Back to Journals » Clinical and Experimental Gastroenterology » Volume 13
Effect of Rikkunshito, a Traditional Japanese Herbal Medicine, on Delayed Gastric Emptying and Oral Dietary Intake After Pancreaticoduodenectomy: A Prospective, Randomized, Single-Center, Open-Labeled Study
Authors Yamaguchi H , Kimura Y , Imamura M, Nagayama M, Ito T, Kyuno D , Kono T, Kimura A, Akizuki E, Nishidate T , Okita K, Nobuoka T, Mizuguchi T, Hirata K, Takemasa I
Received 7 March 2020
Accepted for publication 12 November 2020
Published 9 December 2020 Volume 2020:13 Pages 577—587
DOI https://doi.org/10.2147/CEG.S252913
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Koulaouzidis
Hiroshi Yamaguchi,1 Yasutoshi Kimura,1 Masafumi Imamura,1 Minoru Nagayama,1 Tatsuya Ito,1 Daisuke Kyuno,1 Tsuyoshi Kono,1 Akina Kimura,1 Emi Akizuki,1 Toshihiko Nishidate,1 Kenji Okita,1 Takayuki Nobuoka,1 Toru Mizuguchi,1,2 Koichi Hirata,3 Ichiro Takemasa1
1Department of Surgery, Surgical Oncology and Science, Sapporo Medical University, School of Medicine, Sapporo, Japan; 2Department of Nursing and Surgical Science, Sapporo Medical University School of Health Science, Sapporo, Japan; 3Department of Surgery, JR Sapporo Hospital, Sapporo, Japan
Correspondence: Hiroshi Yamaguchi
Department of Surgery, Surgical Oncology and Science, Sapporo Medical University, South 1, West 16, Sapporo 060-8543, Japan
Tel +81 11-611-2111 Ext 32810
Fax +81 11-613-1678
Email [email protected]
Introduction: Delayed gastric emptying (DGE) remains an important problem after pancreaticoduodenectomy (PD). There is a lack of effective treatments for early recovery of oral dietary intake. Rikkunshito (RKT), a Japanese herbal medicine, has been gaining attention as a facilitator of gastric emptying. We evaluated the effects of RKT on DGE after PD.
Methods: In this prospective, randomized, open-labeled study, patients were randomly allocated before PD in a 1:1 ratio to the RKT group or the control group that received no additional treatment. The RKT group received 2.5 g of RKT three times daily (7.5 g/day) from postoperative day (POD) 1 to POD 21. The primary endpoint was the incidence of DGE. Secondary endpoints were short-term postoperative outcomes including oral dietary intake volume and perioperative changes in levels of the hormones ghrelin and leptin. Patients were observed until hospital discharge.
Results: Twenty-six patients in each group (n = 52) completed the protocol treatment and were included in the analysis set. There were no statistically significant differences in basic characteristics and operative factors. The overall incidence of DGE was not statistically different between the RKT and control groups (30.8% vs 30.8%, p> 0.9999). There were no statistically significant differences in the amount of postoperative oral dietary intake represented by total dietary intake (TDI) up to POD 14 and POD 21, complications, and length of hospital stay. No adverse events related to this study were observed. In the RKT group, total ghrelin and acyl-ghrelin were significantly upregulated and leptin was significantly downregulated earlier than in the control group.
Conclusion: RKT treatment from POD 1 to 21 did not reduce the incidence of DGE and had no clinically beneficial effect on short-term postoperative outcomes irrespective of changes in hormone levels.
Keywords: delayed gastric emptying, pancreaticoduodenectomy, Rikkunshito, ghrelin, leptin
Introduction
Delayed gastric emptying (DGE) remains an important problem after pancreaticoduodenectomy (PD). Effective treatments for early recovery of oral dietary intake are lacking, resulting in prolonged hospital stay and interval before the induction of adjuvant therapy.1,2 The incidence of DGE after PD varies from 4.5% to 100% across studies,3,4 implying difficulties in defining diagnostic criteria and incomplete understanding of its pathophysiology. We previously traced oral dietary intake after PD in detail to clarify the impact on the diagnosis of DGE and short-term postoperative outcomes.1,5 We found that the International Study Group of Pancreatic Surgery (ISGPS) definition of DGE6 based on oral dietary intake and short-term outcomes after PD was reasonable, although some patients with low oral dietary intake were not diagnosed with DGE,1 suggesting that the recovery process after PD is complex. Numerous studies have reported strategies to decrease DGE. In general, surgical procedures influenced DGE, indicating the superiority of pylorus resection,7 antecolic gastrojejunostomy or duodenojejunostomy reconstruction,8 Braun anastomosis,9 Billroth II reconstruction10,11 and side-to-side gastrojejunostomy.12,13 However, recent studies including a meta-analysis reported conflicting findings,2–4,14–16 resulting in a lack of evidence-based effective procedures that decrease DGE consistently. Few studies have focused on pharmacological treatment for DGE after PD. Previous reports have shown the effectiveness of the motilin agonist erythromycin.17,18 Additional effective pharmacological approaches are urgently needed to improve DGE after PD.
