Back to Journals » Clinical Interventions in Aging » Volume 15
Effect of the Casein-Derived Peptide Met-Lys-Pro on Cognitive Function in Community-Dwelling Adults Without Dementia: A Randomized, Double-Blind, Placebo-Controlled Trial
Authors Yuda N , Tanaka M , Yamauchi K, Abe F, Kakiuchi I, Kiyosawa K, Miyasaka M, Sakane N, Nakamura M
Received 11 March 2020
Accepted for publication 7 May 2020
Published 27 May 2020 Volume 2020:15 Pages 743—754
DOI https://doi.org/10.2147/CIA.S253116
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Naoki Yuda,1 Miyuki Tanaka,1 Koji Yamauchi,1 Fumiaki Abe,1 Izumi Kakiuchi,2 Kyoko Kiyosawa,2 Mitsunaga Miyasaka,2 Naoki Sakane,3 Masahiko Nakamura4
1Food Ingredients and Technology Institute, Morinaga Milk Industry Co., Ltd., Zama, Kanagawa, Japan; 2Department of Nursing, Matsumoto Junior College, Matsumoto, Nagano, Japan; 3Division of Preventive Medicine, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, Kyoto, Japan; 4Matsumoto City Hospital, Matsumoto, Nagano, Japan
Correspondence: Naoki Yuda
Food Ingredients and Technology Institute, Morinaga Milk Industry Co., Ltd., 1-83, 5-Chome, Higashihara, Zama, Kanagawa, Japan
Tel +81 46 252 3051
Fax +81 46 252 3017
Email [email protected]
Background: Preventative measures have recently been taken to reduce the incidence of Alzheimer’s disease worldwide. We previously showed that Met-Lys-Pro (MKP), a casein-derived angiotensin-converting enzyme inhibitory peptide with the potential to cross the blood–brain barrier, attenuated cognitive decline in a mouse model of Alzheimer’s disease. However, the effect of MKP on cognitive function improvement in humans remains unknown. This exploratory study sought to investigate whether MKP intake could improve cognitive function in adults without dementia.
Methods: A total of 268 community-dwelling adults without dementia participated in this 24-week randomized controlled trial. Participants were randomly allocated to the MKP (n = 134) or placebo (n = 134) group. The MKP group received four tablets daily, each containing 50 μg MKP, while the placebo group received four dextrin tablets containing no detectable MKP for 24 weeks. Scores on the Japanese version of the cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog) were used as the primary outcome to compare cognitive function between the MKP and placebo groups. The study products were also evaluated for safety.
Results: The intention-to-treat analysis showed that there was no significant difference between the groups in terms of the ADAS-cog total score. Orientation, as measured by the respective ADAS-cog subscale, was significantly improved compared to placebo at 24 weeks post-MKP administration (P = 0.022). No serious adverse events due to MKP intake were observed.
Conclusion: To the best of our knowledge, this is the first study to report the effects of MKP on human cognition. These preliminary results suggested the safety of daily MKP intake and its potential to improve orientation in adults without dementia. Further clinical studies are needed to confirm the present findings and the benefits of MKP on cognitive function.
