurthy M
Received 11 June 2021
Accepted for publication 2 September 2021
Published 18 November 2021 Volume 2021:13 Pages 937—941
DOI https://doi.org/10.2147/CEOR.S321638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Aditi Shirsat,1 Siddhi Pashilkar,2 Ashish Chavan,1 Devanshi Kalra,2 Vikram Gota,1,3 Amit Joshi,2,3 Manjunath Nookala Krishnamurthy1,3
1Department of Clinical Pharmacology, Advanced Centre for Treatment Research and Education in Cancer, Tata Memorial Centre, Navi Mumbai, Maharashtra, India; 2Department of Medical Oncology, Tata Memorial Hospital, Tata Memorial Centre, Mumbai, Maharashtra, India; 3Homi Bhabha National Institute, Mumbai, Maharashtra, India
Correspondence: Manjunath Nookala Krishnamurthy
Department of Clinical Pharmacology, Advanced Centre for Treatment, Research and Education in Cancer, Tata Memorial Centre, Sector- 22, Kharghar, Navi Mumbai, 410210, Maharashtra, India
Tel +91 2268735130
Email [email protected]; [email protected]
Purpose: To understand the difficulties that happen during the quality of life (QoL) data collection in a pandemic and provide measures to overcome them.
Methods: We analyzed the recruitment and follow-up data of patients in one of our ongoing study whose aim was to collect the Adverse drug reactions and QoL (at regular intervals) in prostate cancer patients who were on docetaxel. Before the pandemic, we could enroll 31 patients in the study over four months. We analyzed the difficulties experienced by these patients and consultants in collecting QoL data during the pandemic, especially in situations with limited availability of resources and also where the patients are not technologically advanced.
Results: Due to the pandemic, we could not recruit a single new patient into the study. Complete QoL assessments were available in only two patients, and the disease progressed in five patients. QoL assessment was not possible in 19 of 31 enrolled patients. More than 44% of the enrolled patients had difficulty commuting to the hospital despite transport services to hospitals. Due to the risk of acquiring COVID19 infection during traveling to the hospital, follow-ups were affected.
Conclusion: There should be increased support for novel technologies that can successfully capture and transfer patients’ QoL data to the treating consultant.
Keywords: pandemic, coronavirus, quality of life, COVID 19
Introduction
Cancer incidence is increasing steadily, with continuing improvement in diagnostic measures. Novel treatment modalities have increased the longevity of a cancer patient, impacting the quality of life of cancer patients, leading to an increase in the interest in evaluating the health-related quality of life (HRQOL) of cancer patients.1 Subsequently, a long-term follow-up to assess survival and HRQOL is part of a majority of the clinical trials.2 European Organization for Research and Treatment of Cancer Quality of Life core questionnaire (EORTC QLQ-C30) is commonly used to capture HRQOL. The questionnaire consists of 30 questions arranged into five domains physical, role, cognitive, emotional, and social functioning. This questionnaire is generic, and site-specific questionnaires exist to capture the QOL in specific cancers.3
Prostate cancer is a common type of cancer in males and is predominantly a disease of the elderly. The risk of prostate cancer increases with age, and the peak incidence occurring at just over 70 years of age.4,5 However, prostate cancer is getting diagnosed in early cancer stages in younger men.6 A lot of younger men are asymptomatic and are physically and sexually active at diagnosis.7 These trends have led to increasing numbers of patients undergoing disease management for more extended periods. Quality of life (QOL) is as important as survival;8 Prostate cancer specific questionnaire (PR-25) captures symptoms and functional impairments due to prostate cancer . PR-25 is commonly given to patients in addition to EORTC QLQ-C30 to get comprehensive information on the prostate cancer patient’s QoL.