Rikkunshito (RKT), a Japanese herbal medicine, has been drawing attention as an effective treatment option for anorexia, epigastric distress, and dyspepsia in patients with functional dyspepsia,19,20 proton pump inhibitor (PPI)-refractory non-erosive reflux disease (NERD),21 after gastrectomy,22 or undergoing chemotherapy.23,24 Furthermore, RKT has been reported to improve gastric emptying in patients with Parkinson’s disease25 or profound handicaps26 and after pylorus-preserving gastrectomy.27 Pharmacological studies have revealed that RKT enhances the secretion and function of ghrelin, a 28-amino-acid peptide hormone that enhances appetite, gastric motility, and growth hormone secretion.24,28 The effects of RKT on the ghrelin system might be important mechanisms underlying its clinical benefits.20,22,29 We conducted a prospective, randomized, open-labeled study to elucidate the role of RKT in short-term postoperative outcomes such as the incidence of DGE, amount of oral dietary intake, and changes in plasma ghrelin levels after PD. To the best of our knowledge, no previous studies have evaluated the effects of RKT on DGE after PD.
Methods
This prospective, randomized, open-label study comparing a group of patients who received RKT to a control group who received no additional treatment was conducted at Sapporo Medical University Hospital as a pilot study. The study protocol was approved by the institutional review board (approval number: 25–29) and carried out in accordance with the 2004 revision of the Declaration of Helsinki. This study was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry as UMIN000012052 on October 16, 2013 (https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014089). Written informed consent was obtained from all subjects prior to their participation in the study. The study was conducted and completed by academic surgeons. No funding or staff from pharmaceutical companies was involved in any aspects of the study.
Patient Eligibility
Patients aged 20 to 79 years with Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0 or 1 who were scheduled to undergo PD at Sapporo Medical University Hospital were considered eligible to enroll in the study. Exclusion criteria were as follows: 1) another active cancer outside of the pancreatobiliary-duodenal region; 2) concurrent chemotherapy; 3) use of any herbal medicines within 2 weeks before start of the study; 4) serious comorbidities or hypersensitivities; 5) pregnancy, lactation, or intention to become pregnant during the study; or 6) study participation considered to be inappropriate by the investigators. Patient eligibility was evaluated from November 2014 to May 2017.
Study Protocol
After providing written informed consent, patients were allocated randomly in a 1:1 ratio to the RKT or control group before surgery. Randomization was conducted by researchers according to a table of random numbers generated using Excel software (Microsoft Japan Co., Ltd., Tokyo, Japan) without concealment. The RKT group received 2.5 g of RKT (TJ-43, Tsumura & Co., Tokyo, Japan) three times daily (7.5 g/day) from postoperative day (POD) 1 to POD 21. From POD 1 to 4, RKT and other drugs were administered via tube jejunostomy; oral administration began on POD 5. If patients did not tolerate the taste of RKT, tube jejunostomy administration was used upon request to maintain treatment compliance. In general, we prohibited the concomitant use of prokinetic drugs, herbal medicines, and motilin receptor agonists up to POD 21. Gastric acid secretion inhibitors such as PPIs and histamine H2 blockers were allowed to be administered concomitantly, but changes in medications or dosages were not allowed until POD 21. However, these drugs were allowed if urgently needed due to postoperative condition or complications. Patients were observed up to hospital discharge.
