Back to Journals » Infection and Drug Resistance » Volume 13
Effect of Sub-Minimum Inhibitory Concentrations of Tyrosol and EDTA on Quorum Sensing and Virulence of Pseudomonas aeruginosa
Authors Abdel-Rhman SH , Rizk DE , Abdelmegeed ES
Received 28 May 2020
Accepted for publication 11 September 2020
Published 8 October 2020 Volume 2020:13 Pages 3501—3511
DOI https://doi.org/10.2147/IDR.S264805
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Shaymaa H Abdel-Rhman,1,2 Dina E Rizk,1 Eman S Abdelmegeed1
1Department of Microbiology and Immunology, Faculty of Pharmacy, Mansoura University, Mansoura, Egypt; 2Department of Pharmaceutics and Pharmaceutical Biotechnology, Faculty of Pharmacy, Taibah University, AlMadinah Al Munawwarah, Saudi Arabia
Correspondence: Dina E Rizk
Department of Microbiology and Immunology, Faculty of Pharmacy, Mansoura University, Mansoura 35516, Egypt
Tel +201002605440
Fax +20502247496
Email [email protected]
Introduction: Pseudomonas aeruginosa is considered a dangerous pathogen, as it causes many human diseases, besides that it is resistant to almost all types of antibacterial agents. So, new strategies to overcome P. aeruginosa infection have evolved to attenuate its virulence factors and inhibit its quorum-sensing (QS) activity.
Purpose: This study investigated the effect of tyrosol and EDTA as anti-quorum-sensing and antivirulence agents against P. aeruginosa PAO1.
Methods: Anti-quorum activity of sub-minimum inhibitory concentrations (sub-MICs) of tyrosol and EDTA was tested using Chromobacterium violaceum (CV 12,472) biosensor bioassay. Miller assay was used to assess the inhibition of QS signal molecules by β-galactosidase activity determination. Also, their effects on the production of protease, lipase, lecithinase, and motility were tested. The inhibitory effects of these molecules on QS regulatory genes and exotoxins genes expression were evaluated by real-time PCR.
Results: Tyrosol and EDTA at sub-MICs inhibited the production of violacein pigment. Both compounds inhibited QS molecules production and their associated virulence factors (protease, lipase, lecithinase, and motility) (P≤ 0.05). Besides, the expression levels of QS regulatory genes (lasI, lasR, rhƖI, rhIR, pqsA, and pqsR) and exotoxins genes (exoS and exoY) were significantly reduced (P≤ 0.05).
Conclusion: Both tyrosol and EDTA can be used to fight P. aeruginosa infection as anti-quorum-sensing and antivirulence agents at their sub-MICs.
Keywords: PAO1, C. violaceum, signal molecules, real-time PCR, exotoxins, QS regulatory genes
Introduction
P. aeruginosa is an opportunistic Gram-negative bacterium that can cause several lethal infections including urinary tract, burn, respiratory tract and eye infections. Multidrug-resistant pathogens have emerged due to the unlimited use of antimicrobials. A new strategy to control the infections aims to affect and stop the adaptability of microbes to the host environment and prevent their communication with each other rather than affecting their growth.1,2 Quorum sensing (QS) is defined as communication between cells that relies on the cells density and the concentration of specific signaling molecules known as the autoinducers (AIs).2 AIs bind to their receptors forming a complex that will bind to a promoter and regulate the QS genes. P. aeruginosa contains four distinguished QS systems (Las, Rhl, Pqs and Iqs) that use AIs: N-oxododecanoyl-l-homoserine lactone (OdDHL or 3OC12-HSL), N-butanoyl-l-homoserine lactone (BHL or C4-HSL), the Pseudomonas quinolone signal (PQS), and the integrated quorum-sensing signal (IQS), respectively.1,3 Once significant levels of these signals were reached, they control the transcriptional regulatory protein LasR, RhIR, and Pqs then virulence factors transcription was enhanced.4
P. aeruginosa produces various pathogenicity factors and enzymes including pyocyanin, elastases, rhamnolipids, protease and extracellular exotoxin A that assist in microbial dissemination and interfere with the host immune system.5 Virulence factors expression, production of enzymes, cells adhesion and formation of biofilm are regulated by QS systems.6,7 Inhibitors of QS are an advanced strategy discovered to decrease P. aeruginosa pathogenesis and its virulence, so they can be used in treatment of its infections.8 QS system inhibitors act by different mechanisms including inhibitors of the AIs biosynthetic pathway, interference with the AIs extracellular accumulation (through using enzymes that degrade or modify the signals, and/or antibodies and synthetic polymers that segregate the signals), interference with signal detection (by using compounds that affect binding of signal to receptors), intervention with the binding of transcription factors to DNA, inhibition the quorum-sensing signal synthesis, and the use of structural analogs to the AIs.9
Natural compounds such as ellagic acid,10 ginseng,11 eugenol,12 xanthones,13 penicillanic acid,14 and different plant extracts15,16 inhibit QS associated virulence expression. Halogenated furanones which were isolated from certain macroalgae and different acyl-homoserine lactones (AHLs) analogs were found to inhibit QS signals.17 Also, Signal-degrading or modifying enzymes like lactonase, oxidase, and paraoxonase have been reported.18–20 In addition, vaccines that antagonize QS signaling molecules such as C12-HSL-BSA and C12-HSL-r-PcrV have been settled.21,22
Tyrosol or 2-(4-hydroxyphenyl)ethanol is a natural compound found in the human diet (olives and olive oil)23 and is produced by Candida albicans as QS molecules.24,25 It exhibits antioxidant, anti-inflammatory, anticancer, antitrypanosomal and antileishmanial activities.25 Besides, it has antivirulence activity against P. aeruginosa and Staphylococcus aureus.24,26 It has a potent antibacterial activity that was explained by its ability to bind and inhibit bacterial ATP synthase.27 Ethylenediaminetetraacetic acid (EDTA) which is a polyamine carboxylic acid was utilized as a food preservative,28 metal chelator, anticoagulant29 and in combination with antibiotics and vitamins for several diseases therapy.30
This research was conducted to evaluate the anti-quorum-sensing and antivirulence activity of sub-MICs of tyrosol and EDTA and to investigate their effect on the expression levels of QS-regulatory genes and some virulence factors production.
