Back to Journals » Journal of Inflammation Research » Volume 15
Effect of Telmisartan and Quercetin in 5 Fluorouracil-Induced Renal Toxicity in Rats
Authors Ali HH , Ahmed ZA , Aziz TA
Received 7 September 2022
Accepted for publication 27 October 2022
Published 7 November 2022 Volume 2022:15 Pages 6113—6124
DOI https://doi.org/10.2147/JIR.S389017
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Harzhin Hiwa Ali,1 Zheen Aorahman Ahmed,2 Tavag Ahmed Aziz2
1Center of Diabetic and Endocrine Diseases, Sulaimani, Iraq; 2Department of Pharmacology and Toxicology, College of Pharmacy, University of Sulaimani, Sulaimani, Iraq
Correspondence: Zheen Aorahman Ahmed; Tavag Ahmed Aziz, Tel +9647709724959 ; +9647701523544, Email [email protected]; [email protected]
Purpose: The present study was designed to evaluate the possible synergistic effects of telmisartan and quercetin in 5 fluorouracil (5-FU) induced nephrotoxicity in rats.
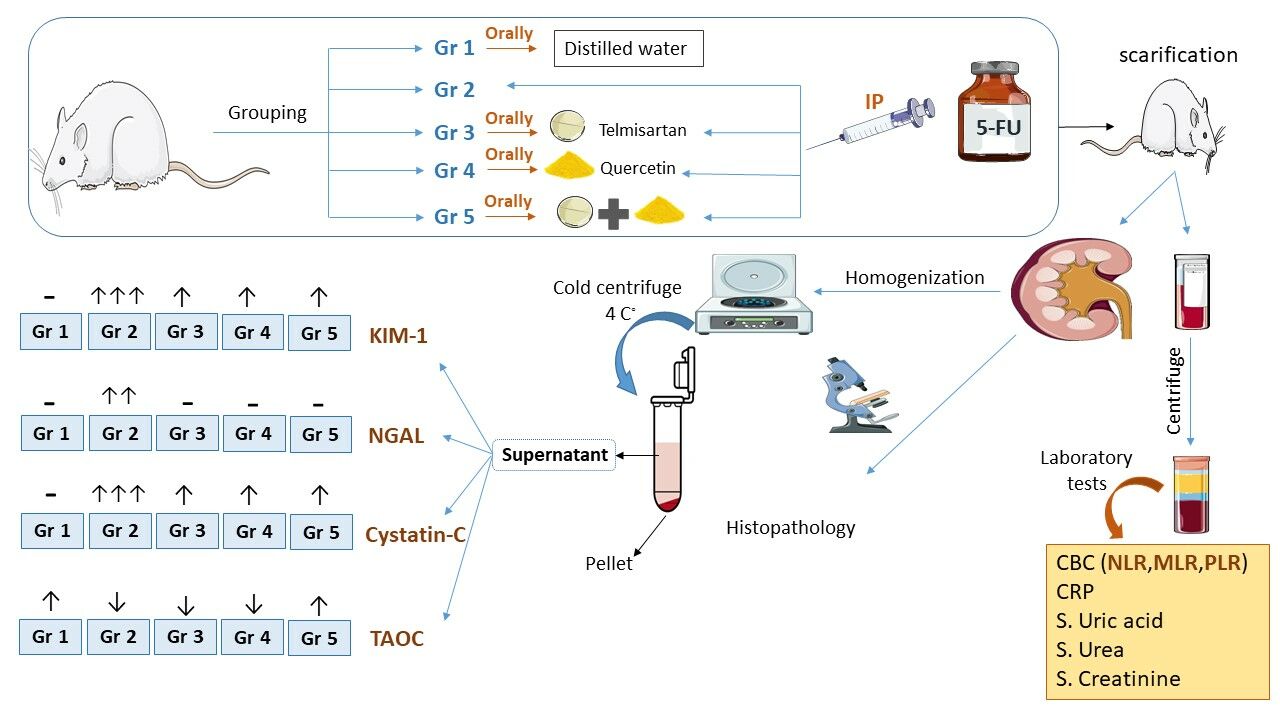
Methodology: Forty male rats were randomly divided into five groups: The negative control group, the positive control group that received 5-FU, the telmisartan group, receiving 10 mg/kg, the quercetin group, receiving 80 mg/kg, and the combination of telmisartan and quercetin group. All the treatments were given orally for 14 days. A single intraperitoneal injection of 5-FU (150 mg/kg) on day 13 of the experiment was given except for the negative control group. On the 15th day after scarification, approximately 5 mL of blood was collected and used for measurement of CBC, urea, creatinine, and uric acid. The kidneys were used for histopathological examination and for the measurement of kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), Cystatin C (Cys-C), and total antioxidant capacity (TAOC).
Results: The combination therapy significantly attenuated the levels of tissue KIM-1, NGAL, Cys-C, and serum uric acid as well as blood inflammatory markers, Neutrophil/Lymphocyte (NLR), Monocyte/Lymphocyte (MLR), and Platelets/Lymphocyte ratios (PLR), and restored the TAOC. The histopathological findings greatly support the biochemical tests.