Keywords: humans, MKP, cognition, cognitive dysfunction, orientation, Alzheimer’s disease
Introduction
As global life expectancy rises, the growth in the number of older adults with dementia is unprecedented. The number of individuals living with dementia is predicted to increase from 47 million in 2015 to 132 million by 2050.1 Alzheimer’s disease (AD) is the most common cause of dementia. Cholinesterase inhibitors and N-methyl D-aspartate receptor antagonists have been approved for the treatment of AD in many countries.2 Although these drugs provide a moderate treatment effect, they do not completely alter the condition.3,4 Prevention and care for AD have become urgent worldwide issues. AD progresses for many years before symptoms appear; and when symptoms become clinically apparent, the condition is too advanced for treatment.5,6 Therefore, taking preventative measures before the onset of clinical symptoms is recommended.7–9
Recently, it was suggested that centrally active angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers may lower AD risk or slow its progression, independent of their blood-pressure-lowering effect.10,11 The activity of ACE is elevated in the brains of patients with AD.12 Angiotensin II generated by ACE may promote oxidative stress and neuroinflammation, leading to neurodegeneration and brain aging.13,14 However, several clinical studies have shown that centrally active ACE inhibitors, which cross the blood–brain barrier, prevent the process of neurodegeneration leading to dementia and the incidence of AD.11,15-17
Food-derived peptides have been identified as ACE inhibitors.18 We have previously identified a novel antihypertensive tripeptide, Met-Lys-Pro (MKP), derived from bovine casein.19 MKP exhibited relatively strong ACE inhibitory activity in vitro (IC50 = 0.43 μM), and orally administrated MKP was absorbed into the plasma and reduced blood pressure (BP) in spontaneously hypertensive rats.20 Moreover, a randomized, double-blind, placebo-controlled, parallel group trial revealed that daily MKP intake is a safe and effective systolic BP (SBP) lowering treatment for individuals with high-normal BP or grade 1 hypertension.21
As MKP exhibited relatively strong ACE inhibitory activity, we examined its potential effects on cognitive function. The results showed that oral administration of MKP significantly attenuated cognitive decline in a mouse model of AD.22 Additionally, the autoradiography data showed that orally administrated14 C-MKP was distributed in the brain.22 Therefore, we hypothesized that MKP may act as a centrally active ACE inhibitor and therapeutic agent for cognitive function.
Improving cognitive function by the daily ingestion of effective food ingredients at a pre-AD stage may either prevent or delay the onset of AD.23 Thus, we sought to conduct a 24-week, randomized, double-blind, placebo-controlled trial to investigate the ability of MKP to improve cognitive function in a population of community-dwelling adults without dementia. Since they represent a population at risk of developing clinical AD, but constitute a group in whom the preclinical disease is believed to be at an early enough stage to still respond to intervention, middle-aged and elderly individuals without dementia were recruited for the study.6 We used the scores of the Japanese version of the cognitive subscale of the AD Assessment Scale (ADAS-cog)24,25 as the primary outcome. ADAS-cog is one of the most frequently used instruments to evaluate general cognitive function in clinical trials. To the best of our knowledge, this is the first study to evaluate the effects of MKP on human cognition. Therefore, we used ADAS-cog in this study to broadly examine the effects of MKP on cognitive function. As secondary outcomes, we assessed participants using the Revised Hasegawa’s Dementia Scale (HDS-R),26 the Japanese version of the Montreal Cognitive Assessment (MoCA-J),27,28 and the eight-item Short-Form Health Survey (SF-8).29 We also evaluated the safety of MKP.
Methods
Participants
Adult volunteers living in Matsumoto, Japan, and the surrounding areas were recruited through website announcements, advertisements, and mailed invitations. Recruitment was conducted from April to June 2018. The inclusion criteria were as follows: age ≥ 40 years and an HDS-R score of 21–30. The exclusion criteria were as follows: 1) history or presence of dementia; 2) suspected dementia; 3) mental disorders such as schizophrenia and depression; 4) serious diseases of the brain, liver, kidney, heart, lung, gastrointestinal tract, blood, or metabolism; 5) serious allergies to medicine or food; 6) pregnancy, lactation, or pregnancy planning during the study period; 7) ineligibility due to physician’s diagnosis based on participant background, physical examination, and interview.
Procedures
The trial was conducted in Matsumoto between June 2018 and February 2019. To investigate the impact of regular MKP intake on cognitive function in community-dwelling adults without dementia, a 24-week randomized, double-blind, placebo-controlled trial was conducted. The study lasted 24 and 2 weeks for treatment and post-treatment observation, respectively. Efficacy assessments were obtained at baseline, week 12, and week 24.
Eligibility was assessed on the basis of interviews, physical examination (BP, height, body weight), self-reported data from health and lifestyle questionnaires, the Geriatric Depression Scale (GDS)30,31 score, and the HDS-R score. Individuals with scores ≥ 7 on the GDS or ≤ 20 on the HDS-R were excluded from participation. Eligible participants were randomly assigned to receive MKP or placebo tablets in a 1:1 ratio by a person not directly involved in the study using computer-generated lists of random numbers via the randomly permuted block method. The participants, physician, researchers assessing outcomes, and researchers conducting statistical analyses were blinded to the treatment group allocation over the study duration.