Materials and Methods
Bacterial Strains, Culture Conditions, and Reagents
C. violaceum (CV 12,472) is a wild-type strain that produces quorum-sensing controlled purple pigment, violacein. It responds to AIs molecules C4 and C1 AHLs. P. aeruginosa PAO1, wild-type strain, was used to test QS and virulence factors. P. aeruginosa PAO-JP2 (QS deficient) (ΔLasI: Tn 10, Tcr, ΔrhII: Tn 501, Hgr) was used as QS negative control. Escherichia coli MG4/pkDT17 (Las reporter, LasB: LacZ plac-LasR; Apr) and Escherichia coli DH5α/pECP61.5 (rhI reporter, rhID rhIA: LacZ Apr) were used to test QS signal molecules. All strains were grown in LB broth/agar at 37°C for 16–18 h except for C. violaceum that was incubated at 28°C.
Tyrosol (Sigma number 79,058) and EDTA (Sigma number 27,285) were used in the preparation of stock solution (500 mM) in water and stored at −20° C.
This research was exempt from approval by the ethics committee of faculty of Pharmacy, Mansoura University, Egypt as it was conducted using reference bacterial strains.
Determination of Minimum Inhibitory Concentrations of Tyrosol and EDTA
The MIC of tyrosol and EDTA were determined by the broth microdilution method.31 Briefly, P. aeruginosa PAO1 overnight culture in LB medium was adjusted to OD600 nm=0.08 to 0.1 and mixed with two-fold serial dilutions of tyrosol (250–16,000 µg mL−1) and EDTA (39–5000 µg mL−1) in 96-well microtiter plate. The plates were incubated at 37°C and the MIC was calculated as the lowest concentration that shows no visible growth of the organism. Sub-MICs (1/2, 1/4, 1/8, 1/16, and 1/32x MIC) were used for further experiments.
Determination of Anti-Quorum-Sensing Activity of Tyrosol and EDTA
The anti-quorum-sensing activity of tyrosol and EDTA was determined against C. violaceum ATCC 12,472 by agar well diffusion assay.32 Briefly, 50 µL of sub-MICs (1/2x −1/32x MIC) of tyrosol and EDTA were added in wells made into LB agar plates that were seeded with C. violaceum (0.5 McFarland) then incubation at 30°C for 48 h was done. The pigment production inhibition without exhibiting antibacterial activity around the well was checked.
Effect of Sub-MICs on the Viability of P. aeruginosa
To determine the non-inhibitory action of tyrosol and EDTA sub-MICs on P. aeruginosa growth; MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reduction assay was conducted on tyrosol and EDTA treated and untreated cells with the predetermined concentrations. Briefly, suspensions of tested strains (100µL) in phosphate-buffered saline (PBS) containing 0.2% glucose were dispensed in 96-well plates with sub-MICs of tyrosol and EDTA in PBS, incubated at 37°C for 24 h. Then, 10 μL of MTT solution (5 mg/mL) was added to the wells and plates were incubated at 37°C for 30 min with shaking. For solubilization of the formed formazan, 100 μL of dimethylsulfoxide (DMSO) solution was added then plates were left for 3 h at room temperature. Absorbance (A540) was recorded using a microtiter plate reader (BioTek ELx800).26,33,34
Inhibition of QS Signal Molecule
Supernatants of P. aeruginosa PAO1 overnight cultures treated with sub-MICs (1/4x −1/32x MIC) of tyrosol and EDTA, in addition to the supernatant of untreated cells and PAO1-JP2 (a negative control) were prepared as mentioned previously by Abdel-Rhman and Rizk.26 Miller assay was used to measure AHLs by β-galactosidase activity determination,35,36 utilizing E. coli DH5α/pECP61.5 and E. coli MG4/pkDT17 for measuring C4-HSL and C12-HSL, respectively.
Effect of Tyrosol and EDTA on Pseudomonas Virulence Factors
The effectiveness of tyrosol and EDTA at sub-MICs on the production of total protease, lipase, and lecithinase enzymes by P. aeruginosa PAO1 was detected. This was conducted as described previously by utilizing the modified skim milk assay for measuring total protease,26 p-nitrophenyl palmitate as the substrate for measuring lipase,24 and egg-yolk tellurite emulsion for lecithinase.26
Motility Assays
The swarming, swimming and twitching motilities of P. aeruginosa were measured in the presence or absence of tyrosol and EDTA sub-MICs as previously described.2,6 Reduction in the motility of P. aeruginosa indicated QS inhibitory activity of the used compounds.
