Back to Journals » Clinical Interventions in Aging » Volume 17
Effect of Pudendal Nerve Block on the Prevention of Postoperative Bladder Spasm and Catheter-Related Bladder Discomfort in Male Patients Undergoing Transurethral Holmium Laser Enucleation of the Prostate
Authors Wang SY , Qiu Q , Shen X
Received 15 August 2022
Accepted for publication 21 November 2022
Published 30 November 2022 Volume 2022:17 Pages 1729—1738
DOI https://doi.org/10.2147/CIA.S384612
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Sheng-Yan Wang,1 Qing Qiu,2 Xu Shen1
1Medical Center for Anesthesia and Pain, First Hospital of Jiaxing, Jiaxing City, People’s Republic of China; 2Anesthesia Medicine, Zhejiang Chinese Medical University, Hangzhou City, People’s Republic of China
Correspondence: Xu Shen, Email [email protected]
Purpose: Bladder spasm (BS) and catheter-related bladder discomfort (CRBD) are complications after transurethral holmium laser enucleation of the prostate (HoLEP). The pudendal nerve (PN) innervates the perineum. Therefore, we evaluated whether PN block (PNB) can reduce the incidence of BS and CRBD in patients undergoing HoLEP.
Methods: In this randomized, parallel, single-blind prospective study, we enrolled 110 patients who were scheduled to undergo HoLEP under general anesthesia. Patients were randomly allocated to the PNB group (ultrasound-guided PNB at the entrance of the pudendal [Alcock’s] canal bilaterally; n = 55) or the control group (general anesthesia only; n = 55). The primary outcome was the incidence of BS and CRBD at 0.5, 1, 2, 4, 6, 12, and 24 hours postoperatively. The CRBD severity, postoperative pain, and analgesic drug use were also assessed. A P value of < 0.05 was considered statistically significant.
Results: A significantly lower incidence of BS (P = 0.023) and CRBD (P < 0.001) was reported within the first 24 hours postoperatively in the PNB group. The incidence of CRBD above a moderate grade at 0.5 (P < 0.001), 1 (P < 0.001), 2 (P < 0.001), and 4 (P = 0.019) hours postoperatively was significantly lower in the PNB group. Additionally, postoperative pain scores and analgesic drug use were significantly lower in the PNB group. No PNB-related complications were observed.
Conclusion: PNB at the entrance of the pudendal canal bilaterally resulted in a lower incidence of BS and CRBD, less postoperative pain, and less analgesic drug use in patients undergoing HoLEP without PNB-related side effects.
Implication Statement: Bladder spasm and catheter-related bladder discomfort can occur in older individuals undergoing transurethral holmium laser enucleation of the prostate. They often result in severe pain and prolonging the hospital stay. We performed a bilateral ultrasound-guided pudendal nerve block at the entrance of the pudendal (Alcock’s) canal and found that the block reduced bladder spasm, catheter-related bladder discomfort, postoperative pain, and use of anesthetics. Besides, pudendal nerve block also decreased the severity of catheter-related bladder discomfort and helped older individuals achieve rapid recovery.
Keywords: transurethral holmium laser enucleation of the prostate, pudendal nerve block, bladder spasm, catheter-related bladder discomfort, pudendal (Alcock’s) canal
Introduction
Benign prostatic hyperplasia (BPH) often causes dysfunction in urination. Up to 50–80% of men over 60 years of age suffer from BPH.1 Transurethral holmium laser enucleation of the prostate (HoLEP) is a commonly used surgical procedure for BPH.2 To prevent thrombosis, all patients require a three-way urinary catheter to allow continuous bladder drainage after surgery.3 The common complications after prostate surgery due to indwelling catheter and bladder drainage include bladder spasm (BS) and catheter-related bladder discomfort (CRBD).3,4 12.9–28.3%5 of patients experience BS. Some patients experience both BS and CRBD.