Study Endpoints
The primary endpoint was the incidence of DGE. DGE was diagnosed based on ISGPS criteria.6 Secondary endpoints were short-term postoperative outcomes including oral dietary intake represented by total dietary intake (TDI), which was calculated as previously reported1 up to POD 14 and POD 21. Briefly, the maximum oral dietary intake for breakfast, lunch, and dinner was evaluated and one representative maximum value ranging from 0 to 1 (0–0.5 for rice and 0–0.5 for main dishes) was assigned to each POD. TDI was calculated as the cumulative maximum oral dietary intake for each POD from POD 1 to POD 14 (range, 0–14) and from POD 1 to POD 21 (range, 0–21). Postoperative complications were defined based on the Clavien-Dindo classification system.30 The International Study Group for Pancreatic Fistula (ISGPF) definition was used to diagnose postoperative pancreatic fistula (POPF).31 Secondary endpoints included changes in serum hormones such as acyl-ghrelin, desacyl-ghrelin, leptin, insulin, and C-peptide. Ghrelin concentration was measured as previously reported.32 Blood samples were obtained preoperatively and on PODs 7, 14, and 21 at rest during the early morning before breakfast.
Adverse Events and Criteria for Study Discontinuation
In this study, an adverse event was defined as any unfavorable or unexpected symptom or sign with the possibility of a causal relationship with RKT treatment. Adverse events were monitored through daily medical examinations and regular perioperative blood tests and imaging studies. The criteria for study discontinuation were as follows: 1) withdrawal of consent, 2) decline in the use of RKT until POD 21, 3) low treatment compliance, 4) adverse event, worsened medical condition, postoperative complication, pregnancy, or another situation considered inappropriate for continued study participation by the investigators.
Operative Procedures and Postoperative Management
All surgeries and postoperative management were performed by two surgeons certified by the Japanese Society of Hepato-Biliary-Pancreatic Surgery (YK and MI) or under their supervision. Subtotal stomach-preserving pancreaticoduodenectomy (SSPPD) or pylorus-preserving pancreaticoduodenectomy (PPPD) was performed in a standardized manner by a specialized surgical team depending on the preoperative and intraoperative diagnoses. The stomach was transected 2 to 3 cm proximal to the pylorus ring in SSPPD. The duodenum was transected 3 to 5 cm distal to the pylorus ring in PPPD. Lymph node dissection was performed according to the classification of pancreatic cancer by the Japan Pancreas Society33 for pancreatic diseases and the Union for International Cancer Control classification system34 for biliary tract diseases. D2 or complete dissection of prescribed locoregional lymph nodes was defined as the standard.
After resection, a modified child’s reconstruction was performed. The proximal jejunal stump was brought to the pancreatic stump through the posterior space of the mesenterium or behind the colon. An end-to-side anastomosis between the bile duct and jejunum was made with interrupted sutures and the distal pancreas was reconstructed via pancreaticojejunostomy using a modified Blumgart method.35 The stomach was transposed anterior to the transverse colon for antecolic and Billroth II reconstructions. A side-to-side gastrojejunal anastomosis was made with staplers in SSPPD and a hand-sewed end-to-side duodenojejunal anastomosis was made using the Gambee method in PPPD. A Braun anastomosis and tube jejunostomy were always created. A 3.0 mm jejunostomy catheter (Covidien Japan, Tokyo, Japan) was inserted 30 cm into the jejunum and through the Braun anastomosis. The catheter was fixed to the jejunal wall using the Witzel technique. Next, the part of the jejunum with the catheter insertion site was fixed to the left abdominal wall. Two closed-system drains were placed, in the foramen of Winslow and around the pancreaticojejunostomy.
Postoperative management was based on the team’s standardized clinical pathway. The nasogastric tube was immediately removed after surgery on POD 0. Drinking water was allowed starting on POD 1. Enteral feeding through the tube jejunostomy started on POD 2. The antibiotic flomoxef sodium was used until POD 2. Liquefied rice and easily digestible main dishes were started on POD 5. Solid food intake was gradually increased. Rice gruel and solid main dishes, which were considered solid foods, were started on POD 7 to assess tolerability. Amylase concentration of the fluid from the drains was measured on PODs 1, 3, and 5 to monitor for POPF. Computed tomography was performed to evaluate postoperative status on POD 6. If there were no signs and symptoms of POPF or other complications, the drains were removed by POD 6.
Sample Size Calculation
In a previous study at our institution, the incidence of DGE after PD based on the ISGPS criteria was 43.9%.1 Erythromycin was associated with a 37% reduction in the incidence of DGE.17 We speculated that RKT may be twice as effective as erythromycin based on numerous promising findings.19–27,29 We hypothesized that the incidence of DGE in the control group would be 50%. We expected that the incidence of DGE in the RKT group would decrease to 15%, an estimated 70% reduction. Applying 1:1 allocation to the control and RKT groups with a two-sided α of 0.05 and statistical power of 0.80 while taking into account that 15% of participants might be excluded from the analysis, we determined that 25 patients would be required for each group (50 in total).