The MKP-containing tablets were prepared using casein hydrolysate manufactured by the Morinaga Milk Industry (Tokyo, Japan).19 The participants in the MKP and placebo groups received four tablets daily, each containing 50 μg MKP in 0.25 g casein hydrolysate, and 0.25 g dextrin with no detectable MKP, respectively. MKP and placebo tablets were matched for appearance.
All participants were encouraged to continue with their usual daily activities and diet throughout the study period. The participants were also asked to maintain a record using diaries, which included items related to supplementation of study products, illness, use of medications or other nutritional supplements, and hospital visits. Study staff interviewed participants before and throughout the study to ensure their compliance with these lifestyle requirements based on the participant diaries. Treatment compliance was assessed by counting the number of tablets returned at the time of the final study visit and inspecting participant diaries.
Outcome Measures
The primary outcome measure was the ADAS-cog score.24,25 The test, which yields a score ranging from 0 (no errors) to 70 (maximum impairment), assesses memory, language, praxis, and orientation and is composed of 11 subscales (word recall, spoken language ability, comprehension of spoken language, word-finding difficulty, following commands, naming objects and fingers, constructions, ideational praxis, orientation, word recognition, and recall of test instructions) to evaluate general cognitive function.
The secondary outcome measures were the HDS-R, MoCA-J, and SF-8 scores. In Japan, the HDS-R is a neuropsychological battery commonly used for the screening of dementia.26 Individuals with scores of ≤ 20 out of 30 are diagnosed with suspected dementia. The MoCA-J is a useful screening tool for detecting mild cognitive impairment (MCI).27,28 The cut-off point of the test for MCI and AD is 25/26 out of 30. To correct for the educational background, 1 point is added for participants with a total score of < 30 and an educational background of < 12 years. The ADAS-cog and MoCA-J were performed at baseline and after 12 and 24 weeks of supplementation. The HDS-R was performed at baseline and after 24 weeks of supplementation. The participants self-reported assessment of physical and mental health at baseline and week 24 was obtained using the SF-8.29 The Mental Component Summary (MCS) and Physical Component Summary (PCS) scores were calculated.
Safety Monitoring
All intervened participants were monitored throughout the study for adverse events (AEs) and side effects. Safety monitoring comprised a questionnaire that assessed general health and occurrence of any health-related events. The relation of AEs to ingestion of the study products was determined by the physician while remaining blinded to group allocation. The severity of AEs was evaluated according to the Common Terminology Criteria for Adverse Events version 4.0 JCOG/JSCO.
Sample Size
The effect size (d) of the ADAS-cog total score at 24 weeks after MKP intake was estimated to be 0.40. The sample size required to detect a mean ADAS-cog total score difference at α = 0.05 and power = 0.90 by the unpaired t-test was calculated at 133 study participants per group, making up a total of 266 study participants. The target sample size was calculated using G*Power 3.1.9.2 (Heinrich Heine Universitat, Dusseldorf, Germany). Considering a 10% dropout rate, approximately 150 participants per group needed to be recruited.
Statistical Analysis
The statistical analysis was based on the intention-to-treat population, which included all randomly assigned participants with at least one observation. Missing data were handled by the available case analysis. Data are presented as means (along with standard deviations). The baseline characteristics of the study groups were compared with the use of the Fisher’s exact test for categorical variables and the unpaired t-test for continuous variables. We assessed the continuous variables of efficacy using the analysis of covariance models to adjust for baseline values, using the week 24 values as the dependent variables. The cognitive test data were also analyzed according to age, MoCA-J score, or medication status by dividing the participants into predefined subgroups. Age was divided by 65, the standard for elderly people in Japan. The MoCA-J score was divided by 26, the standard for suspected MCI. Medication status was divided by whether the participants were using regular medication; temporary medication, such as for colds, was not included. Safety analyses were carried out based on summary listings of AEs, with the Fisher’s exact test used for pairwise comparisons. All comparisons were two-tailed, and the statistical significance level was set to P < 0.05. All analyses were performed using IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY, USA).