Conclusion: The results strongly suggest the renoprotective effects of telmisartan and quercetin in combination against the nephrotoxic effect of 5-FU through decreasing the levels of KIM-1, NGAL, and cys-C, and the novel inflammatory markers of kidney injury like NLP, MLR, and PLR, as well as decreasing uric acid and restoring the TAOC. The proposed mechanism could be the additive inhibitory effect on RAS provided by both telmisartan and quercetin.
Keywords: nephrotoxicity, telmisartan, quercetin, inflammatory markers, KIM-1, NGAL
Graphical Abstract:
Introduction
Renal impairment may take the form of acute or chronic. Epidemiological studies show that drug-induced nephrotoxicity is the third most common cause of acute kidney disease,1 and it is one of the major contributing factors to chronic kidney disease and end-stage renal disease.2
The pivotal roles of the kidney including the secretion, reabsorption, filtration, bioactivation, and excretion of most of the substances that enter the biological system, render it to be a good target for many medications to induce their toxicity. The increased and sustained exposure to numerous medicines and drug metabolites can cause harm to the functional unit of the kidney, including the vascular element, tubules, and the glomeruli.3 5-FU, the uracil nucleotide analog, is one of the cytotoxic medications used in various types of cancers such as stomach, colorectal, and pancreatic cancers in addition to cervical and breast cancer.4 The toxicities of 5-FU appear in several organs such as the kidney,5,6 liver,7 CNS,8 and the heart.9 Many mechanisms have been proposed for the nephrotoxic effect of 5-FU; DNA damage and nontargeting apoptotic cell death via increasing caspase-3 activity in the kidneys are the most deleterious effect of 5-FU.10,11 Additionally, attenuating the kidney’s ability to scavenge and neutralize free radicals, particularly ROS, predisposes it to different types of oxidative stress assaults, which play a key role in triggering 5-FU-induced renal damage.6,12 Furthermore, triggering inflammatory response is another proposed mechanism of 5-FU-induced nephrotoxicity because it has been shown to increase activities of proinflammatory mediators such as tumor necrosis factor-alpha, interleukin-1, and interleukin-6 in the organs of rats treated with 5-FU.10,11
The local effect of the renin-angiotensin system (RAS) has taken pronounced attention, since the components of this system have been found in various tissues, suggesting their role in cellular growth and differentiation.13 Prorenin uptake and intrarenal activation of the kidney RAS is thought to be the cause of renal injury and microvascular alterations in the kidney.14 Angiotensin II may cause pressure-induced renal injury via its ability to induce systemic and glomerular hypertension or cause ischemia-induced renal injury secondary to intrarenal vasoconstriction and decreased renal blood flow.15 Targeting this system is one of the strategies for protecting renal damage. The suppression of RAS by angiotensin receptor blockers (ARBs) has been shown to have renoprotective benefits.16 Telmisartan is one of the ARBs, characterized by the longest half-life that extends to 24 hours and a high affinity to AT1 receptor when compared to other older ARBs.17,18
Combining ARBs with natural products like curcumin and resveratrol has been proven to be more successful in lowering metabolic disturbances, inflammation, insulin resistance (IR), hypertension, and a variety of other diabetic problems.19,20
Flavonoids found in medicinal plants, vegetables, and fruits have the potential to be a novel medication source. In this regard, quercetin (C15H10O7) is a well-known flavonoid with antihypertensive21 and renoprotective properties.22 Adjuvant use of quercetin with other medications like sitagliptin has proven to exert cardioprotective effects23 and protect against testicular damage induced by doxorubicin.24 Another study showed that the concomitant use of quercetin with valsartan was effective in enhancing intestinal absorption and attenuating valsartan outflow.25 All these effects, together with the approved regulatory effect on RAS offered by quercetin,26 suggest the use of quercetin with a drug like telmisartan for the possible synergistic renoprotective effects induced by 5-FU. Accordingly, the present study was designed to evaluate the effect of telmisartan and quercetin alone and in combination in a rat model of nephrotoxicity induced by 5-FU.
Materials and Methods
Experimental Animals
Male Wistar albino rats weighing 150–230 g were purchased from the animal house of the University of Tikrit. The rats were kept in the animal house of the College of Pharmacy/University of Sulaimani in a well-ventilated plastic cage, in normal conditions (temperature 25±2°C) and light–dark cycles. They were fed a standard pellet diet and had free access to water. The rats were acclimatized for 2 weeks before the beginning of the experiment. The experimental protocols met the Guidelines for Animal Experimentation and were approved by the ethical committee of the University of Sulaimani (Certificate no.6 on the date 14/11/2021) following the institutional Animal Ethics Committee. The study was performed by the Canadian Council on Animal Care (CCAC) guidelines.
Study Design
Forty fully-grown adult male rats were randomly divided into five groups, comprising eight animals each as follows:
Group 1: The negative control group received only 1 mL distilled water (D.W) by oral gavage for 14 days.
Group 2: The positive control group received 1 mL D.W by oral gavage for 14 days and a single intraperitoneal injection of 5-fluorouracil (150 mg/kg) on the 13th day of the experiment.
Group 3: The Telmisartan group received telmisartan (10 mg/kg) by oral gavage for 14 days with the protocol of 5-FU on the 13th day of the experiment.