BS6 and CRBD7 are induced by involuntary bladder contraction mediated by muscarinic receptors. BS manifests as suprapubic and perineal pain, urine leakage,8 and rebleeding.4 Some severe patients require transfusion or reoperation to stop hemorrhage. Patients experience more CRBD after urological surgery than other surgery, with an incidence of 47–90%,9 and they are more prone to moderate-to-severe CRBD. CRBD is defined as urinary urgency and urethral pain. Severe CRBD is usually accompanied by behavioral responses10 which may result in removal of urinary catheter or other venous access, and hemodynamic instability. Plenty of drugs have demonstrated effectiveness in reducing BS and CRBD, including anticholinergics,11 antiepileptic drugs,7 and anesthetics.12 However, systemic administration often leads to side effects, such as sedation, nausea and vomiting, and xerostomia.7,13
Reoccurrence of BS and CRBD can prolong the length of hospital stay and increase medical costs. Thus, identifying effective ways to prevent these complications is urgent. The urethra and bladder epithelia are rich in nerve endings, which are sensitive to pressure and mechanical stimulation. Indwelling catheter and bladder irrigation excite the stretch receptors, which leads to BS and CRBD. Various regional block modalities have been used for CRBD, such as spinal anesthesia,14 epidural anesthesia,15 dorsal penile nerve block,9 and bladder flushing with bupivacaine.16 Intrathecal bupivacaine and morphine8 are also effective in reducing BS, suggesting that blocking sensory nerves in the urethra and bladder may prevent BS and CRBD.
Afferents to the urethra and vesical trigone originate from the ventral branch of the second to fourth sacral nerves.17 Neuroanatomical studies demonstrated that the afferent fibers of the bladder can travel with the pudendal nerve (PN).18 The PN derives from the anterior branches of the second to fourth sacral nerves19 and innervates the perineum.20–22 PNB has been used to reduce the pain of circumcision surgery,23 prostate biopsy24 and hemorrhoidectomy.25
We hypothesize that PNB may be effective and safe to prevent BS and CRBD after HoLEP. We adopted ultrasound-guided PNB at the entrance of the pudendal (Alcock’s) canal20 with 0.3% ropivacaine (10 mL) unilaterally. The primary outcome was the incidence of BS and CRBD. The secondary outcomes were the CRBD severity, postoperative pain and PNB-related side effects, etc.
Methods
Ethical approval for this study (LS2021-KY-045) was provided by the Ethical Committee of Jiaxing First Hospital, (Chairperson Prof Gang Qian) on 17 May 2021. It was registered with the Chinese Clinical Trials Registry (ChiCTR2200058372, http://www.chictr.org.cn/showproj.aspx?proj=162569) before any patient enrollment. The principal investigator was Shengyan Wang. All participants provided written informed consent before enrollment. The trial was conducted in accordance with the Declaration of Helsinki. The CONSORT 2010 checklist is available as a supplement.
Patients aged 50–90 years of age with an American Society of Anesthesiologists (ASA) Physical Status of II–III and who were scheduled to undergo elective HoLEP for BPH were enrolled. Patients with a history of prostate or bladder neck surgery, severe urinary tract infection, neurogenic bladder, prostate cancer, end-stage renal disease (urine output of < 500 mL/24 h), an overactive bladder, recent use of anticholinergics, diabetes mellitus (bladder involvement causing bladder irritation), severe cardiovascular disease, mental illness or language impairment, allergies to ropivacaine or any other anesthetic, or infection at the site of injection were excluded.
Eligible participants were randomly assigned to two groups based on computer-generated random numbers. Patients in the PNB group underwent general anesthesia combined with ultrasound-guided PNB at the entrance of the pudendal canal bilaterally, while the control group underwent general anesthesia only. An anesthesiologist blinded to randomization opened the opaque envelope with a group code, performed PNB, and did not involve in any other management of the patients. The patient’s postoperative pain, BS and CRBD were managed by another anesthesiologist blinded to patient grouping.
No premedication was administered before anesthetic induction. After entering the operating theater, pulse oximetry, heart rate (HR), non-invasive blood pressure (NIBP), electrocardiography, and the bispectral index were continuously monitored. Before induction, the patient was placed in the lateral decubitus position. A low-frequency curvilinear transducer was sterilized and placed at the midpoint of the line connecting the greater trochanter of the femur and the posterior superior iliac spine. The long axis of the transducer was positioned parallel to the line, and a continuous hyper-echoic hip bone line was identified. Moving the transducer caudally and medially, a continuous hyper-echoic fracture of the hip bone could be observed. When the ultrasound beam intersected the ischial spine, a hyper-echoic shadow appeared again, which revealed the sacrospinous and sacrotuberous ligaments. The two ligaments and the ischial tubercle formed a similar triangular pattern. Then, moving the transducer further, the PN and the pudendal artery and vein were visualized in the proximal portion of the pudendal canal. The PN accompanied the internal pudendal artery. After positioning, an 100-mm (22-G) needle was inserted in-plane from the medial end of the transducer and advanced until the needle tip was adjacent to the PN. The needle was inserted medially to avoid contact with the ischial tuberosity and to minimize the risk of rectal perforation. When the needle passed through the sacrotuberous ligament, there was a clear sense of loss. Ten milliliters of 0.3% ropivacaine was slowly injected with intermittent aspiration. The dose of ropivacaine was determined based on previous studies.9 The local anesthetic spread in a fusiform pattern between the sacrospinous ligament, the sacrotuberous ligament, and the internal pudendal artery. After unilateral block, the position of the patient was changed to block the contralateral side using the same ultrasound-guided technology. After 10 minutes, the degree of block was measured by puncture. Block was deemed effective when the perineum and anus were numb and no pain was felt by puncture.