Data Analysis and Statistical methods
Short-term postoperative outcomes were analyzed by blinded researchers who fixed the data, which was then linked to group allocation for further analysis. Continuous valuables related to short-term postoperative outcomes were reported as medians (range) and analyzed using the Mann–Whitney U-test. Perioperative changes in serum hormone levels were reported as means (SEM) and analyzed using two-way ANOVA when differences between groups were compared or the paired t-test when preoperative versus postoperative changes were compared within each group. Categorical valuables were analyzed using Fisher’s exact test. p<0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism 7 software (GraphPad Software, San Diego, CA).
Results
The study flow chart is shown in Figure 1. Patient eligibility was evaluated from November 2014 to May 2017. Written informed consent for study enrollment was obtained from 60 patients randomly allocated to the RKT and control groups in a 1:1 ratio before surgery. One patient in the RKT group and four patients in the control group were excluded based on intraoperative findings of unresectable disease or the need for major liver resection concomitantly. Postoperatively, three patients in the RKT group were excluded because of withdrawal of consent (n=1) and discontinuation due to postoperative delirium (n=1) or bleeding from a pseudoaneurysm (n=1). No adverse events defined as related to this study were observed. Ultimately, 26 patients in each group (52 patients total) completed the protocol treatment and were included in the analysis set, fulfilling the calculated sample size requirement for the study.
 |
Figure 1 Study flow chart. Abbreviations: PD, Pancreaticoduodenectomy; RKT, rikkunshito. |
Basic Characteristics and Operative Factors of the Study Participants
There were no statistically significant differences between the RKT and control groups in basic characteristics (Table 1) and operative factors (Table 2). Because diabetes is an important cause of gastroparesis, the homeostasis model assessment of insulin resistance (HOMA-R) was calculated to evaluate insulin resistance and the potential risk for diabetes among patients not taking insulin. In the RKT group, the incidence of combined resection during PD tended to be higher than in the control group (p=0.0751), although this result did not reach statistical significance.
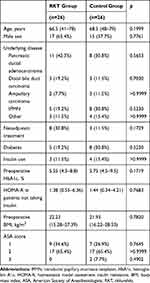 |
Table 1 Characteristics of the Study Participants |
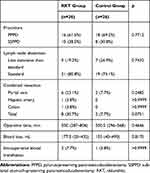 |
Table 2 Operative Factors of the Study Participants |
Primary Endpoint (Table 3)
The overall incidence of DGE in the RKT and control groups was 30.8% and 30.8%, respectively (p>0.9999). In addition, the severity of DGE based on ISGPS grade was also not statistically significant between the two groups.
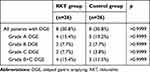 |
Table 3 Primary Endpoint |
Secondary Endpoints for Short-Term Postoperative Outcomes (Table 4)
Postoperative oral dietary intake as represented by TDI up to POD 14 and POD 21 and hospital stay were not statistically significantly different between the two groups. In addition, there were no statistically significant differences in terms of postoperative complications, incidence of POPF, and duration of parenteral nutrition. We checked the distribution of TDI values of all patients by DGE grade (Supplemental Figure 1). TDI values among patients with grade B or C DGE were significantly lower than values among patients without DGE or with grade A DGE. This finding suggested that TDI is a reliable and reproducible indicator of postoperative oral dietary intake after PD by DGE grade, as previously reported.1
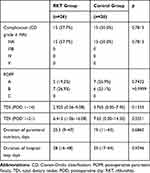 |
Table 4 Secondary Endpoints for Short-Term Postoperative Outcomes |
Hormonal Changes
There were no significant differences between the two groups in levels of total ghrelin (sum of acyl-ghrelin and desacyl-ghrelin: p=0.3999); acyl-ghrelin, an active form of ghrelin (p=0.4059); and leptin (p=0.3931) or the ratio of acyl-ghrelin to total ghrelin (p=0.5499) (Supplemental Figure 2). When postoperative values of these hormonal factors were compared with preoperative values in each group, total ghrelin was significantly upregulated at POD 14 and POD 21 in the RKT group, but no significant changes were observed in the control group (Figure 2A and B). Acyl-ghrelin was also significantly upregulated at POD 14 and POD 21 in the RKT group and at POD 21 in the control group (Figure 2C and D). The ratio of acyl-ghrelin to total ghrelin was significantly upregulated in the RKT group from POD 7 to POD 21, but upregulation was observed only on POD 7 in the control group (Figure 2E and F). Levels of leptin were significantly lower from POD 7 to POD 21 in the RKT group but levels of leptin were lower only on POD 21 in the control group than preoperative levels, respectively (Figure 2G and H).