Group 4: The Quercetin group received quercetin (80 mg/kg) by oral gavage for 14 days with the protocol of 5-FU on the 13th day of the experiment.
Group 5: The combination group received both telmisartan (10 mg/kg) and quercetin (80 mg/kg) by oral gavage for 14 days with the protocol of 5-FU on the 13th day of the experiment.
All institutional and national guidelines for the care and use of laboratory animals were followed. The doses of quercetin,27 telmisartan,28 and 5-FU29 were chosen depending on previous studies.
Measurement of the Weight and Relative Organ Weight of the Rats
The rats utilized in the experiment were weighed before starting the treatment and on scarification day, using a weight measurement scale. The kidneys were removed carefully, cleaned of all other tissue, and weighed. Relative organ weight was measured using the following equation:
Relative organ weight = Organ weight (g) x [1,000/Body weight (g)].
Tissue Homogenization and Blood Sample Collection
On the 15th day of the experiment the animals were anesthetized using chloroform. After euthanizing the animals, approximately 5 mL of blood was collected by cardiac puncture, the blood was placed in a tube and allowed to clot, then centrifuged at 3,000 rpm for 20 minutes and the separated serum directly went through laboratory testing.
Both kidneys were harvested: one of the kidneys was washed with ice-cold normal saline and put in 25 mL of 10% formaldehyde for later histopathological examination. The other kidney was removed and washed with ice-cold phosphate buffer saline PBS solution, then the whole kidney was weighed, dissected, and homogenized with every 1 g of the kidney, 9 mL of PBS was added according to the instruction from bioassay technology Elisa kits, homogenization was done with an homogenizer machine. The homogenized samples were immediately centrifuged at 5,000 g for 5 min in a cold centrifuge and the supernatant fluids were collected and stored at −80°C for later kidney biomarker analysis.
Measurement of Biochemical Markers
Determination of Renal Function, CRP, and CBC
Serum levels of blood urea nitrogen (BUN), creatinine (Cr), uric acid, and C-reactive protein (CRP) determined using Cobas c 311 analyzers for Clinical Chemistry and Homogeneous Immunology (HIA) and CBC were measured using an automated hematology analyzer.
Kidney-Specific Biomarker Analysis
The supernatant of kidney tissue homogenate was used using ELISA kits (Bioassay technology laboratory, Shanghai, China) for measuring KIM-1 (Cat.No E0549Ra), NGAL (Cat.No E0762Ra), Cys-C (Cat.No E0145Ra), and TAOC (Cat NoSH0242).
Histopathological Protocol
Initially, animals were euthanized in a humane practice, continuously, after animal sacrificing necropsy started by collecting tissue samples for histological preparation. Briefly, kidney samples were cut and immobilized into tissue cassettes and then fixed with 10% buffered formaldehyde solution for about 48 hours. Afterward, the sections were dehydrated by passing through a series of ascending concentrations of ethanol alcohol (50%, 60%, 70%, 80%, 90%, and 100%), followed by three phases of xylene clearance. Next, the processed kidney tissues were impregnated and embedded in melted paraffin casts using an automated wax embedder. Paraffinized tissues were sectioned to 6 µm using a semi-automated rotary microtome. Then after, tissue sections were fixed on glass slides and dried using a hot plate tissue holder. Later on, glass slides with their mounted tissue sections were deparaffinized and cleaned with two xylene changes then dried in a hot oven at 50°C for 15 minutes. Finally, tissue sections were stained with Harris’s hematoxylin and eosin solution, cleaned with xylene and cover slipped, then viewed under a bright field light microscope.
Quantitative Lesion Scoring
Lesion scoring was evaluated quantitatively via image analyzer software (AmScope, 3.7) using an ocular lens camera (MD500, 2019) fixed with a bright field light microscope (NOVEL XSZ-N107T, China). In kidney sections, renal tubular cellular degeneration was estimated and measured in the percentage of calculated cell numbers from randomly selected different fields, whereas vascular congestion was assessed in micrometers and statistically evaluated as the mean percentage. While inflammatory cells were counted in 10 randomly chosen fields under high power magnification (100X), the mean average was calculated statistically as a percentage. Lastly, necrotic cells with their pyknotic nuclei were counted from randomly selected fields, then evaluated as the calculated average mean percentage. In conclusion, the mean percentage of all calculated values was expressed as following the lesion scoring-grading system (score 0–10% as no lesions; score 10–25% as mild; score 25–50% as moderate; score 50–75% as severe; score 75–100 as critical).30
Statistical Analysis
The statistical analysis was performed using GraphPad Prism8. The values of the measured parameters were expressed as mean ± standard deviation (SD). For the comparisons between different groups, one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons test were performed. The results were considered statistically significant when the p-value was less than 0.05.
Results
Effect of Telmisartan and Quercetin Alone or in Combination on Total Weight and Relative Weight of the Rats
In the present study a significant reduction was seen in the positive control group in comparison with the negative control group, (p=0.0003). Meanwhile, telmisartan and quercetin each alone and in combination groups significantly increased the weight of the rats when compared to the positive control group (p=0.0028, 0.0057, and 0.0014, respectively) (Figure 1A). Regarding the relative weight; no significant changes were detected in any of the treatment groups (Figure 1B).