Quantitative Real-Time PCR
P. aeruginosa was treated with tyrosol and EDTA sub-MICs, then TRI Reagent (T9424 Sigma-Aldrich) was used to extract the total RNA which was converted into cDNA using QuantiTect Reverse Transcription kit (QIAGEN, USA) as previously described.33 The level of (lasR, lasI, rhƖI, rhIR, pqsR, pqsA, exoS, and exoY) genes expression was measured by RT-PCR utilizing the primer sequences which are listed in Table 1. rpoD gene was used as an internal P. aeruginosa housekeeping gene. The reaction mixture was prepared using 5x FIREPol EvaGreen, qPCR Mix, ROX Dye; Solis BioDyne; Tartu, Estonia; and the program was run as described previously,37 using Rotor-Gene Q thermocycler (QIAGEN, Hilden, Germany). The level of expression of the tested genes was measured in treated relative to untreated samples using the 2−ΔΔCt method.33 Expression in the PAO1-JP2 strain was also assessed.
 |
Table 1 The Sequence of Primers Used in the RT-PCR Amplification |
Statistical Analysis
Results were analyzed using GraphPad Prism 5. One-way analysis of variance (ANOVA) for multiple comparisons, followed by Dunnett's posttest to compare the treated isolates versus the control group. A P value of ≤0.05 was considered statistically significant.
Results
Screening of Anti-Quorum-Sensing Activity of Sub-MICs of Tyrosol and EDTA
The MICs of tyrosol and EDTA were determined to estimate the effect of their sub-MICs on bacterial growth, virulence factors’ production, and expression of QS genes. The MICs of tyrosol and EDTA against P. aeruginosa PAO1 were 4000 µg mL−1 and 1250 µg mL−1, respectively. The sub-MICs of tyrosol and EDTA were used throughout the study.
The quorum-sensing inhibitory (QSI) activity of sub-MICs of tyrosol and EDTA were evaluated against C. violaceum ATCC 12,472 by agar well diffusion assay. The 1/2x MIC of either tyrosol or EDTA inhibited the production of the purple pigment violacein (QS system indicator) and inhibited the bacterial growth. The other used concentrations (1/4x −1/32x MIC) were found to inhibit the violacein pigment production without inhibiting bacterial growth indicating their anti-quorum-sensing activity.
Effect of Sub-MICs of Tyrosol and EDTA on the Viability of P. aeruginosa.
The sub-MICs (1/4x −1/32x MIC) of tyrosol and EDTA were tested for their effect on P. aeruginosa PAO1 viability by MTT reduction assay. The results showed that the viability of treated cells with these compounds was not significantly affected as compared to the untreated cells over the whole course of growth indicating that these compounds did not affect the metabolic activity of the strain.
Effect of Tyrosol and EDTA on Quorum-Sensing Signals
The ability of tyrosol and EDTA at sub-MICs to inhibit QS signal molecules (C4-HSL and C12-HSL) was tested using reporter strain assay. Untreated PAO1 cells produce high levels of C4-HSL (2707 Miller units) and C12-HSL (11,120 Miller units). The P. aeruginosa double mutant PAO-JP2 did not produce any detectable quantity of QS molecules. On the other hand, tyrosol (Figure 1A) and EDTA (Figure 1B) treated P. aeruginosa cells exhibited a significant reduction in C4-HSL (P< 0.001) and C12-HSL (P< 0.05).
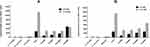 |
Figure 1 Effect of sub-MICs of tyrosol (A) and EDTA (B) on quorum-sensing signals molecules using reporter strain assay. |
The production of C4-HSL was decreased by 72%, 60%, and 39% with 1/4x, 1/8x, and 1/16x MIC of tyrosol, respectively. While with EDTA, the reduction was 70%, 41%, and 22% with 1/4x, 1/8x, and 1/16x MIC, respectively.
The C12-HSL level was also decreased with all used concentrations of tyrosol and EDTA (P-value<0.001). The reduction was 75% by 1/4x, 1/8x, and 1/16x MIC and 60% by 1/32x MIC of tyrosol. EDTA reduced it by 72%, 70%, 63%, and 40% for 1/4x, 1/8x, 1/16x and 1/32x MIC, respectively.
Effect of Tyrosol and EDTA on the Production of Some Virulence Factors
Tyrosol at 1/4x and 1/8x MIC can significantly (P<0.001) reduce the total protease production by 80% and 52%, respectively (Figure 2A). EDTA can only decrease the protease production by 35% at 1/4x MIC (P<0.05) (Figure 2B). Interestingly, tyrosol and EDTA can significantly decrease lipase production by ≥63% at sub-MICs (1/4x, 1/8x, and 1/16x MIC) (P<0.001). EDTA gave a higher effect than tyrosol as the reduction ranged between 81% and 73%. EDTA (1/4x and 1/8x MIC) significantly reduced lecithinase production (P<0.05).
 |
Figure 2 Effect of sub-MICs of tyrosol (A) and EDTA (B) on the production of lipase, protease, and lecithinase enzymes of PAO1. |
Effect of Tyrosol and EDTA on Motility
The motility (swimming, swarming, and twitching) of PAO1 was measured in the presence and absence of tyrosol and EDTA. Tyrosol can significantly reduce the swarming motility at 1/4x, 1/8x (P<0.0001), and 1/16x MIC (P-value<0.05). EDTA (1/4x and 1/8x MIC) significantly decreased both swimming (P=0.001) and swarming motility (P<0.0001 and P<0.05, respectively). Both tyrosol and EDTA decreased twitching motility but non-significantly as illustrated in Table 2.
 |
Table 2 Comparison of the Swimming, Swarming and Twitching Motilities Which Were Shown by PAO1 and PAO-JP2 and Treated PAO1 with Sub-MICs of Tyrosol and EDTA |
Real-Time PCR Analysis of P. aeruginosa Exotoxins and QS Regulatory Genes
The expression level of QS regulatory genes was significantly reduced by 80–50% by tyrosol (1/4x and 1/8x MIC). Tyrosol (1/4x MIC) reduced the expression of LasI and LasR by 75% and 80%, respectively; while (1/8x MIC) decreased their expression by 60% and 64%, respectively (Figure 3A). For RhƖI/RhIR, tyrosol at 1/4x MIC reduced their expression by 78% and 75%, respectively; while at 1/8x MIC, they were reduced by 60% and 63%, respectively (Figure 3B). PqsA and PqsR were also decreased by tyrosol; 1/4x MIC caused a reduction in expression of PqsA and PqsR by 64% and 70%, respectively, while 1/8x MIC caused reduction by 50% and 57%, respectively (Figure 3C).