Subgroup Analysis
We hypothesized that RKT may improve the outcomes of patients diagnosed with DGE. We evaluated short-term postoperative outcomes in patients with and without DGE (Table 5). Treatment with RKT did not improve short-term postoperative outcomes in patients with or without DGE. In a previous study,21 RKT showed significant effects against PPI-refractory NERD in patients with a body mass index (BMI) under 22 kg/m2. Patients were divided into the BMI < 22 kg/m2 group or BMI ≥ 22 kg/m2 group for additional analysis. There were no significant differences in the incidence and severity of DGE and short-term postoperative outcomes including TDI and duration of parenteral nutrition and hospital stay between the RKT and control groups after stratification by BMI (Supplemental Table 1). A previous study7 also showed a relationship between pylorus preservation and DGE. We separated patients who underwent PPPD or SSPPD and reanalyzed. There were no statistically significances in primary and secondary endpoints between the RKT and control groups when stratified by procedure type (Supplemental Table 2).
 |
Table 5 Short-Term Postoperative Outcomes of Patients with and without DGE |
Discussion
We conducted a prospective, open-labeled randomized controlled trial to evaluate the effect of RKT on DGE after PD. Treatment with RKT did not show significant effects on the incidence or severity of DGE. Similarly, short-term postoperative outcomes did not significantly improve with RKT. We first reported the importance of TDI after PD for evaluating postoperative outcomes reflecting actual oral dietary intake. TDI decreased significantly with DGE severity. Furthermore, in patients who did not satisfy the ISGPS criteria for DGE, TDI was associated with the length of hospital stay.1 In this study, to evaluate oral dietary intake in detail, we calculated TDI values at both POD 14 and 21. Of note, the study findings suggest that TDI is a reliable and reproducible indicator of oral dietary intake after PD. Treatment with RKT did not significantly improve TDI values at either time point. In addition, RKT did not have significant effects on TDI in patients with or without DGE in the subgroup analysis.
Hayakawa et al29 reported that administration of RKT via a nasogastric tube had a positive effect on reaching 50% of the target enteral feeding volume earlier and increased plasma active ghrelin levels more than metoclopramide in critically ill patients in a small prospective RCT. Furthermore, intragastric administration of RKT improved gastric emptying in profoundly handicapped patients26 and dogs.36 In this study, we allowed continuation of RKT administration via tube jejunostomy upon patient request to maintain treatment compliance. There is a possibility that strict oral administration might change the pharmacokinetics of RKT, which might have impacted the results. In this study, relative changes before and after surgery in levels of total ghrelin, acyl-ghrelin, and leptin differed between the two groups, suggesting that RKT affects the ghrelin system. In the RKT group, total ghrelin and acyl-ghrelin were significantly upregulated and leptin was significantly downregulated earlier than in the control group, although significant differences were not observed in these values between the two groups. Leptin was reported to have an antagonist effect on ghrelin in animal experiments.37 Earlier postoperative downregulation of leptin in the RKT group compared with the control group may warrant further analysis to reveal the role of RKT on leptin. Irrespective of changes in hormone levels from POD 7 to POD 21 in the RKT group, RKT did not have any observed clinical impact, reflecting the complex pathophysiology of DGE after PD. Recently, Sasaki et al38 focused on the anti-inflammatory effects of ghrelin. They showed that a decrease in total ghrelin concentration on POD 1 to <82% of the preoperative value is associated with postoperative complications and longer hospital stay after PD. In the RKT group, it took 2 weeks from the initiation of the treatment to observe a significant increase in total ghrelin concentrations. Preoperative intervention and earlier upregulation of the ghrelin system, before POD 1, might be important for improving outcomes after PD. Further studies are needed to evaluate the optimal timing of the RKT treatment. Regarding improvements in outcomes after PD, it should be noted that the median duration of hospital stay in this study was longer than that in most Western countries, although the results were almost compatible with hospital stay durations from other studies in Japan, which ranged from 18 to 39.5 days.1,7,8,12,13,38 Differences in the health care insurance systems and medical expenses might influence postoperative care and patients behaviors, as discussed in other studies from Japan.1,7,12,13