Effect of Telmisartan and Quercetin Alone or in Combination on Serum Levels of Urea, Creatinine, and Uric Acid
The use of 5-FU produced a significant increase in the serum level of urea in comparison with the negative control group (p=0.0008). Only quercetin alone produced a significant reduction when compared with the positive control group (p=0.015), (Figure 2A). Regarding the effect on serum creatinine, only telmisartan alone showed a significant reduction when compared to the positive control group (p=0.024) (Figure 2B). Serum uric acid significantly increased in the 5-FU treated group in comparison with the negative control (p=0.018). Both telmisartan and the combination group significantly decreased the level when compared with the positive control (p=0.0023 and 0.0013, respectively) (Figure 2C).
Effect of Telmisartan and Quercetin Alone or in Combination on CRP, Neutrophil/Lymphocyte (NLR), Monocyte/Lymphocyte (MLR), and Platelets/Lymphocyte Ratio (PLR)
C-reactive protein has been increased significantly (p=0.003) in the positive control group when compared to the negative control group, and all the treatment groups; telmisartan, quercetin, and the combination group resulted in significant attenuation of CRP level (p=0.001, 0.007, and 0.001, respectively) (Figure 3A). Meanwhile, significant elevation was observed in each NLR, MLR, and PLR in 5-FU treated group when compared with the negative control (p=0.012, 0.005, and 0.0001, respectively). The combination treatment was able to decrease NLR and MLR significantly in comparison with the positive control group (p=0.01 and 0.01, respectively) (Figure 3B and C). Concerning the PLR; all the treatment groups; telmisartan, quercetin, and the combination group, resulted in significant attenuation of PLR (p=0.02, 0.006, and 0.001, respectively) (Figures 3D).
Effect of Telmisartan and Quercetin Alone or in Combination KIM-1, NGAL, Cys-C, and TAOC Levels in Kidney Tissue
The use of 5-FU produced a significant increase in the level of KIM-1 in comparison with the negative control group (p<0.0001). All the treatment groups including telmisartan, quercetin alone, and in combination were able to produce a significant reduction in the KIM-1 value in the kidneys tissue when compared to the positive control group (p=0.0034, 0.0066, and 0.0004, respectively) (Figure 4A). Concerning NGAL level; 5-FU produced a significant increase when compared with the negative control group (p=0.01). Meanwhile, a significant decrease has been observed with telmisartan (p=0.008) and quercetin (p0.009), with a significant reduction in the combination group when compared to the positive control group (p=0.006) (Figure 4B). The tissue level of cystatin-C has increased significantly in the positive control group when compared to the negative control group (p=0.0001). Telmisartan alone decreased the level of Cys-C significantly (p=0.0022). Quercetin alone and the combination group were also able to decrease its level significantly (p=0.0009, and 0.0002, respectively) (Figure 4C). Regarding TAOC level in the tissue, a significant decline in its level has been achieved by the use of 5-FU when compared to the negative control group (p=0.046). Only the combination group was able to increase the level of TAOC significantly (p=0.047) (Figure 4D).
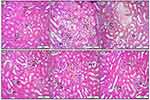
Histopathology Findings
Generally speaking, the quantitative morphometric assay and lesion scoring of kidney sections are illustrated in Table 1. Interestingly, animals in treatment group 5 (G5) which is a combination of telmisartan 10 mg/kg and quercetin 80 mg/kg, show enormous improvement in the abnormal morphological changes induced by a single dose of 5-FU 150 mg/kg, which is evident by a significant (p<0.05) reduction in the percentage of acute cellular swelling (hydropic degeneration) within the renal tubular epithelia, as well as remarkable mitigation in the number of necrotic cells, along with the number of inflammatory cells (Figure 5). Moreover, tissue sections from animals treated with telmisartan 10 mg/kg (G3) and quercetin 80 mg/kg (G4) alone show significant alleviation in lesion scoring in comparison with the positive control group G2 (5-FU 150 mg/kg a single dose) apparent by a clear reduction in the number of degenerative and necrotic cells as well as vascular disorders.