Results
Participants
From a total of 468 participants screened for the study, 268 were enrolled and randomly allocated into the MKP (n = 134) and placebo (n = 134) groups (Figure 1). Out of the 268 enrolled participants, 256 and 253 remained enrolled in the study for 12 weeks and until the end of the study period, respectively. Three randomized participants withdrew before the intervention for personal reasons unrelated to the trial and 12 (six in the MKP and the placebo group, respectively) discontinued; nine (six and three in the MKP and the placebo group, respectively) dropped out during the intervention period due to personal reasons unrelated to the trial, and three (all in the placebo group) due to AEs unrelated to the treatment. The overall dropout rate was 5.6% (15 of 268). The compliance rates were 96.7% and 96.5% in the MKP and placebo group, respectively, with the difference being nonsignificant. Table 1 shows the baseline characteristics, including sex, age, BP, body mass index (calculated as weight in kg divided by height in m2), education years, and SF-8, GDS, ADAS-cog, MoCA-J, and HDS-R scores. The two groups did not differ significantly in the baseline demographic variables. In the overall population, the mean age was 68.3 years, the mean ADAS-cog score was 4.1, the mean MoCA-J score was 25.8, and the mean HDS-R score was 28.6. Considering the cut-off threshold of the MoCA-J score (25/26), 58% of all enrolled participants were considered cognitively healthy, and 42% were considered as having a suspected MCI.
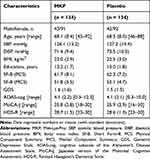 |
Table 1 Baseline Characteristics of the Participants |
 |
Figure 1 Study flow diagram. Abbreviation: MKP, Met-Lys-Pro. |
Outcomes
A summary of the cognitive test data at baseline and after 12 and 24 weeks is presented in Table 2. After 24 weeks, there was no significant MKP treatment effect on the ADAS-cog total score compared to placebo. Orientation of the participants in the MKP group, as measured by the respective ADAS-cog subscale, significantly improved (P = 0.022, d = 0.30). There were no significant differences between the groups in the other cognitive variables.
 |
Table 2 Summary of the Cognitive Tests in the Intention-to-Treat Population |
We performed a subgroup analysis of age, MoCA-J score, and medication status. The analysis results are shown in Tables 3–5. The study of the subgroup of elderly participants (age ≥ 65 years) revealed a statistically significant treatment effect between the two groups with regard to construction (P = 0.049, d = 0.28) and orientation (P = 0.039, d = 0.34), as measured by the respective subscales of the ADAS-cog (Table 3). There were no significant differences between the groups in terms of other cognitive variables in the subgroup analysis by age. The P values for the interaction between treatment and age were 1.000 for construction and 0.869 for orientation. The study of the subgroup of cognitively healthy participants (MoCA-J score ≥ 26) revealed a statistically significant treatment effect for orientation (P = 0.029, d = 0.37) and HDS-R score (P = 0.033, d = 0.37) between the two groups (Table 4). There were no significant differences between groups in terms of the other cognitive variables in the subgroup analysis by MoCA-J score. The P values for the interaction between treatment and MoCA-J scores were 0.181 for orientation and 0.140 for the HDS-R score. The analysis of the subgroup “without medication” revealed a statistically significant treatment effect for orientation (P = 0.003, d = 0.62) between the two groups (Table 5). There were no significant differences between the groups in terms of the other cognitive variables in the subgroup analysis by medication status. The P value for the interaction between treatment and medication status was 0.235 for orientation. Table 6 presents the MCS and PCS values of SF-8 before and after daily intake of MKP or placebo. There was no significant MKP treatment effect on SF-8 compared to placebo.
 |
Table 3 Subgroup Analysis of the Cognitive Tests by Age |
 |
Table 4 Subgroup Analysis of the Cognitive Tests by MoCA-J Score |
 |
Table 5 Subgroup Analysis of the Cognitive Tests by Medication Status |
 |
Table 6 Summary of the SF-8 in the Intention-to-Treat Population |
Safety
As shown in Table 7, there was no significant difference between the groups in the incidence of AEs during the 24 weeks of treatment and 2 weeks of post-treatment observation. In total, 306 AEs were reported by the 243 participants throughout the study; 145 were reported by 114 participants in the MKP group and 161 by 129 participants in the placebo group. Upper respiratory infection was the most common AE (32.1% in the MKP vs 33.6% in the placebo group, P = 0.896). No AE was related to the study products.