Pandemic can affect every part of the world, and the ongoing COVID-19 is not an exception, leading to global slowdown.9 The devastating and unpredictable spread of COVID-19 across the globe has caused unprecedented global lockdowns and an immense burden for healthcare systems. A nationwide lockdown on 24th March 2020 was implemented in India, limiting the movement of India’s entire 1.3 billion population as a preventive measure against the COVID-19 pandemic spread in India. The lockdown restricted people from stepping out of their homes. All transport services–road, air, and rail–were suspended, except for transporting essential goods, fire, police, and emergency services. Educational institutions, industrial establishments, and hospitality services were all arrested. Public services such as banks and ATMs, petrol pumps, other essentials, and manufacturing are exempted.10
COVID-19 pandemic has caused an immense burden on the healthcare systems, which was an enormous challenge for clinicians, researchers, and patients. Although the hospital services continued during the lockdown, lack of transportation has affected the patient’s hospital visits during the pandemic. In its direct effect, the recruitment of patients in many clinical trials too got affected. In this article, we discussed the issues faced by the patients and study team of a study whose main objective was to capture the HRQOL of prostate cancer patients.
Study Description
The study was a prospective observational study to assess the quality of life in prostate cancer patients receiving docetaxel as a standard of care. The study was initiated in a tertiary cancer hospital after the Institutional Ethics Committee approved it. Patients’ HRQOL was captured with EORTC QLQc30 and PR 25 questionnaires.
Docetaxel was administered to the prostate cancer patients as a 2-weekly or 3-weekly regimen as decided by their treating consultant. QoL questionnaires were filled before the first dose and then regularly at 2 or 3 monthly intervals depending on their treatment regimen.
Patient Recruitment
Within the first four months of the start of the study, we were able to recruit 31 patients in the study after obtaining written Informed consent from the patients (Table 1). Patients completed the EORTC QLQ-C30 and PR-25 as per the protocol.
 |
Table 1 Baseline Characteristics of Patients |
Results
Follow-Up of Enrolled Patients During the Pandemic
None of the patients enrolled was coronavirus vaccinated. None of the patients acquired coronavirus infection during the study. However, their cancer treatment and follow-ups were affected the most (Figure 1). Due to the pandemic, transportation of the patients to the hospital was affected – 10% of patients’ had to stop docetaxel, as the drug was unavailable in their hometown; more than 30% of the patients underwent treatment at their local places which had inadequate cancer management facilities. More than 30% of the patients could come to the hospital, but due to the fear of acquiring Covid-19, they were not willing for their QoL assessments (Figure 2). Furthermore, not a new patient got enrolled in the study (Figure 1).
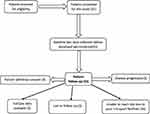 |
Figure 1 Flow chart of patients enrolled in the study. |
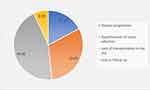 |
Figure 2 Factors affecting the patient follow-up to the study site. |
Discussion
The risk-benefit balance of a scenario is critical. Clinical services were curtailed to a minimum in most cancer centers due to the fear of infection spread among vulnerable populations. Patients feared acquiring the COVID-19 infection in the hospitals to themselves and to their caregivers, not realizing the effects of cancer progression and the resultant poorer prognosis that could be more severe. Only very few new cancer cases were registered in most hospitals, not learning that the delay in diagnosis and treatment would affect morbidity and mortality. Routine clinical services in oncology relegated as the second priority. COVID-19 pandemic significantly impacted cancer surgeries, leading to their postponement in many cancer centres in India.11 The delays have shifted patients’ management from the curative to the palliative stage. Both these scenarios can adversely affect long-term survival and possibly lead to worse outcomes.12,13
The majority of the tertiary care cancer hospitals are located in cities, while limited availability in the small villages and towns.14,15 Patients with their hometowns in villages were unable to travel to the cancer hospitals for follow-up due to the travel restrictions. Patients had to follow up at their local places with no experienced oncology consultants. Furthermore, anti-cancer drugs were limited in the small villages, which further complicated their management. However, ours being an exclusive tertiary care cancer centre in Mumbai, India, continued to offer complete services for patients who could access it even during the lockdown period. Teleconsultation was also available to provide patient support. Our hospital administration took precautionary measures to prevent infection spread like screening patients and attendants for any coronavirus infection symptoms before they enter the hospital, restricting the number of attendants with each patient, banning visitors for inpatients, protecting vulnerable staff, maintaining drug supply chains, and so on. These measures have secured patients and staff from spreading COVID-19 infection among the patients, team, and attendants.