 |
Table 1 Histological Quantitative Evaluation of Kidney Sections |
Discussion
The use of herbal medicine as an adjuvant with other medications has widely increased nowadays, and the results are promising in the management of many diseases such as hypertension,31 diabetes mellitus,32 and rheumatoid arthritis.33 Alternative medicines have also shown great effectiveness when used as add-ons with cancer chemotherapeutic agents to ameliorate the toxicities associated with their use that directly contribute to the limited effectiveness.34 In the current study, combining quercetin with telmisartan aimed to attenuate the toxic effects produced by 5-FU; this effect appeared to maintainthe normal growth of the animals during the period of the study. A study revealed an increase in the levels of anabolic growth factors as well as protection against muscle damage in subjects who used quercetin for 2 weeks.35 Other studies showed the protective effect of quercetin against 5-FU-induced toxicities.36,37 Furthermore, another study conducted on the nephroprotective effect of telmisartan in diabetic rats showed noticeable maintenance of renal functions.38 In the present study, telmisartan significantly decreased the level of serum creatinine and this finding was in tune with another study.39 Meanwhile, quercetin maintained a normal function of the kidney by decreasing serum levels of urea. Another biomarker of kidney injury is the elevation of uric acid level. Uric acid buildup in the renal interstitium can draw in inflammatory cells, lead to persistent interstitial inflammation, and result in fibrosis.40 The significant increased which occurred in the level of uric acid by 5-FU was reversed by each of telmisartan and the combination group. High uric acid concentrations can encourage the generation of reactive oxygen species and oxidative stress in renal tubular epithelial cells, which can lead to a number of clinical consequences.41 A study has demonstrated the ability of quercetin to inhibit the activity of xanthine oxidoreductase and eventually decrease the production of uric acid.42 Additionally, telmisartan has been proven to attenuate the level of uric acid and its deleterious consequences.43
Inflammatory reaction plays a critical role in the pathogenesis of kidney damage and the condition is known as nephritis. An intricate web of interactions between renal parenchymal cells and local immune cells, such macrophages and dendritic cells, as well as the enlistment of circulating monocytes, lymphocytes, and neutrophils, constitutes inflammation, a condition closely related to renal illness.44 Monocytes are crucial for tissue remodeling, inflammation, and innate and adaptive immunity.45 Potential substitute indicators for inflammation include neutrophils and lymphocytes.46 The results of the current study clearly revealed significant elevation in the levels of CRP, NLR, MLR, and PLR in the 5-FU treated group. These inflammatory biomarkers are considered as novel predictors of acute kidney injury,47 and the combination therapy produced a maximum anti-inflammatory effect in this regard. This could be attributed to the documented anti-inflammatory effect of telmisartan48 and quercetin.49 This combination was also effective in attenuating the levels of KIM-1, NGAL, and cystatin C. KIM-1 is a membrane glycoprotein that serves as a new biomarker for kidney injury.50 The level of KIM-1 usually increases during toxic damage to the kidney.51,52 The use of telmisartan, quercetin, and the combination therapy was effective in attenuating the tissue level of KIM-1. Another biomarker of kidney injury is NGAL.53 Kidney damage produced by 5FU presented as high levels of kidney biomarkers such as KIM-1, NGAL, and cystatin C. All the treatment groups were able to ameliorate tissue level of NGAL with maximum effect achieved by the combination therapy. NGAL is produced by both kidney tubular cells and neutrophils during stress and inflammation. It’s been proposed as a biomarker for acute kidney damage.54 Another biomarker of renal function is cystatin-C.55 In the current study, 5FU markedly increased the level of cystatin C, quercetin and the combination groups were able to decrease its level. The synergistic effect of quercetin and telmisartan was also notified in maintaining the total antioxidant status in the present work. The effectiveness of telmisartan could be attributed to increased blood flow to the kidneys and antioxidant, anti-inflammatory, and antiapoptotic effects.56,57 Quercetin, on the other hand, has been shown in other studies to improve endothelial function by activating nitric oxide synthase,58 and through the interference with RAS59 via downregulating the expression of the Ag II 1a receptor.26 Additionally, the combination therapy was successful in maintaining the antioxidant capacity. The antioxidant capacity of quercetin is very well documented previously,60,61 and the nephroprotective effect of telmisartan is partially attributed to its ability to attenuate renal oxidative stress.56,62 Many studies proved the superiority of telmisartan as nephroprotective in comparison with other antihypertensive agents via protecting the renovascular function and improving atherosclerosis.63,64 Meanwhile a large body of evidence demonstrated the renoprotective effects of quercetin through anti-inflammatory activity and attenuation of apoptosis and fibrosis.65–67 The histopathological finding of the current study greatly supports the biochemical test; where a pronounced improvement has been observed in the combination therapy via clear improvement in the lesion scoring, tissue degeneration, and necrosis.
Conclusion
The results of the current study strongly demonstrate the renoprotective effects of the combination of telmisartan and quercetin against the cytotoxic effect of 5-FU via attenuating the levels of kidney damage specific markers such as KIM-1, NGAL, and cystatin C, and the novel inflammatory markers of kidney injury like NLR, MLR, and PLR as well as restoring total antioxidant capacity. Furthermore, the combination therapy also ameliorated tissue degeneration and lesion scoring in the histopathological finding. The proposed mechanism could be the additive inhibitory effect on RAS provided by both telmisartan and quercetin. These finding suggest the use of this combination as add-on therapy with 5-FU to minimize the toxic effects afforded by the later one.