This study had several limitations. First, this study was conducted in an open-label setting without placebo drugs. Although there were no placebo effects because the control group did not receive any interventions, some biases during the study should be considered. A recent meta-analysis of RKT treatment pointed out a high risk of bias among analyzed studies, especially due to the lack of blinding.39 We allowed continuation of RKT administration via tube jejunostomy to maintain treatment compliance and reduce attrition bias. During data analysis, blinded researchers analyzed the results and fixed the data before linking it to treatment status in order to reduce detection bias. We did not use control drugs for the study because standard pharmacological treatment for DGE after PD has not been established. RKT is an established drug for upper gastrointestinal symptoms covered by the Japanese healthcare insurance system. This study was conducted at a single institution as a pilot study. The second limitation is the small sample size, which included patients with a variety of diseases who underwent one of two operative procedures, PPPD or SSPPD. In our prospective, nonrandomized study, SSPPD was associated with a comparable incidence of DGE and postoperative dietary intake as PPPD.5 However, a larger sample size and more precise specification of underlying diseases and operative procedures are important to balance baseline characteristics and reduce bias. To compensate for these study limitations, we analyzed TDI in addition to the incidence of DGE based on ISGPS criteria as an alternative reliable indicator of postoperative dietary intake.
Conclusions
RKT treatment from POD 1 to 21 did not reduce the incidence or severity of DGE as defined using the ISGPS criteria. Irrespective of hormonal changes from POD 7 to POD 21 in the RKT group, RKT therapy was not associated with clinical benefits in terms of short-term postoperative outcomes, suggesting that DGE after PD has a complicated pathophysiology. Earlier intervention and upregulation of the ghrelin system before POD 1 should be evaluated in future studies.
Abbreviations
BMI, body mass index; DGE, delayed gastric emptying; HOMA-R, homeostasis model assessment of insulin resistance; ISGPS, International Study Group of Pancreatic Surgery; NERD, non-erosive reflux disease; PD, pancreaticoduodenectomy; POD, postoperative day; POPF, postoperative pancreatic fistula; PPI, proton pump inhibitor; PPPD, pylorus-preserving pancreaticoduodenectomy; RKT, Rikkunshito; SSPPD, subtotal stomach-preserving pancreaticoduodenectomy; TDI, total dietary intake.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study protocol was approved by the institutional review board (approval number: 25–29). Written informed consent was obtained from all subjects prior to their participation in the study.
Author Contributions
YK and KH designed the study. HY, YK, MI, MN, TI, DK, and TK contributed to the acquisition of data. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by a KAKENHI (Grant-in-Aid for Scientific Research (C)), (number 25462122) from Japan Society for the Promotion of Science (JSPS) for the collection and analysis of data.
Disclosure
Dr Hiroshi Yamaguchi reports grants from Japan Society for the Promotion of Science and The Akiyama Life Science Foundation, during the conduct of the study and outside the submitted work. The authors declare that they have no other competing interests in this work.
References
1. Akizuki E, Kimura Y, Nobuoka T, et al. Reconsideration of postoperative oral intake tolerance after pancreaticoduodenectomy: prospective consecutive analysis of delayed gastric emptying according to the ISGPS definition and the amount of dietary intake. Ann Surg. 2009;249(6):986–994. doi:10.1097/SLA.0b013e3181a63c4c
2. Glowka TR, Webler M, Matthaei H, et al. Delayed gastric emptying following pancreatoduodenectomy with alimentary reconstruction according to Roux-en-Y or Billroth-II. BMC Surg. 2017;17(1):24.
3. Imamura M, Kimura Y, Ito T, et al. Effects of antecolic versus retrocolic reconstruction for gastro/duodenojejunostomy on delayed gastric emptying after pancreatoduodenectomy: a systematic review and meta-analysis. J Surg Res. 2016;200(1):147–157. doi:10.1016/j.jss.2015.08.004
4. Klaiber U, Probst P, Strobel O, et al. Meta-analysis of delayed gastric emptying after pylorus-preserving versus pylorus-resecting pancreatoduodenectomy. Br J Surg. 2018;105(4):339–349. doi:10.1002/bjs.10771
5. Akizuki E, Kimura Y, Nobuoka T, et al. Prospective nonrandomized comparison between pylorus-preserving and subtotal stomach-preserving pancreaticoduodenectomy from the perspectives of DGE occurrence and postoperative digestive functions. J Gastrointest Surg. 2008;12(7):1185–1192. doi:10.1007/s11605-008-0513-z
6. Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761–768.