After confirmation of complete bilateral block, standardized general anesthesia was administered. Etomidate (0.2–0.4 mg/kg), cis-atracurium (0.15 mg/kg), and sufentanil (0.5 μg/kg) were administered, and the trachea was intubated after pre-oxygenation. The ventilation parameters were adjusted to ensure that the end-tidal carbon dioxide partial pressure was maintained at 35–45 mmHg. Anesthesia was maintained with sevoflurane, remifentanil (0.1–1 μg/kg/min), and propofol (50–150 µg/kg/min) and maintained the bispectral index at 40–60. Cis-atracurium (0.15 mg/kg) was administered every 40–50 minutes to maintain muscle relaxation. A total of 250 mL 0.9% physiological saline (PS) and ephedrine (0.1 mg/kg) was administered if the NIBP decreased by > 20% from baseline, and atropine (0.01 mg/kg) was administered if the HR decreased by > 20% from baseline. Standard medication, including 0.5 mg hydromorphone for postoperative analgesia and 3 mg granisetron for postoperative nausea and vomiting, was administered intravenously before the end of surgery. A thermal blanket was used to prevent hypothermia from continuous intraoperative bladder irrigation. The anesthesia protocol was identical in both groups.
After surgery, a 20-F three-lumen urinary catheter with a balloon inflated with 30 mL PS was inserted and fixed in the suprapubic area to ensure no traction. Postoperatively, the patient was moved to the post-anesthesia care unit, and tramadol was used as a rescue drug. If BS occurred, the speed of bladder irrigation was adjusted, and the catheter was gently squeezed and repositioned or flushed repeatedly with PS until the blocked blood clot was drained. If the above measures were ineffective and the pain score was ≥ 4, intravenous tramadol 1.5 mg/kg was supplemented as rescue therapy. Bladder irrigation was performed with 0.9% PS at room temperature, and the irrigation rate was 120–150 gtt/min and adjusted in time based on the color of the drainage fluid. Intermittent bladder irrigation was discontinued after confirming that there was no further risk of active bleeding or clot retention. The catheter was removed when the urine was clear.
The primary outcome was the incidence of BS and CRBD at 0.5, 1, 2, 4, 6, 12, and 24 hours postoperatively. The diagnostic criteria for BS included both subjective and observable symptoms. Subjective symptoms included incontinence-like symptoms, such as the frequent urge to urinate and anal bulging, accompanied by pain in the suprapubic region, lower abdomen, urethra, and myoclonus of the pelvic floor and lower extremities. Observable symptoms included deepening of the drainage fluid and bladder fluid reflux into the irrigation tube or squirting around the urinary catheter.
The secondary outcomes were CRBD severity and postoperative pain, which was assessed using the visual analog scale (VAS) score (0 = no pain, 10 = unbearable pain) at 0.5, 1, 2, 4, 6, 12, and 24 hours postoperatively. Analgesic drug use, catheter acceptance, the irrigation time, the catheter removal time, the length of postoperative hospital stay, and the changes in hemoglobin and hematocrit were also assessed. After the catheter was removed, we asked the patients about their acceptance of the catheter. Unacceptable patients indicated that they could not tolerate the discomfort caused by the catheter, and acceptable patients indicated that they could tolerate or have no discomfort.
In the PNB group, the complications related to PNB were also assessed. The severity of CRBD was assessed on a 4-point scale, as follows: 0 points = no symptoms of urethral or bladder discomfort on questioning; 1 point = mild discomfort in the urethra that was tolerable on questioning; 2 points = chief complaint of lower abdominal distension, and urgency and dysuria without questioning, but not accompanied by behavioral reactions; 3 points = chief complaint of strong urgency, dysuria, and lower abdominal distension without questioning, accompanied by a spontaneous behavioral response, such as limb movement, a strong language reaction, an attempt to remove the catheter, or an attempt to get up to urinate. We defined a score of 1 as mild CRBD, 2 as moderate CRBD, and 3 as severe CRBD.