Patient recruitment in the clinical studies was seriously affected, and our study on QoL data collection was no exception to it. We could not enroll even a single patient in the study during the pandemic. Our study patients being elderly and fall under the high-risk category for coronavirus infection spread; their treatment follow-up was affected. Researchers faced difficulties in collecting QoL data from the patients at scheduled intervals, as few patients stopped the treatment on their own and few patients lost to follow-up. All of these difficulties are attributable to the pandemic. Despite the early steps taken at our centre to prevent the spread of the infection, the high infectivity rate of the coronavirus led to poor follow-up of the patients who came for treatment follow-up. Cancer patients who visited the hospital during the pandemic were attended to and managed according to the standard treatment practices.
Possible Ways to Overcome the Difficulties in QoL Data Collection
QoL data collection captures a patient’s current clinical situation due to the underlying disease and its treatment through a predefined questionnaire.16 This data collection does not require the actual presence of the physician with a patient while completing the form because the presence of the treating physician may bias the patient’s responses to the questionnaire. If the time-point for QoL data collection is missed (due to either avoidable or unavoidable reasons), it is less meaningful even if obtained later. This collection of data at scheduled time stresses the importance of the timely collection of QoL data. If the patient cannot visit the physician to complete the QoL questionnaires in the western world, they send their QoL data electronically. The Paper and pencil method and electronic modes of QOL questionnaire administrations have comparable results.17–19 Patient counseling on the study activities can improve compliance. Flyers and videos will create increased awareness in patients about the importance of QoL.
Questionnaires can be shared with the patients by email or by mail and requesting them to return the filled questionnaire by the same means. The other option is to consider the availability of social networking means, by which the questionnaires are sent and received back from the patients. The proxy method is another method to get patients’ QoL data from the patient’s relatives or caretakers, who will complete the QOL questionnaire.20–22 As our study was conducted on elderly patients, and we could not get the email addresses of the patients or their relatives. Furthermore, due to complete lockdown, all the non-essential services were on halt. Patient relatives were reluctant to accept the option of getting a hard copy of the questionnaire from outside of their residence. Hence, we did not approach the ethics committee to consider these options for the study data collection.
Telemedicine can overcome poor data collection when the patient cannot physically come to the physician. Here, QOL will be filled by the consultant or a deputed coordinator while discussing telephonically with the patient. The world has advanced much in information technology; web-based QOL data collection tools are developed and used regularly in western countries.23 There are various software platforms on which the questionnaires exist, and the patient can provide their responses to the questionnaire questions. Furthermore, by these means, patients’ responses to the questions will be immediately available to the health care personnel. One of these is the Computer-based Health Evaluation System (CHES) which electronically captures patient responses to the questionnaires. European Organization for Research and Treatment of Cancer (EORTC) Quality of Life group developed CHES.24 With CHES software, the longitudinal data collection and its simultaneous analysis, help interpret changes in different domains of the patient QOL over time.
To conclude, patients' quality of life data is central for their management, and there exist various modes that help in data capture even in pandemics. Our observations can be seen in any situations where the technology and resources are limited.
Ethical Approval and Informed Consent
The study was approved by the institutional ethics committee 1 of Tata Memorial Centre, Mumbai, Maharashtra, India. Patients signed the informed consent form before any study-related procedures were carried out and were willing to use the study data for scientific purposes. We followed all the ethical standards of the national and institutional research guidelines during the study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Adolfsson J. Health-related quality-of-life assessments in patients with advanced cancer of the prostate. Pharmacoeconomics. 2003;21(4):241–247. doi:10.2165/00019053-200321040-00002
2. Van Leeuwen M, Husson O, Alberti P, et al. Understanding the quality of life (QOL) issues in survivors of cancer: towards the development of an EORTC QOL cancer survivorship questionnaire. Health Qual Life Outcomes. 2018;16(1):1–15. doi:10.1186/s12955-018-0920-0
3. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNCI. 1993;85(5):365–376. doi:10.1093/jnci/85.5.365
4. Heinzer H, Steuber T. Prostate cancer in the elderly. Urol Oncol. 2009;27(6):668–672. Elsevier. doi:10.1016/j.urolonc.2009.07.015
5. Zhou CK, Check DP, Lortet‐Tieulent J, et al. Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int J Cancer. 2016;138(6):1388–1400. doi:10.1002/ijc.29894
6. Bleyer A, Spreafico F, Barr R. Prostate cancer in young men: an emerging young adult and older adolescent challenge. Cancer. 2020;126(1):46–57. doi:10.1002/cncr.32498
7. Dimitropoulou P, Lophatananon A, Easton D, et al. Sexual activity and prostate cancer risk in men diagnosed at a younger age. BJU Int. 2009;103(2):178–185. doi:10.1111/j.1464-410X.2008.08030.x