EDTA (1/4x and 1/8x MIC) decreased the expression of LasI by 73% and 64%, respectively, and LasR by 75% and 47%, respectively (Figure 3A). While for the other genes, only 1/4x MIC EDTA can significantly reduce the expression of rhƖI, rhIR (Figure 3B), pqsA and pqsR (Figure 3C) by 71%, 73%, 51%, and 58%, respectively.
The reduction in the QS genes expression level indicated the inhibitory activity of tyrosol and EDTA on the transcriptional level of these genes. For exotoxins, our results revealed that only EDTA (1/4x MIC) resulted in a significant reduction (P< 0.05) in the expression level of ExoS and ExoY (80% and 40%, respectively).
Discussion
The increased prevalence of antibiotic resistance in Gram-negative bacteria necessitates finding new strategies for bacterial infections treatment.38 New approaches focused on targeting Gram-negative virulence factors to decrease their pathogenicity without causing drug resistance such as inhibition of quorum sensing.39 QS regulates many bacterial physiological processes including virulence characteristics, production of proteolytic enzymes and motility.40 Attenuation of microbial virulence can be achieved by disruption of this communication system.41,42 P. aeruginosa is considered a highly pathogenic bacterium because of its high resistance to many classes of antibiotics and development of intrinsic, acquired and adaptive resistance mechanisms,43,44 as well as secretions of several virulence factors.45
A new anti-Pseudomonas strategy is targeting QS to reduce its pathogenicity without affecting microbial resistance inducement.46 Various QS inhibitors have been reported including natural47 or synthetic compounds.17 Several previous studies reported that sub-MICs of ciprofloxacin, ceftazidime, and azithromycin reduces the QS of P. aeruginosa.47,48 Other studies indicated that tyrosol, one of major olive oil phenolic compounds, has potent activity against many bacteria causing respiratory and intestinal infections.49 EDTA was also reported to have an antibacterial activity by destroying the outer membrane of bacterial cells,50 and prevention of biofilm formation by chelation of several divalent cations which are essential for stabilization of the biofilm.51
This is the first study to investigate the effect of tyrosol and EDTA on QS. Our results showed that tyrosol and EDTA have anti-QS activity at sub-MICs when they were screened using C. violaceum (CV 12,472) biosensor bioassay. The selected tested concentrations (1/4x −1/32x MIC) inhibited AHL-mediated violacein production without any bactericidal effect on the cells. To evaluate the impact of tyrosol and EDTA on QS signals and the associated virulence factors of P. aeruginosa PAO1 and to be sure that the results are not attributed to a decrease in the cell viability, we studied the effect of sub-MICs on the metabolic activity of P. aeruginosa PAO1. Interestingly, no decrease in the viability of tested strains was observed compared to the control untreated cells. This finding was consistent with previous studies reported by Abdel-Rhman et al, 2015 and Abdel-Rhman and Rizk, 2016 who found the same effectiveness of tyrosol sub-MICs on the viability of P. aeruginosa and Staphylococcus aureus, respectively.24,26 Also, Aboelenin et al, 2017 reported a similar effect of sub-MICs of EDTA on the viability of E. coli.51
Many studies reported that several antibacterial agents (tobramycin,52 ciprofloxacin,53 azithromycin,54 ceftazidime,48 aminoglycosides, tetracyclines55 and piperacillin-tazobactam56), natural plant-derived compounds as gall extract2,57 and onion extract58 and drugs such as aspirin37 can decrease QS signals (HSL) in P. aeruginosa at sub-MICs without any bactericidal effect. Our study showed the same result whereas both tyrosol and EDTA at sub-MICs verified a significant reduction of C4-HSL and C12-HSL signal molecules production in P. aeruginosa PAO1.
P. aeruginosa produces various exotoxins and enzymes that have important role in tissue injury and causing dissemination of the infection. QS coordinates its virulence factors release.59 So, inhibition of C4-HSL and C12-HSL signals in P. aeruginosa leads to a marked reduction in its QS mediated virulence factors. For this reason, we evaluated the effect of tyrosol and EDTA sub-MICs on the production of some QS regulated virulence factors in P. aeruginosa PAO1 strain.