7. Kawai M, Tani M, Hirono S, et al. Pylorus ring resection reduces delayed gastric emptying in patients undergoing pancreatoduodenectomy: a prospective, randomized, controlled trial of pylorus-resecting versus pylorus-preserving pancreatoduodenectomy. Ann Surg. 2011;253(3):495–501. doi:10.1097/SLA.0b013e31820d98f1
8. Tani M, Terasawa H, Kawai M, et al. Improvement of delayed gastric emptying in pylorus-preserving pancreaticoduodenectomy: results of a prospective, randomized, controlled trial. Ann Surg. 2006;243(3):316–320. doi:10.1097/01.sla.0000201479.84934.ca
9. Hochwald SN, Grobmyer SR, Hemming AW, et al. Braun enteroenterostomy is associated with reduced delayed gastric emptying and early resumption of oral feeding following pancreaticoduodenectomy. J Surg Oncol. 2010;101(5):351–355.
10. Shimoda M, Kubota K, Katoh M, Kita J. Effect of Billroth II or Roux-en-Y reconstruction for the gastrojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a randomized controlled study. Ann Surg. 2013;257(5):938–942. doi:10.1097/SLA.0b013e31826c3f90
11. Yang J, Wang C, Huang Q. Effect of Billroth II or Roux-en-Y reconstruction for the gastrojejunostomy after pancreaticoduodenectomy: meta-analysis of randomized controlled trials. J Gastrointest Surg. 2015;19(5):955–963. doi:10.1007/s11605-015-2751-1
12. Nakamura T, Ambo Y, Noji T, et al. Reduction of the incidence of delayed gastric emptying in side-to-side gastrojejunostomy in subtotal stomach-preserving pancreaticoduodenectomy. J Gastrointest Surg. 2015;19(8):1425–1432. doi:10.1007/s11605-015-2870-8
13. Murata Y, Tanemura A, Kato H, et al. Superiority of stapled side-to-side gastrojejunostomy over conventional hand-sewn end-to-side gastrojejunostomy for reducing the risk of primary delayed gastric emptying after subtotal stomach-preserving pancreaticoduodenectomy. Surg Today. 2017;47(8):1007–1017. doi:10.1007/s00595-017-1504-z
14. Hackert T, Probst P, Knebel P, et al. Pylorus resection does not reduce delayed gastric emptying after partial pancreatoduodenectomy: a blinded randomized controlled trial (PROPP study, DRKS00004191). Ann Surg. 2018;267(6):1021–1027. doi:10.1097/SLA.0000000000002480
15. Busquets J, Martín S, Fabregat J, Secanella L, Pelaez N, Ramos E. Randomized trial of two types of gastrojejunostomy after pancreatoduodenectomy and risk of delayed gastric emptying (PAUDA trial). Br J Surg. 2019;106(1):46–54. doi:10.1002/bjs.11023
16. Schorn S, Demir IE, Vogel T, et al. Mortality and postoperative complications after different types of surgical reconstruction following pancreaticoduodenectomy-a systematic review with meta-analysis. Langenbecks Arch Surg. 2019;404(2):141–157. doi:10.1007/s00423-019-01762-5
17. Yeo CJ, Barry MK, Sauter PK, et al. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann Surg. 1993;218(3):228–229. doi:10.1097/00000658-199309000-00002
18. Ohwada S, Satoh Y, Kawate S, et al. Low-dose erythromycin reduces delayed gastric emptying and improves gastric motility after Billroth I pylorus-preserving pancreaticoduodenectomy. Ann Surg. 2001;234(5):668–674. doi:10.1097/00000658-200111000-00013
19. Suzuki H, Matsuzaki J, Fukushima Y, et al. Randomized clinical trial: rikkunshito in the treatment of functional dyspepsia–a multicenter, double-blind, randomized, placebo-controlled study. Neurogastroenterol Motil. 2014;26(7):950–961. doi:10.1111/nmo.12348
20. Arai M, Matsumura T, Tsuchiya N, et al. Rikkunshito improves the symptoms in patients with functional dyspepsia, accompanied by an increase in the level of plasma ghrelin. Hepatogastroenterology. 2012;59(113):62–66.