 |
Table 7 Intervened Participants with Adverse Events by System Organ Class |
Discussion
In recent years, the search for the best strategy to reduce AD incidence and prevalence in cognitively healthy individuals at a risk of developing AD has attracted marked attention.8 In fact, a considerable body of epidemiological evidence supports that modifiable lifestyle-related factors are associated with the development of pre-dementia and dementia syndromes in later life.32 Furthermore, several healthy dietary plans and food compositions have preventive effects on cognitive decline.23,33-35 The present study showed that MKP supplementation may have the potential to improve orientation in community-dwelling adults without dementia, with good tolerability, and no treatment-related AEs, during the 24 weeks of treatment and 2 weeks after treatment. In addition, although our data were exploratory, the results of the pre-specified subgroup analyses suggested that the benefits of MKP may be more likely to appear in elderly individuals aged ≥ 65 years, those with healthy cognitive function, and those who do not use regular medications. Therefore, this study’s results suggested that MKP may be effective in treating individuals in the preclinical stage of AD or dementia, especially elderly people who are not suffering from any disease. Orientation is the ability to correctly identify one’s own location in space and time and serves as a useful indicator of cognitive decline.36 Therefore, systematic dietary supplementation with MKP may be a promising intervention toward safe and improved orientation before the onset of AD or dementia.
We previously described the effect of MKP, an ACE inhibitory peptide with the potential to cross the blood–brain barrier, on cognitive function in an AD mouse model induced by intracerebroventricular injection of amyloid-β (Aβ) 42 using the Morris water maze.22 In addition, the hippocampus was collected after behavioral testing, and inflammatory cytokine and nicotinamide adenine dinucleotide phosphate oxidase subunit expression were measured.22 Consequently, daily administration of casein hydrolysate containing MKP markedly attenuated Aβ42-induced cognitive decline and reduced Aβ42-induced tumor necrosis factor-α, monocyte chemoattractant protein-1, inducible nitric oxide synthase, p47phox, and gp91phox expression. A clinicopathological study of patients with AD found that spatial and temporal disorientations were associated with neurofibrillary tangle densities in the Brodmann areas 7 and 23, and the CA1 field of the hippocampus.37 Although the precise nature of the relationship between MKP and neurofibrillary tangles remains unknown, MKP may improve orientation by improving hippocampal function.
In this study, the ADAS-cog was used as the primary endpoint. Although the ADAS-cog is the gold standard for confirming general cognitive function in AD trials, it has been suggested to be less sensitive in individuals with normal cognitive function and MCI.38 In this study, the effect size of MKP intake on the ADAS-cog total score was estimated to be 0.40, whereas the actual effect size was 0.12 (Table 2). Therefore, it may be necessary to use more sensitive neuropsychological tests for cognitively healthy individuals and individuals with MCI to investigate the impact of MKP on cognitive function in more detail.
We previously reported that regular MKP intake was a safe and effective SBP-lowering treatment for individuals with high-normal BP or grade 1 hypertension in clinical trial.21 In this study, BP was measured only at baseline; thus, the relationship between the MKP effect on orientation and BP was not elucidated. Alternatively, other tests using the AD mouse model showed that MKP intake did not affect BP.22 Besides, BP before intervention in the present participants was not high (Table 1). Therefore, this result may be independent of the effect of MKP on BP. In the future, to clarify this mechanism of action, it will be crucial to examine how MKP intake affects BP and cognitive function.
Our study had several further limitations. First, considering the effects observed in this trial, the sample size might have been too small to fully evaluate the effect of MKP intake on cognitive function in adults without dementia. In addition, the intervention period was limited to a 24-week period and a single MKP-dose protocol. Furthermore, the MKP impact on patients with manifested dementia remains unknown. It may be necessary to conduct a larger-scale, longer-duration, and multi-dose intervention to detect the MKP effects on adults with and without dementia.
Conclusion
To the best of our knowledge, this is the first study to report the effects of MKP, a casein-derived ACE inhibitory peptide with the potential to cross the blood–brain barrier, on human cognitive function. The results of the present study suggested the safety of daily MKP intake and its potential to improve orientation in adults without dementia. However, this study was exploratory. Therefore, further studies are warranted to confirm these findings and the beneficial effects of MKP on cognitive function.