Proteases, lipases, and lecithinase are considered to be remarkable virulence factors of P. aeruginosa because they have an important role in Pseudomonas pathogenesis.60 In the present research, we reported that EDTA and tyrosol at sub-MICs significantly quenched the production of protease. Also, sub-MICs of both compounds significantly reduced the production of lipase enzyme which consequently results in a decreased pathogenicity of the bacterial cells. In accordance with our results, previous studies reported that EDTA had an inhibitory effect on lipase activity.61
A significant reduction in lecithinase production was observed only with 1/4x and 1/8x MIC of EDTA. These findings were similar to what was reported by previous studies on other compounds such as that conducted by Mattmann and Blackwell about furanone C-30,62 Musthafa et al about phenylacetic acid,63 Quecan et al about onion extract58 and Ahmed et al about cinnamaldehyde and salicylic acid.64
Motility is an important factor in both adhesion and biofilm formation.65 P. aeruginosa has three types of motility that are swimming, swarming and twitching which are positively regulated by both LasIR and RhIR QS systems, where las control swimming, swarming and twitching and rhI regulates swarming and twitching.66 In this study both swimming and swarming motility were significantly attenuated by tyrosol (1/4x - 1/16x MIC) and EDTA (1/4x and 1/8x MIC). However, both compounds demonstrated no significant reduction of twitching motility. The effect of different compounds on motility varied. Similar to our results, berberine was reported to significantly reduce both swimming and swarming without affecting twitching motility.67 While azithromycin was reported to affect only swimming motility,54 other compounds such as tobramycin,52 FL fraction of Psidium guajava68 and clove oil69 impair swarming motility. Abbas et al reported a similar effect of Sitagliptin as QS inhibitor of P. aeruginosa and it blocked its twitching motility.70 Gupta et al reported that ciprofloxacin can significantly reduce all three types of motility.53
In addition to the role of sub-MICs of tyrosol and EDTA in inhibiting virulence production, it was found that both compounds inhibited the expression of QS regulatory and exotoxin genes. The expression level of lasR, lasI, rhƖI, rhIR, pqsR and pqsA genes was significantly inhibited by tyrosol (1/4x and 1/8x MIC) and EDTA (1/4x MIC), while EDTA (1/8x MIC) significantly reduced the expression of lasR and lasI genes only. This result was following that found by Ouyang et al and Okusa et al who reported the significant inhibition of QS-related genes expression by quercetin (a naturally occurring flavonol) and C. gilletii extracts, respectively.71,72
The type III secretion system (T3SS) in P. aeruginosa includes four exoenzymes S, U, T, and Y which lead to cell death by the destruction of cellular machinery.73 In the current study, the expression level of exoS and exoY genes was significantly reduced by EDTA (1/4x MIC) only. Similarly, naringenin down-regulated type III secretion system genes expression.74 Although the expression of T3SS in P. aeruginosa is negatively regulated by RhlI/RhlR system,75,76 our results showed that EDTA repressed both RhlI/RhlR system and T3SS exotoxins which suggests the QS independent decrease of their secretion. In agreement, Zhang et al and Kim et al reported that coumarin and 6-gingerol, respectively, are QS inhibitors that repressed T3SS genes.77,78
P. aeruginosa combines several QS systems to integrate different signals hierarchically in which four QS systems act in a network.79 The Las system is at the top of the signaling cascade since LasR/3O-C12-HSL controls the expression of the Rhl and Pqs systems.80 Tyrosol was found to provide two types of interactions with the LasR receptor, two hydrogen bonds, and the π-π interaction, while EDTA exhibited five intermolecular hydrogen bonds by molecular docking using MOE v102015.10 software (Data not shown). These interactions between tyrosol and EDTA with the LasR receptor may aid in an explanation of their QS inhibitory effect. Accordingly, tyrosol and EDTA may act by targeting the Las system firstly then the following cascade includes RhI and Pqs systems and as a consequence, the virulence factors production under their control will be reduced. The other possible QS inhibition mechanisms of these compounds necessitate further investigation in future studies.
Conclusion
Our data illustrated that tyrosol and EDTA reduced the production of QS controlled virulence factors (protease, lipase, lecithinase, and motility) and T3SS exotoxins (exoS and exoY). Also, they downregulated the key genes involved in QS (lasI, lasR, rhƖI, rhIR, pqsR and pqsA). Also, this is the first study that indicates the potential effect of tyrosol and EDTA at sub-MICs as anti-quorum-sensing and antivirulence compounds in P. aeruginosa. So the present data suggest the possible application of EDTA and tyrosol in P. aeruginosa infections therapy. Further investigations are required to confirm such inhibitory effects in clinical isolates, study their effect on other virulence factors, and the exact mechanism of such inhibitory action at the molecular level.
Acknowledgments
The authors are grateful to Dr. Mona I. Shaaban (Faculty of Pharmacy, Mansoura University, Egypt) who kindly provided standard strains used in this study. We also thank Dr. Youssif M. Ali (School of Biological Science, University of Cambridge, United Kingdom) who kindly copyedited the manuscript for language usage, spelling, and grammar.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Scoffone VC, Trespidi G, Chiarelli LR, Barbieri G, Buroni S. Quorum sensing as antivirulence target in cystic fibrosis pathogens. Int J Mol Sci. 2019;20:8. doi:10.3390/ijms20081838
2. Ahmed AA, Salih FA. Quercus infectoria gall extracts reduce quorum sensing-controlled virulence factors production and biofilm formation in Pseudomonas aeruginosa recovered from burn wounds. BMC Complement Altern Med. 2019;19(1):177. doi:10.1186/s12906-019-2594-5
3. D’Angelo F, Baldelli V, Halliday N, et al. Identification of FDA-approved drugs as antivirulence agents targeting the pqs quorum-sensing system of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2018;62:11.