21. Tominaga K, Kato M, Takeda H, et al. A randomized, placebo-controlled, double-blind clinical trial of rikkunshito for patients with non-erosive reflux disease refractory to proton-pump inhibitor: the G-PRIDE study. J Gastroenterol. 2014;49(10):1392–1405. doi:10.1007/s00535-013-0896-9
22. Takiguchi S, Hiura Y, Takahashi T, et al. Effect of rikkunshito, a Japanese herbal medicine, on gastrointestinal symptoms and ghrelin levels in gastric cancer patients after gastrectomy. Gastric Cancer. 2013;16(2):167–174. doi:10.1007/s10120-012-0164-3
23. Ohno T, Yanai M, Ando H, et al. Rikkunshito, a traditional Japanese medicine, suppresses cisplatin-induced anorexia in humans. Clin Exp Gastroenterol. 2011;4:291–296. doi:10.2147/CEG.S26297
24. Fujitsuka N, Uezono Y. Rikkunshito, a ghrelin potentiator, ameliorates anorexia-cachexia syndrome. Front Pharmacol. 2014;5:271. doi:10.3389/fphar.2014.00271
25. Doi H, Sakakibara R, Sato M, et al. Dietary herb extract rikkunshi-to ameliorates gastroparesis in Parkinson’s disease: a pilot study. Eur Neurol. 2014;71(3–4):193–195. doi:10.1159/000355608
26. Kawahara H, Mitani Y, Nomura M, et al. Impact of rikkunshito, an herbal medicine, on delayed gastric emptying in profoundly handicapped patients. Pediatr Surg Int. 2009;25(11):987–990. doi:10.1007/s00383-009-2453-4
27. Takahashi T, Endo S, Nakajima K, Souma Y, Nishida T. Effect of rikkunshito, a chinese herbal medicine, on stasis in patients after pylorus-preserving gastrectomy. World J Surg. 2009;33(2):296–302. doi:10.1007/s00268-008-9854-8
28. Matsumura T, Arai M, Yonemitsu Y, et al. The traditional Japanese medicine Rikkunshito increases the plasma level of ghrelin in humans and mice. J Gastroenterol. 2010;45(3):300–307. doi:10.1007/s00535-009-0166-z
29. Hayakawa M, Ono Y, Wada T, et al. Effects of Rikkunshito (traditional Japanese medicine) on enteral feeding and the plasma ghrelin level in critically ill patients: a pilot study. J Intensive Care. 2014;2(1):53. doi:10.1186/s40560-014-0053-4
30. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
31. Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. doi:10.1016/j.surg.2005.05.001
32. Akamizu T, Shinomiya T, Irako T, Fukunaga M, Nakai Y, Kangawa K. Separate measurement of plasma levels of acylated and desacyl ghrelin in healthy subjects using a new direct ELISA assay. J Clin Endocrinol Metab. 2005;90(1):6–9. doi:10.1210/jc.2004-1640
33. Japan Pancreas Society ed. Classificaation of Pancreatic Carcinoma.
34. Brierley JD, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours.
35. Fujii T, Sugimoto H, Yamada S, et al. Modified blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg. 2014;18(6):1108–1115. doi:10.1007/s11605-014-2523-3
36. Yanai M, Mochiki E, Ogawa A, et al. Intragastric administration of rikkunshito stimulates upper gastrointestinal motility and gastric emptying in conscious dogs. J Gastroenterol. 2013;48(5):611–619. doi:10.1007/s00535-012-0687-8
37. Briggs DI, Lockie SH, Benzler J, et al. Evidence that diet-induced hyperleptinemia, but not hypothalamic gliosis, causes ghrelin resistance in NPY/AgRP neurons of male mice. Endocrinology. 2014;155(7):2411–2422. doi:10.1210/en.2013-1861
38. Sasaki K, Asaoka T, Eguchi H, et al. Plasma ghrelin suppression as an early predictor for postoperative complications after pancreatoduodenectomy. Pancreatology. 2018;18(1):73–78. doi:10.1016/j.pan.2017.12.002
39. Hoshino N, Nishizaki D, Hida K, Obama K, Sakai Y. Rikkunshito for upper gastrointestinal symptoms: a systematic review and meta-analysis. Complement Ther Med. 2019;42:255–263. doi:10.1016/j.ctim.2018.11.025
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