Patients were educated preoperatively about the VAS score and how to distinguish the symptoms of BS and CRBD. In addition, the general characteristics of the patients, such as height, weight, the duration of anesthesia, and the duration of surgery were collected.
According to the findings of the literature review, we hypothesized that PNB would decrease the incidence of bladder-related complications (BS and CRBD) by approximately 35% (70% to 36%) within 24 hours. Based on this assumption, our calculation showed that 44 patients in each group would be necessary to acquire sufficient power to detect statistical significance, with a two-sided α value of 0.05 and a β value of 0.10. Considering a 20% dropout rate, 55 patients were included in each group.
SPSS version 25.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. The data are expressed either as mean ± standard deviation, median (interquartile range), or number (percent). Data with a normal distribution were analyzed using the Shapiro–Wilk test. Categorical variables, such as postoperative BS and CRBD incidence and severity, were compared using the chi-square test or Fisher’s exact test, as appropriate. Continuous variables, such as the irrigation time, catheter removal time, and length of postoperative hospital stay, were compared using the t-test (for normally distributed data) or the Mann–Whitney U-test (for non-normally distributed data). Postoperative pain was compared between the two groups at each evaluation time using a repeated measures ANOVA, which was performed at an adjusted significance level of < 0.007 (0.05/7) after post-hoc analysis using the Bonferroni method. Otherwise, a P value of < 0.05 was considered statistically significant.
Results
Among the 116 patients who were enrolled, three patients were excluded for neurogenic bladder and three for prostate cancer. A total of 110 patients were randomly grouped and included in the final analysis (Figure 1). No significant between-group differences were observed in age (P = 0.654), ASA score (P = 0.606), height (P = 0.902), weight (P = 0.623), body mass index (P = 0.526), duration of anesthesia (P = 0.190), and duration of surgery (P = 0.149), (Table 1). The average age of patients in the control group was 70.0 ± 6.9 years, while the average age of patients in the PNB group was 70.6 ± 7.1 years (P = 0.654). The clinical and surgical characteristics of the patients are shown in Table 1.
 |
Table 1 Patients’ Characteristics |
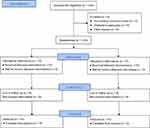 |
Figure 1 A CONSORT flow diagram. |
The irrigation time was significantly shorter in the PNB group [2 (1.0) days vs 3 (1.0) days; P < 0.001]. Moreover, the postoperative catheter removal time was significantly shorter in the PNB group [6 (1.0) days vs 6 (2.0) days; P = 0.011]. After catheter removal, we observed a significantly greater rate of catheter acceptance in the PNB group [17 (30.9%) vs 28 (50.9%), respectively; P = 0.033]. Besides, the length of postoperative hospital stay was significantly shorter [6 (1.0) days vs 6 (2.0) days; P = 0.009] in the PNB group.
We observed a significant reduction in the incidence of BS within 24 hours postoperatively in the PNB group (9% vs 25%; P = 0.023; Table 2). The rate of CRBD in the PNB group was remarkably lower than in the control group at 0.5 (36.4% vs 76.4%), 1 (30.9% vs 76.4%), 2 (23.6% vs 74.5%), 4 (21.8% vs 70.9%), 6 (10.9% vs 58.2%), 12 (3.6% vs 38.2%), and 24 (0% vs 20%) hours (P < 0.001 for all; Table 3; Figure 2). The incidence of CRBD above a moderate grade during the first 4 hours postoperatively was significantly lower in the PNB group (0.5 hours: 10.9% vs 41.8%, P < 0.001; 1 hour: 5.5% vs 40%, P < 0.001; 2 hours: 1.8% vs 32.7%, P < 0.001; 4 hours: 0.0% vs 12.7%, P = 0.019; Table 3, Figure 3). Moreover, none of the patients in the PNB group developed moderate-to-severe CRBD after 4 hours postoperatively.
 |
Table 2 Incidence of Bladder Spasm in the First 24 Hours and Irrigation and Catheter Removal Time |
 |
Table 3 Incidence and Severity of CRBD in Patients Undergoing HoLEP |
The VAS scores differ significantly between the two groups throughout the 24-hour postoperative period (main effect of time: F = 11.68, P < 0.001, main effect of group: F = 5.14, P < 0.001). A total of 28 tramadol administration was required in two groups. Whereas 21 of them (75%) were administered to the patients without PNB, 7 (25%) were in the PNB group (P = 0.002). There were no significant differences between the two groups in terms of the changes in hemoglobin and hematocrit (P = 0.112 and P = 0.240, respectively). None of the patients developed PNB-related complications.