4. Toder DS, Ferrell SJ, Nezezon JL, Rust L, Iglewski BH. lasA and lasB genes of Pseudomonas aeruginosa: analysis of transcription and gene product activity. Infect Immun. 1994;62(4):1320–1327. doi:10.1128/IAI.62.4.1320-1327.1994
5. Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76(1):46–65. doi:10.1128/MMBR.05007-11
6. Bahari S, Zeighami H, Mirshahabi H, Roudashti S, Haghi F. Inhibition of Pseudomonas aeruginosa quorum sensing by subinhibitory concentrations of curcumin with gentamicin and azithromycin. J Glob Antimicrob Resist. 2017;10:21–28. doi:10.1016/j.jgar.2017.03.006
7. Bala A, Kumar R, Harjai K. Inhibition of quorum sensing in Pseudomonas aeruginosa by azithromycin and its effectiveness in urinary tract infections. J Med Microbiol. 2011;60(Pt 3):300–306. doi:10.1099/jmm.0.025387-0
8. Rasmussen TB, Givskov M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol. 2006;296(23):149–161. doi:10.1016/j.ijmm.2006.02.005
9. Fleitas Martinez O, Rigueiras PO, Pires ADS, et al. Interference with quorum-sensing signal biosynthesis as a promising therapeutic strategy against multidrug-resistant pathogens. Front Cell Infect Microbiol. 2018;8:444. doi:10.3389/fcimb.2018.00444
10. Sarabhai S, Sharma P, Capalash N. Ellagic acid derivatives from Terminalia chebula Retz. Downregulate the expression of quorum sensing genes to attenuate Pseudomonas aeruginosa PAO1 virulence. PLoS One. 2013;8(1):e53441. doi:10.1371/journal.pone.0053441
11. Schneper L, Maricic N, Mathee K. Anti-quorum sensing, anti-bacterial, and immunomodulatory properties of Panax ginseng. Int J Res Pharm Biomed Sci. 2012;6:11–24.
12. Zhou L, Zheng H, Tang Y, Yu W, Gong Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol Lett. 2013;35(4):631–637. doi:10.1007/s10529-012-1126-x
13. Mohamed GA, Ibrahim SR, Shaaban MI, Ross SA. Mangostanaxanthones I and II, new xanthones from the pericarp of Garcinia mangostana. Fitoterapia. 2014;98:215–221. doi:10.1016/j.fitote.2014.08.014
14. Rasmussen TB, Skindersoe ME, Bjarnsholt T, et al. Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology. 2005;151(Pt 5):1325–1340. doi:10.1099/mic.0.27715-0
15. Adonizio A, Kong KF, Mathee K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother. 2008;52(1):198–203. doi:10.1128/AAC.00612-07
16. Koh CL, Sam CK, Yin WF, et al. Plant-derived natural products as sources of anti-quorum sensing compounds. Sensors. 2013;13(5):6217–6228. doi:10.3390/s130506217
17. Wang Y, Ma S. Small molecules modulating AHL-based quorum sensing to attenuate bacteria virulence and biofilms as promising antimicrobial drugs. Curr Med Chem. 2014;21(3):296–311. doi:10.2174/09298673113206660294
18. Lin YH, Xu JL, Hu J, et al. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol Microbiol. 2003;47(3):849–860. doi:10.1046/j.1365-2958.2003.03351.x
19. Liu D, Momb J, Thomas PW, et al. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 1. Product-bound structures. Biochemistry. 2008;47(29):7706–7714. doi:10.1021/bi800368y
20. Chun CK, Ozer EA, Welsh MJ, Zabner J, Greenberg EP. Inactivation of a Pseudomonas aeruginosa quorum-sensing signal by human airway epithelia. Proc Natl Acad Sci U S A. 2004;101(10):3587–3590. doi:10.1073/pnas.0308750101
21. Golpasha ID, Mousavi SF, Owlia P, Siadat SD, Irani S. Immunization with 3-oxododecanoyl-L-homoserine lactone-r-PcrV conjugate enhances survival of mice against lethal burn infections caused by Pseudomonas aeruginosa. Bosn J Basic Med Sci. 2015;15(2):15–24. doi:10.17305/bjbms.2015.292
22. Miyairi S, Tateda K, Fuse ET, et al. Immunization with 3-oxododecanoyl-L-homoserine lactone-protein conjugate protects mice from lethal Pseudomonas aeruginosa lung infection. J Med Microbiol. 2006;55(Pt 10):1381–1387. doi:10.1099/jmm.0.46658-0
23. Napolitano A, De Lucia M, Panzella L, D’ Ischia M. The Chemistry of Tyrosol and Hydroxytyrosol. Olives and Olive Oil in Health and Disease Prevention. academic press; 2010.
24. Abdel-Rhman SH, El-Mahdy AM, El-Mowafy M. Effect of tyrosol and farnesol on virulence and antibiotic resistance of clinical isolates of Pseudomonas aeruginosa. Biomed Res Int. 2015;2015:456463.
25. Bernini R, Carastro I, Santoni F, Clemente M. Synthesis of lipophilic esters of tyrosol, homovanillyl alcohol and hydroxytyrosol. Antioxidants. 2019;8:6.
26. Abdel-Rhman SH, Rizk DE. Effect of tyrosol on Staphylococcus aureus antimicrobial susceptibility, biofilm formation and virulence factors. African J Microbiol Res. 2016;10(20):687–693. doi:10.5897/AJMR2016.8001
27. Rodrigues CF, Cernakova L. Farnesol and tyrosol: secondary metabolites with a crucial quorum-sensing role in Candida biofilm development. Genes. 2020;11:4. doi:10.3390/genes11040444
28. Lavilla Lerma L, Benomar N, Valenzuela AS, Casado Munoz Mdel C, Galvez A, Abriouel H. Role of EfrAB efflux pump in biocide tolerance and antibiotic resistance of Enterococcus faecalis and Enterococcus faecium isolated from traditional fermented foods and the effect of EDTA as EfrAB inhibitor. Food Microbiol. 2014;44:249–257. doi:10.1016/j.fm.2014.06.009
29. Pullar JM, Bayer S, Carr AC. Appropriate handling, processing and analysis of blood samples is essential to avoid oxidation of vitamin c to dehydroascorbic acid. Antioxidants. 2018;7:2.