Data Sharing Statement
The datasets used in the present study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study protocol was examined and approved by the institutional review board and the Ethics Committee of Matsumoto Junior College (approval code: 201704). The study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labor and Welfare, Japan). After receiving a detailed explanation regarding the study objectives and procedures, all participants provided written informed consent and were informed that they were free to withdraw at any time without obligation. This trial was registered at the University Hospital Medical Information Network Clinical Trials Registry as UMIN000032833 on June 1, 2018.
Acknowledgments
We would like to thank the participants and staff, including Matsumoto City and Matsumoto Health Lab staff, for their cooperation in this study. We would also like to thank Dr. Ryuji Takeda of the Kansai University of Welfare Sciences for providing useful comments on the statistical analysis.
Funding
The study was financially supported by the Morinaga Milk Industry Co., Ltd., Tokyo, Japan, the manufacturer of the study substances. The funder did not play any role in the study design, data collection or analysis, decision to pub- 445 lish, or preparation of the manuscript.
Disclosure
NY, MT, KY, and FA are employed by Morinaga Milk Industry Co., Ltd., Tokyo, Japan. NS received a supervision fee from Morinaga Milk Industry Co., Ltd, Tokyo, Japan. The other authors have no conflicts of interest to report.
References
1. Global action plan on the public health response to dementia 2017 – 2025. Geneva: World Health Organization; 2017. Available from: http://www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/.
2. Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging. 2008;3(2):211–225.
3. Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer’s disease: past, present and future. Neuropharmacol. 2014;76(Part A):27–50. doi:10.1016/j.neuropharm.2013.07.004
4. Schneider LS, Mangialasche F, Andreasen N, et al. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med. 2014;275(3):251–283. doi:10.1111/joim.12191
5. Jack CR
6. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi:10.1016/j.jalz.2011.03.003
7. McDade E, Bateman RJ. Stop Alzheimer’s before it starts. Nature. 2017;547(7662):153–155. doi:10.1038/547153a
8. Crous-Bou M, Minguillón C, Gramunt N, Molinuevo JL. Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimers Res Ther. 2017;9:71. doi:10.1186/s13195-017-0297-z
9. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi:10.1016/S0140-6736(17)31363-6
10. Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM. Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimers Dis. 2011;26(4):699–708. doi:10.3233/JAD-2011-110347
11. O’Caoimh R, Healy L, Gao Y, et al. Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with Alzheimer’s disease. J Alzheimers Dis. 2014;40(3):595–603. doi:10.3233/JAD-131694
12. Miners JS, Ashby E, Van Helmond Z, et al. Angiotensin-converting enzyme (ACE) levels and activity in Alzheimer’s disease, and relationship of perivascular ACE-1 to cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2008;34(2):181–193. doi:10.1111/j.1365-2990.2007.00885.x
13. Dong YF, Kataoka K, Tokutomi Y, et al. Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer’s disease. FASEB J. 2011;25(9):2911–2920. doi:10.1096/fj.11-182873
14. Labandeira-Garcia JL, Rodríguez-Perez AI, Garrido-Gil P, Rodriguez-Pallares J, Lanciego JL, Guerra MJ. Brain renin-angiotensin system and microglial polarization: implications for aging and neurodegeneration. Front Aging Neurosci. 2017;9:129. doi:10.3389/fnagi.2017.00129
15. Sink KM, Leng X, Williamson J, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med. 2009;169(13):1195–1202. doi:10.1001/archinternmed.2009.175
16. Gao Y, O’Caoimh R, Healy L, et al. Effects of centrally acting ACE inhibitors on the rate of cognitive decline in dementia. BMJ Open. 2013;3(7):e002881. doi:10.1136/bmjopen-2013-002881
17. Fazal K, Perera G, Khondoker M, Howard R, Stewart R. Associations of centrally acting ACE inhibitors with cognitive decline and survival in Alzheimer’s disease. Br J Psych Open. 2017;3(4):158–164. doi:10.1192/bjpo.bp.116.004184
18. Martínez-Maqueda D, Miralles B, Recio I, Hernández-Ledesma B. Antihypertensive peptides from food proteins: a review. Food Funct. 2012;3(4):350–361. doi:10.1039/c2fo10192k
19. Yamada A, Sakurai T, Ochi D, Mitsuyama E, Yamauchi K, Abe F. Novel angiotensin I-converting enzyme inhibitory peptide derived from bovine casein. Food Chem. 2013;141(4):3781–3789. doi:10.1016/j.foodchem.2013.06.089
20. Yamada A, Sakurai T, Ochi D, Mitsuyama E, Yamauchi K, Abe F. Antihypertensive effect of the bovine casein-derived peptide Met-Lys-Pro. Food Chem. 2015;172:441–446. doi:10.1016/j.foodchem.2014.09.098
21. Yuda N, Tanaka M, Yamada A, et al. Antihypertensive effect of the casein-derived peptide Met-Lys-Pro in individuals with high-normal blood pressure or grade 1 hypertension: a randomized, double-blind, placebo-controlled, parallel-group trial. Jpn Pharmacol Ther. 2018;46(4):529–537.