Acknowledgments
The authors appreciate the College of Pharmacy, University of Sulaimani for its support and for providing facilities for this project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Teixeira G, Sales M, Foresto RD. Drug-induced nephrotoxicity. Rev Assoc Med Bras. 2020;66(1):82–90. doi:10.1590/1806-9282.66.S1.82
2. Wu H, Huang J. Drug-induced nephrotoxicity: pathogenic mechanisms, biomarkers and prevention strategies. Curr Drug Metab. 2018;19(7):559–567. doi:10.2174/1389200218666171108154419
3. Luciano RL, Perazella MA. Drug-induced acute kidney injury. In: Core Concepts in Acute Kidney Injury. New York, NY: Springer; 2018:145–163. doi:10.1007/978-1-4939-8628-6_9
4. Ison G, Beaver JA, McGuinn WD, et al. FDA approval: uridine triacetate for the treatment of patients following fluorouracil or capecitabine overdose or exhibiting early-onset severe toxicities following administration of these drugs. Clin Cancer Res. 2016;22(18):4545–4549. doi:10.1158/1078-0432.CCR-16-0638
5. Adikwu E, Ebinyo N, Amgbare B. Protective activity of selenium against 5-fluorouracil-induced nephrotoxicity in rats. Cancer Transl Med. 2019;5(3):50. doi:10.4103/CTM.CTM_26_19
6. Gelen V, Şengül E, Yıldırım S, Senturk E, Tekin S, Kükürt A. The protective effects of hesperidin and curcumin on 5-fluorouracil–induced nephrotoxicity in mice. Environ Sci Pollut Res. 2021;28(34):47046–47055. doi:10.1007/S11356-021-13969-5
7. Gelen V, Şengül E, Yıldırım S, Atila G. The protective effects of naringin against 5-fluorouracil-induced hepatotoxicity and nephrotoxicity in rats. Iran J Basic Med Sci. 2018;21(4):404–410. doi:10.22038/IJBMS.2018.27510.6714
8. Jules R, Maciel C, Busl K, et al. 5-fluorouracil neurotoxicity in patient without dihydropyrimidine dehydrogenase deficiency. Neurology. 2021;96(15Supplement):100.
9. Yuan C, Parekh H, Allegra C, George TJ, Starr JS. 5-FU induced cardiotoxicity: case series and review of the literature. Cardio-Oncology. 2019;5(1):1–7. doi:10.1186/S40959-019-0048-3/TABLES/3
10. Rashid S, Ali N, Nafees S, Hasan SK, Sultana S. Mitigation of 5-Fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in Wistar rats. Food Chem Toxicol. 2014;66:185–193. doi:10.1016/J.FCT.2014.01.026
11. Famurewa AC, Asogwa NT, Aja PM, et al. Moringa oleifera seed oil modulates redox imbalance and iNOS/NF-ĸB/caspase-3 signaling pathway to exert antioxidant, anti-inflammatory and antiapoptotic mechanisms against anticancer drug 5-fluorouracil-induced nephrotoxicity in rats. South African J Bot. 2019;127:96–103. doi:10.1016/J.SAJB.2019.08.038
12. Arab HH, Salama SA, Maghrabi IA. Camel milk ameliorates 5-fluorouracil-induced renal injury in rats: targeting MAPKs, NF-κB and PI3K/Akt/eNOS pathways. Cell Physiol Biochem. 2018;46(4):1628–1642. doi:10.1159/000489210
13. Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86(3):747–803. doi:10.1152/physrev.00036.2005
14. Ichihara A, Hayashi M, Kaneshiro Y, et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114(8):1128–1135. doi:10.1172/JCI21398
15. Long DA, Price KL, Herrera-Acosta J, Johnson RJ. How does angiotensin ii cause renal injury? Hypertension. 2004;43(4):722–723. doi:10.1161/01.HYP.0000120964.22281.3e
16. Burgess E, Muirhead N, De Cotret PR, Chiu A, Pichette V, Tobe S. Supramaximal dose of candesartan in proteinuric renal disease. J Am Soc Nephrol. 2009;20(4):893–900. doi:10.1681/ASN.2008040416
17. Burnier M. Telmisartan: a different angiotensin II receptor blocker protecting a different population? J Int Med Res. 2009;37(6):1662–1679. doi:10.1177/147323000903700602
18. Kakuta H, Sudoh K, Sasamata M, Yamagishi S. Telmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockers. Int J Clin Pharmacol Res. 2005;25(1):41–46.
19. Zhu H, Chen X, Cai G, et al. Telmisartan combined with probucol effectively reduces urinary protein in patients with type 2 diabetes: a randomized double-blind placebo-controlled multicenter clinical study. J Diabetes. 2016;8(5):677–685. doi:10.1111/1753-0407.12347
20. Hassanin AE, Elmasry K, Saleh DM, Bondok AA. Possible synergistic effect of curcumin on captopril /losartan combined therapy in diabetic nephropathy. Zagazig Univ Med J. 2022. doi:10.21608/ZUMJ.2022.111158.2433