30. WE G, OM H, WM T, AM. H. Effect of EDTA on biofilm formation and antibiotic susceptibility of multidrug resistant uropathogenic Escherichia coli clinical isolates in Egypt. African J Microbiol Res. 2017;11(38):1445–1458. doi:10.5897/AJMR2017.8700
31. CLSI. Performance Standards for Antimicrobial Susceptibility Testing.
32. Reina JC, Perez-Victoria I, Martin J, Llamas I. A quorum-sensing inhibitor strain of vibrio alginolyticus blocks qs-controlled phenotypes in chromobacterium violaceum and Pseudomonas aeruginosa. Mar Drugs. 2019;17:9. doi:10.3390/md17090494
33. Abdel-Rhman SH. Role of Pseudomonas aeruginosa lipopolysaccharides in modulation of biofilm and virulence factors of Enterobacteriaceae. Ann Microbiol. 2019;69:299–305. doi:10.1007/s13213-018-1420-5
34. Benov L. Effect of growth media on the MTT colorimetric assay in bacteria. PLoS One. 2019;14(8):e0219713. doi:10.1371/journal.pone.0219713
35. Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory; 1972.
36. Schaefer J, Jovanovic G, Kotta-Loizou I, Buck M. Single-step method for beta-galactosidase assays in Escherichia coli using a 96-well microplate reader. Anal Biochem. 2016;503:56–57. doi:10.1016/j.ab.2016.03.017
37. El-Mowafy SA, Abd El Galil KH, El-Messery SM, Shaaban MI. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb Pathog. 2014;74:25–32. doi:10.1016/j.micpath.2014.07.008
38. Giamarellou H. Multidrug-resistant Gram-negative bacteria: how to treat and for how long. Int J Antimicrob Agents. 2010;36(Suppl 2):S5054. doi:10.1016/j.ijantimicag.2010.11.014
39. Woodford N, Turton JF, Livermore DM. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev. 2011;35(5):736–755. doi:10.1111/j.1574-6976.2011.00268.x
40. Asfour HZ. Anti-quorum sensing natural compounds. J Microsc Ultrastruct. 2018;6(1):1–10.
41. Finch RG, Pritchard DI, Bycroft BW, Williams P, Stewart GS. Quorum sensing: a novel target for anti-infective therapy. J Antimicrob Chemother. 1998;42(5):569–571. doi:10.1093/jac/42.5.569
42. Smith RS, Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol. 2003;6(1):56–60. doi:10.1016/S1369-5274(03)00008-0
43. Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264(5157):382–388. doi:10.1126/science.8153625
44. Poole K. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J Mol Microbiol Biotechnol. 2001;3(2):255–264.
45. Murray PR, Rosenthal KS, Pfaller MA. Medical Microbiology.
46. Ben Haj Khalifa A, Moissenet D, Vu TH, Khedher M. [Virulence factors in Pseudomonas aeruginosa: mechanisms and modes of regulation]. Ann Biol Clin (Paris). 2011;69(4):393–403. French.
47. Nalca Y, Jansch L, Bredenbruch F, Geffers R, Buer J, Haussler S. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob Agents Chemother. 2006;50(5):1680–1688. doi:10.1128/AAC.50.5.1680-1688.2006
48. Skindersoe ME, Alhede M, Phipps R, et al. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52(10):3648–3663. doi:10.1128/AAC.01230-07
49. Karkovic Markovic A, Toric J, Barbaric M, Jakobusic Brala C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules. 2019;24:10. doi:10.3390/molecules24102001
50. Abd AH, Abu-Raghif AR, Al-Azzawi ANF. The effect of EDTA with single or combination of antibiotics on Pseudomonas aeruginosa isolates in vitro. Iraq J Med Sci. 2011;9:285–291.
51. Aboelenin AM, Abdelmegeed ES, Hassan R. Studies on the effect of EDTA as antibiofilm agent on the production of biofilm by some clinical Gram negative bacteria. N Egypt J Microbiol. 2017;46:67–80.
52. Babic F, Venturi V, Maravic-Vlahovicek G. Tobramycin at subinhibitory concentration inhibits the RhlI/R quorum sensing system in a Pseudomonas aeruginosa environmental isolate. BMC Infect Dis. 2010;10:148. doi:10.1186/1471-2334-10-148
53. Gupta P, Chhibber S, Harjai K. Subinhibitory concentration of ciprofloxacin targets quorum sensing system of Pseudomonas aeruginosa causing inhibition of biofilm formation & reduction of virulence. Indian J Med Res. 2016;143(5):643–651. doi:10.4103/0971-5916.187114
54. Xu ZG, Gao Y, He JG, Xu WF, Jiang M, Jin HS. Effects of azithromycin on Pseudomonas aeruginosa isolates from catheter-associated urinary tract infection. Exp Ther Med. 2015;9(2):569–572.
55. Deryabin DG, Inchagova KS. Inhibitory effect of aminoglycosides and tetracyclines on quorum sensing in Chromobacterium violaceum. Microbiology. 2018;87:1–8. doi:10.1134/S002626171801006X
56. Fonseca AP, Extremina C, Fonseca AF, Sousa JC. Effect of subinhibitory concentration of piperacillin/tazobactam on Pseudomonas aeruginosa. J Med Microbiol. 2004;53(Pt 9):903–910. doi:10.1099/jmm.0.45637-0
57. Mohabi S, Kalantar-Neyestanaki D, Mansouri S. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by Quercus infectoria gall extracts. Iran J Microbiol. 2017;9(1):26–32.