22. Min LJ, Kobayashi Y, Mogi M, et al. Administration of bovine casein-derived peptide prevents cognitive decline in Alzheimer disease model mice. PLoS One. 2017;12(2):e0171515. doi:10.1371/journal.pone.0171515
23. Solfrizzi V, Panza F, Frisardi V, et al. Diet and Alzheimer’s disease risk factors or prevention: the current evidence. Expert Rev Neurother. 2011;11(5):677–708. doi:10.1586/ern.11.56
24. Mohs RC, Rosen WG, Davis KL. The Alzheimer’s disease assessment scale: an instrument for assessing treatment efficacy. Psychopharmacol Bull. 1983;19(3):448–450.
25. Homma A, Fukuzawa K, Tsukada Y, Ishii T, Hasegawa K, Mohs RC. Development of a Japanese version of the Alzheimer’s disease assessment scale (ADAS) [in Japanese]. Jpn J Geriatr Psychiatr. 1992;3(6):647–655.
26. Imai Y, Hasegawa K. The Revised Hasegawa’s Dementia Scale (HDS-R) - Evaluation of its usefulness as a screening test for dementia. J Hong Kong Coll Psychiatr. 1994;4(SP2):20–24.
27. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
28. Fujiwara Y, Suzuki H, Yasunaga M, et al. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr Gerontol Int. 2010;10(3):225–232. doi:10.1111/j.1447-0594.2010.00585.x
29. Ware JE, Kosinski M, Dewey JE, Gandek B. How to Score and Interpret Single-Item Health Status Measures: A Manual for Users of the SF-8 Health Survey. Lincoln, RI: QualityMetric, Inc., Boston; 2001.
30. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17(1):37–49. doi:10.1016/0022-3956(82)90033-4
31. Sugishita K, Sugishita M, Hemmi I, Asada T, Tanigawa T. A validity and reliability study of the Japanese version of the geriatric depression scale 15 (GDS-15-J). Clin Gerontol. 2017;40(4):233–240. doi:10.1080/07317115.2016.1199452
32. Solfrizzi V, Capurso C, D’Introno A, et al. Lifestyle-related factors in predementia and dementia syndromes. Expert Rev Neurother. 2008;8(1):133–158. doi:10.1586/14737175.8.1.133
33. Yurko-Mauro K, Alexander DD, Van Elswyk ME. Docosahexaenoic acid and adult memory: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0120391. doi:10.1371/journal.pone.0120391
34. Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. 2016;103(2):330–340. doi:10.3945/ajcn.115.124081
35. Wu L, Sun D. Meta-analysis of milk consumption and the risk of cognitive disorders. Nutrients. 2016;8(12):E824. doi:10.3390/nu8120824
36. Guerrero-Berroa E, Luo X, Schmeidler J, et al. The MMSE orientation for time domain is a strong predictor of subsequent cognitive decline in the elderly. Int J Geriatr Psychiatr. 2009;24(12):1429–1437. doi:10.1002/gps.2282
37. Giannakopoulos P, Gold G, Duc M, Michel JP, Hof PR, Bouras C. Neural substrates of spatial and temporal disorientation in Alzheimer’s disease. Acta Neuropathol. 2000;100(2):189–195. doi:10.1007/s004019900166
38. Kueper JK, Speechley M, Montero-Odasso M. The Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog): modifications and responsiveness in pre-dementia populations. A narrative review. J Alzheimers Dis. 2018;63(2):423–444. doi:10.3233/JAD-170991
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