21. Ożarowski M, Mikołajczak PL, Kujawski R, et al. Pharmacological effect of quercetin in hypertension and its potential application in pregnancy-induced hypertension: review of in vitro, in vivo, and clinical studies. Evid Based Complement Altern Med. 2018;2018:1–19. doi:10.1155/2018/7421489
22. Gomes IBS, Porto ML, Santos MC, et al. Renoprotective, anti-oxidative and anti-apoptotic effects of oral low-dose quercetin in the C57BL/6J model of diabetic nephropathy. Lipids Health Dis. 2014;13(1):1–10. doi:10.1186/1476-511X-13-184/FIGURES/6
23. Aziz TA. Cardioprotective effect of quercetin and sitagliptin in doxorubicin-induced cardiac toxicity in rats. Cancer Manag Res. 2021;13:2349–2357. doi:10.2147/CMAR.S300495
24. Ahmed ZA, Abtar AN, Othman HH, Aziz TA. Effects of quercetin, sitagliptin alone or in combination in testicular toxicity induced by doxorubicin in rats. Drug Des Devel Ther. 2019;13:3321–3329. doi:10.2147/DDDT.S222127
25. Challa VR, Ravindra Babu P, Challa SR, Johnson B, Maheswari C. Pharmacokinetic interaction study between quercetin and valsartan in rats and in vitro models. Drug Dev Ind Pharm. 2013;39(6):865–872. doi:10.3109/03639045.2012.693502
26. Mackraj I, Govender T, Ramesar S. The antihypertensive effects of quercetin in a salt-sensitive model of hypertension. J Cardiovasc Pharmacol. 2008;51(3):239–245. doi:10.1097/FJC.0B013E318162011F
27. Chen LI, Wu XI, Wang WE, Wang XI, Ma J. Quercetin with lycopene modulates enzymic antioxidant genes pathway in isoproterenol cardiotoxicity in rats. Libyan J Med. 2021;16(1). doi:10.1080/19932820.2021.1943924
28. Zhou X, Zezi MY, Li D, et al. Telmisartan ameliorates LPS-induced pneumonia in rats through regulation of the PPARγ/NF-κB pathway. Microbiol Immunol. 2022;66(7):371–378. doi:10.1111/1348-0421.12981
29. Al-Asmari AK, Khan AQ, Al-Masri N. Mitigation of 5-fluorouracil-induced liver damage in rats by Vitamin C via targeting redox-sensitive transcription factors. Hum Exp Toxicol. 2016;35(11):1203–1213. doi:10.1177/0960327115626583
30. Hadi Mohammed A, Obaid Hossain A, Gradus-Pizlo I, Hadi MA, Wozniak TC, Malik AS. Minar study the effects of anadrol overdose on kidney in rat model. Europace. 2013;15:11–17. doi:10.47832/2717-8234.11.28
31. Azizah N, Halimah E, Puspitasari IM, Hasanah AN. Simultaneous use of herbal medicines and antihypertensive drugs among hypertensive patients in the community: a review. J Multidiscip Healthc. 2021;14:259. doi:10.2147/JMDH.S289156
32. Aziz TA, Hussain SA, Mahwi TO, Ahmed ZA, Rahman HS, Rasedee A. The efficacy and safety of Ginkgo biloba extract as an adjuvant in type 2 diabetes mellitus patients ineffectively managed with metformin: a double-blind, randomized, placebo-controlled trial. Drug Des Devel Ther. 2018;12(4):735–742. doi:10.2147/DDDT.S157113
33. Ji JJ, Lin Y, Huang SS, Zhang HL, Diao YP, Li K. Quercetin: a potential natural drug for adjuvant treatment of rheumatoid arthritis. African J Tradit Complement Altern Med. 2013;10(3):418. doi:10.4314/ajtcam.v10i3.5
34. Hedigan K. Herbal medicine reduces chemotherapy toxicity. Nat Rev Drug Discov. 2010;9(10):765. doi:10.1038/nrd3280
35. Sgrò P, Ceci R, Lista M, et al. Quercetin modulates IGF-I and IGF-II levels after eccentric exercise-induced muscle-damage: a placebo-controlled study. Front Endocrinol. 2021;12:1412. doi:10.3389/FENDO.2021.745959/BIBTEX
36. Gelen V, Şengül E, Gedikli S, Atila G, Uslu H, Makav M. The protective effect of rutin and quercetin on 5-FU-induced hepatotoxicity in rats. Asian Pac J Trop Biomed. 2017;7(7):647–653. doi:10.1016/J.APJTB.2017.06.013
37. Lotfi M, Kazemi S, Ebrahimpour A, et al. Protective effect of quercetin nanoemulsion on 5-fluorouracil-induced oral mucositis in mice. J Oncol. 2021;2021:1–10. doi:10.1155/2021/5598230
38. Zhang Q, Xiao X, Li M, et al. Telmisartan improves kidney function through inhibition of the oxidative phosphorylation pathway in diabetic rats. J Mol Endocrinol. 2012;49(1):35–46. doi:10.1530/JME-12-0020
39. Najim SM, Ulaiwy MAA, Intesar T. Nephroprotective effects of artichoke extract against 5- fluorouracil induced nephrotoxicity in rats: a comparative study with telmisartan. Int J Pharm Sci Rev Res. 2018;48(1):70–74.