58. Quecan BXV, Santos JTC, Rivera MLC, Hassimotto NMA, Almeida FA, Pinto UM. Effect of quercetin rich onion extracts on bacterial quorum sensing. Front Microbiol. 2019;10:867. doi:10.3389/fmicb.2019.00867
59. Tang K, Zhang XH. Quorum quenching agents: resources for antivirulence therapy. Mar Drugs. 2014;12(6):3245–3282. doi:10.3390/md12063245
60. Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177(14):3998–4008. doi:10.1128/JB.177.14.3998-4008.1995
61. Mobarak-Qamsari E, Kasra-Kermanshahi R, Moosavi-Nejad Z. Isolation and identification of a novel, lipase-producing bacterium, Pseudomonas aeruginosa KM110. Iran J Microbiol. 2011;3(2):92–98.
62. Mattmann ME, Blackwell HE. Small molecules that modulate quorum sensing and control virulence in Pseudomonas aeruginosa. J Org Chem. 2010;75(20):6737–6746. doi:10.1021/jo101237e
63. Musthafa KS, Sivamaruthi BS, Pandian SK, Ravi AV. Quorum sensing inhibition in Pseudomonas aeruginosa PAO1 by antagonistic compound phenylacetic acid. Curr Microbiol. 2012;65(5):475–480. doi:10.1007/s00284-012-0181-9
64. Ahmed S, Rudden M, Smyth TJ, Dooley JSG, Marchant R, Banat IM. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl Microbiol Biotechnol. 2019;103(8):3521–3535. doi:10.1007/s00253-019-09618-0
65. Sun Y, Sun F, Feng W, et al. Hyperoside inhibits biofilm formation of Pseudomonas aeruginosa. Exp Ther Med. 2017;14(2):1647–1652. doi:10.3892/etm.2017.4641
66. Yeung AT, Torfs EC, Jamshidi F, et al. Swarming of Pseudomonas aeruginosa is controlled by a broad spectrum of transcriptional regulators, including MetR. J Bacteriol. 2009;191(18):5592–5602. doi:10.1128/JB.00157-09
67. Aswathanarayan JB, Vittal RR. Inhibition of biofilm formation and quorum sensing mediated phenotypes by berberine in Pseudomonas aeruginosa and Salmonella typhimurium. RSC Adv. 2018;8(63):36133–36141. doi:10.1039/C8RA06413J
68. Vasavi HS, Arun AB, Rekha PD. Anti-quorum sensing activity of Psidium guajava L. flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1. Microbiol Immunol. 2014;58(5):286–293. doi:10.1111/1348-0421.12150
69. Khan MS, Zahin M, Hasan S, Husain FM, Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett Appl Microbiol. 2009;49(3):354–360. doi:10.1111/j.1472-765X.2009.02666.x
70. Abbas HA, Shaldam MA, Eldamasi D. Curtailing quorum sensing in Pseudomonas aeruginosa by sitagliptin. Curr Microbiol. 2020;77(6):1051–1060. doi:10.1007/s00284-020-01909-4
71. Ouyang J, Sun F, Feng W, et al. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J Appl Microbiol. 2016;120(4):966–974. doi:10.1111/jam.13073
72. Okusa PN, Rasamiravaka T, Vandeputte O, Stevigny C, Jaziri ME, Duez P. Extracts of Cordia gilletii de wild (Boraginaceae) quench the quorum sensing of Pseudomonas aeruginosa PAO1. J Intercult Ethnopharmacol. 2014;3(4):138–143. doi:10.5455/jice.20140710031312
73. Galle M, Jin S, Bogaert P, Haegman M, Vandenabeele P, Beyaert R. The Pseudomonas aeruginosa type III secretion system has an exotoxin S/T/Y independent pathogenic role during acute lung infection. PLoS One. 2012;7(7):e41547. doi:10.1371/journal.pone.0041547
74. Vikram A, Jayaprakasha GK, Jesudhasan PR, Pillai SD, Patil BS. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J Appl Microbiol. 2010;109(2):515–527.
75. Bleves S, Soscia C, Nogueira-Orlandi P, Lazdunski A, Filloux A. Quorum sensing negatively controls type III secretion regulon expression in Pseudomonas aeruginosa PAO1. J Bacteriol. 2005;187(11):3898–3902. doi:10.1128/JB.187.11.3898-3902.2005
76. Kong W, Liang H, Shen L, Duan K. [Regulation of type III secretion system by Rhl and PQS quorum sensing systems in Pseudomonas aeruginosa]. Wei Sheng Wu Xue Bao. 2009;49(9):1158–1164.Chinese.
77. Zhang Y, Sass A, Van Acker H, et al. Coumarin reduces virulence and biofilm formation in Pseudomonas aeruginosa by affecting quorum sensing, Type III secretion and C-di-GMP levels. Front Microbiol. 2018;9:1952. doi:10.3389/fmicb.2018.01952
78. Kim HS, Lee SH, Byun Y, Park HD. 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci Rep. 2015;5:8656. doi:10.1038/srep08656
79. Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2015;6(1):26–41. doi:10.1007/s13238-014-0100-x
80. Meng X, Ahator SD, Zhang LH. Molecular mechanisms of phosphate stress activation of Pseudomonas aeruginosa quorum sensing systems. mSphere. 2020;5:2. doi:10.1128/mSphere.00119-20
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