8. Rossetti SR, Terrone C. Quality of life in prostate cancer patients. Eur Urol. 1996;30:44–48. doi:10.1159/000474246
9. Madhav N, Oppenheim B, Gallivan M, Mulembakani P, Rubin E, Wolfe N. Pandemics: risks, impacts, and mitigation; 2017.
10. Sharma DC. Lockdown poses new challenges for cancer care in India. Lancet Oncol. 2020;21(7):884. doi:10.1016/S1470-2045(20)30312-0
11. Kumar D, Dey T. Treatment delays in oncology patients during COVID-19 pandemic: a perspective. J Glob Health. 2020;10(1):1. doi:10.7189/jogh.10.010367
12. Hinz A, Weis J, Faller H, et al. Quality of life in cancer patients—a comparison of inpatient, outpatient, and rehabilitation settings. Support Care Cancer. 2018;26(10):3533–3541. doi:10.1007/s00520-018-4211-4
13. Hartman HE, Sun Y, Devasia TP, et al. Integrated survival estimates for cancer treatment delay among adults with cancer during the COVID-19 pandemic. JAMA Oncol. 2020;6(12):1881–1889. doi:10.1001/jamaoncol.2020.5403
14. Das S, Patro KC. Cancer care in the rural areas of India: a firsthand experience of a clinical oncologist and review of literatures. J Cancer Res Ther. 2010;6(3):299. doi:10.4103/0973-1482.73369
15. Banavali SD. Delivery of cancer care in rural India: experiences of establishing a rural comprehensive cancer care facility. Indian J Med Paediatr Oncol. 2015;36(2):128. doi:10.4103/0971-5851.158848
16. Basch E. The rise of patient-reported outcomes in oncology. ASCO Daily News; 2016.
17. Campbell N, Ali F, Finlay AY, Salek SS. Equivalence of electronic and paper-based patient-reported outcome measures. Qual Life Res. 2015;24(8):1949–1961. doi:10.1007/s11136-015-0937-3
18. Muehlhausen W, Doll H, Quadri N, et al. Equivalence of electronic and paper administration of patient-reported outcome measures: a systematic review and meta-analysis of studies conducted between 2007 and 2013. Health Qual Life Outcomes. 2015;13(1):1–20. doi:10.1186/s12955-015-0362-x
19. Gwaltney CJ, Shields AL, Shiffman S. Equivalence of electronic and paper-and-pencil administration of patient-reported outcome measures: a meta-analytic review. Value Health. 2008;11(2):322–333. doi:10.1111/j.1524-4733.2007.00231.x
20. Cella D, Hahn EA, Jensen SE, Butt Z, Nowinski CJ, Rothrock N. Methodological issues in the selection, administration and use of patient-reported outcomes in performance measurement in health care settings. In: Patient Reported Outcomes (Pros) in Performance Measurement. National Quality Forum (NQF); 2013.
21. Fairclough DL. Practical considerations in outcomes assessment for clinical trials. In: Outcomes Assessment in Cancer: Measures, Methods, and Applications. Cambridge, UK: Cambridge University Press; 2005:346–361.
22. Simes RJ, Greatorex V, Gebski VJ. Practical approaches to minimize problems with missing quality of life data. Stat Med. 1998;17(5‐7):725–737. doi:10.1002/(SICI)1097-0258(19980315/15)17:5/7<725::AID-SIM817>3.0.CO;2-1
23. Basch E, Artz D, Dulko D, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol. 2005;23(15):3552–3561. doi:10.1200/JCO.2005.04.275
24. Holzner B, Giesinger JM, Pinggera J, et al. The Computer-based Health Evaluation Software (CHES): a software for electronic patient-reported outcome monitoring. BMC Med Inform Decis Mak. 2012;12(1):1–11. doi:10.1186/1472-6947-12-126
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