40. Tsai CW, Lin SY, Kuo CC, Huang CC. Serum uric acid and progression of kidney disease: a longitudinal analysis and mini-review. PLoS One. 2017;12(1):e0170393. doi:10.1371/JOURNAL.PONE.0170393
41. Verzola D, Ratto E, Villaggio B, et al. Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4. PLoS One. 2014;9(12):e115210. doi:10.1371/JOURNAL.PONE.0115210
42. Shi Y, Williamson G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: a randomised, double-blinded, placebo-controlled, cross-over trial. Br J Nutr. 2016;115(5):800–806. doi:10.1017/S0007114515005310
43. Zha D, Wu S, Gao P, Wu X. Telmisartan attenuates uric acid-induced epithelial-mesenchymal transition in renal tubular cells. Biomed Res Int. 2019;2019:1–12. doi:10.1155/2019/3851718
44. Andrade-Oliveira V, Foresto-Neto O, Watanabe IKM, Zatz R, Câmara NOS. Inflammation in renal diseases: new and old players. Front Pharmacol. 2019;10:1192. doi:10.3389/FPHAR.2019.01192/BIBTEX
45. Kratofil RM, Kubes P, Deniset JF. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol. 2017;37(1):35–42. doi:10.1161/ATVBAHA.116.308198
46. Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol. 2004;18(3):385–405. doi:10.1016/J.BPA.2003.12.002
47. Jiang F, Lei J, Xiang J, et al. Monocyte-to-lymphocyte ratio: a potential novel predictor for acute kidney injury in the intensive care unit. Ren Fail. 2022;44(1):1004. doi:10.1080/0886022X.2022.2079521
48. Klinghammer L, Urschel K, Cicha I, et al. Impact of telmisartan on the inflammatory state in patients with coronary atherosclerosis--influence on IP-10, TNF-α and MCP-1. Cytokine. 2013;62(2):290–296. doi:10.1016/J.CYTO.2013.02.001
49. Saeedi-Boroujeni A, Mahmoudian-Sani MR. Anti-inflammatory potential of Quercetin in COVID-19 treatment. J Inflamm. 2021;18(1):1–9. doi:10.1186/S12950-021-00268-6/FIGURES/1
50. Karmakova TA, Sergeeva NS, Kanukoev KY, Alekseev BY, Kaprin AD. Kidney injury molecule 1 (KIM-1): a multifunctional glycoprotein and biological marker (review). Mod Technol Med. 2021;13(3):64. doi:10.17691/STM2021.13.3.08
51. Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–4142. doi:10.1074/JBC.273.7.4135
52. Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286(3):F552–F563. doi:10.1152/AJPRENAL.00285.2002
53. Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(2):337. doi:10.2215/CJN.03530708
54. Törnblom S, Nisula S, Petäjä L, et al. Urine NGAL as a biomarker for septic AKI: a critical appraisal of clinical utility—data from the observational FINNAKI study. Ann Intensive Care. 2020;10(1):1–10. doi:10.1186/S13613-020-00667-7/FIGURES/3
55. Larki RA, Jamali B, Meidani M, Mousavi S. Serum cystatin C for evaluation of acute kidney injury in adults treated with colistin. J Res Pharm Pract. 2018;7(4):178. doi:10.4103/JRPP.JRPP_18_53
56. Fouad AA, Qureshi HA, Al-Sultan AI, Yacoubi MT, Al-Melhim WN. Nephroprotective effect of telmisartan in rats with ischemia/reperfusion renal injury. Pharmacology. 2010;85(3):158–167. doi:10.1159/000269779
57. Bin DS, Wang YH, Liu FY, et al. The protective role of telmisartan against nephrotoxicity induced by x-ray contrast media in rat model. Acta radiol. 2009;50(7):754–759. doi:10.1080/02841850902995544
58. Shen Y, Croft KD, Hodgson JM, et al. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem Pharmacol. 2012;84(8):1036–1044. doi:10.1016/J.BCP.2012.07.016
59. Häckl LPN, Cuttle G, Sanches Dovichi S, Lima-Landman MT, Nicolau M. Inhibition of angiotensin-converting enzyme by quercetin alters the vascular response to bradykinin and angiotensin I. Pharmacology. 2002;65(4):182–186. doi:10.1159/000064341
60. D’Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–271. doi:10.1016/j.fitote.2015.09.018
61. Zhang M, Swarts SG, Yin L, et al. Antioxidant properties of quercetin. Adv Exp Med Biol. 2011;701:283–289. doi:10.1007/978-1-4419-7756-4_38
62. Sugiyama H, Kobayashi M, Wang DH, et al. Telmisartan inhibits both oxidative stress and renal fibrosis after unilateral ureteral obstruction in acatalasemic mice. Nephrol Dial Transplant. 2005;20(12):2670–2680. doi:10.1093/NDT/GFI045
63. Morimoto S, Yano Y, Maki K, Sawada K. Renal and vascular protective effects of telmisartan in patients with essential hypertension. Hypertens Res. 2006;29(8):567–572. doi:10.1291/HYPRES.29.567
64. Nakamura T, Inoue T, Suzuki T, et al. Comparison of renal and vascular protective effects between telmisartan and amlodipine in hypertensive patients with chronic kidney disease with mild renal insufficiency. Hypertens Res. 2008;31(5):841–850. doi:10.1291/HYPRES.31.841
65. Andreucci M, Faga T, Pisani A, et al. Quercetin protects against radiocontrast medium toxicity in human renal proximal tubular cells. J Cell Physiol. 2018;233(5):4116–4125. doi:10.1002/JCP.26213
66. Almaghrabi OA. Molecular and biochemical investigations on the effect of quercetin on oxidative stress induced by cisplatin in rat kidney. Saudi J Biol Sci. 2015;22(2):227–231. doi:10.1016/J.SJBS.2014.12.008
67. Chen YQ, Chen HY, Tang QQ, et al. Protective effect of quercetin on kidney diseases: from chemistry to herbal medicines. Front Pharmacol. 2022;13:3304. doi:10.3389/FPHAR.2022.968226/BIBTEX
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.