Discussion
In this study, we investigated the effects of ultrasound-guided PNB at the entrance of the pudendal (Alcock’s) canal on BS, CRBD, and postoperative analgesia. We included 110 patients and found that PNB reduced the incidence of BS to 9.0%, which was significantly lower than 25.0% in the control group. PNB reduced the incidence of CRBD by at least half, as well as decreasing the severity of CRBD and the VAS score.
The most general complications early after HoLEP include BS and CRBD.4 Most patients with BPH are older men.1 Older individuals often accompany by underlying diseases, and the severe pain and psychological burden of BS can easily lead to hemodynamic instability. Most cases of mild CRBD are tolerable, but the incidence of moderate-to-severe CRBD is 27–55%,12 and a strong behavioral reaction can lead to secondary bleeding and catheter prolapse. The CRBD incidence in the control group in our study was 76.4%, which is consistent with previous studies on patients undergoing urological surgery.26,27 BS often occurs in patients with long-term indwelling catheters or after urological surgery. The incidence of BS in the control group in this study was 25.00%, which is similar to the incidence of 28.57% in a previous study.4 Bladder irrigation,4 male sex, and the use of thicker catheters may be factors that predispose to BS and CRBD28 after HoLEP. BS and CRBD29 are both mediated by muscarinic receptor activation. CRBD can also be mediated by prostaglandin release.12 In this study, 20 patients (36.36%) in the control group developed both BS and CRBD simultaneously, compared with only 4 patients (7.27%) in the PNB group.
There are currently a number of drugs with different mechanisms used to treat both BS and CRBD. However, these drugs have many adverse reactions.13 Anticholinergics have significant side effects for all patients, especially the elderly, and are linked to cognitive impairment and dementia.30 Antiepileptic drugs such as valium may lead to hangover effect or dependence. So, it should be used with caution in the elderly. Besides, opioids are over medicating for the symptom and often do not provide appropriate relief because of different mechanisms. And, it can cause ileus or respiratory depression. In addition to drug therapy, various regional block modalities have been used for CRBD, which may be accompanied by nausea and vomiting, affecting early postoperative mobility and local anesthetic poisoning. Despite these complications, they all reduce the occurrence and severity of CRBD to some extent. Intrathecal bupivacaine and morphine are also effective in reducing the discomfort associated with BS.8 This evidence provides new ideas for the management of BS and CRBD; specifically, block of sensory nerves in the bladder and urethra.
We found BS was significantly reduced after HoLEP in the PNB group. No similar studies have previously demonstrated that PNB can be used for BS. Yoo et al31 reported that efferent somatic nerve branches originating from the PN can cause bladder contraction, and afferent fibers that conduct pain from the bladder floor, urethral sphincter, and prostate capsule propagate through the PN.18 Koning et al8 found that intrathecal bupivacaine and morphine could reduce BS by reducing bladder contraction after robot-assisted radical prostatectomy. However, many patients reported pruritus after intervention. Young et al6 found that intravesical injection of botulinum neurotoxin significantly reduced BS and urine leakage, with 94% of patients reporting that their symptoms were controlled. However, 38% of patients reported urinary tract infections after receiving botulinum neurotoxin. Some scholars4 used a self-made speed-adjusting card to adjust the speed of bladder irrigation after prostate surgery. They found that the incidence of BS and the irrigation time were significantly lower/shorter in the experimental group. Our study demonstrated a significantly lower incidence of BS and a shorter irrigation time, similar to the conclusion of this study. Another significant finding of the present study was the significant reduction in postoperative pain score and tramadol consumption in the PNB group, which further confirmed the positive effect of PNB on pain.
We also found that after PNB, postoperative CRBD was significantly reduced and less severe, and the catheter acceptance was high. The PN is the main nerve that innervates the perineum, and the dorsal nerve of the penis, which is a branch of the PN, innervates the membranous urethra,9 which may reduce urethral stimulation by the catheter. In a study conducted in 2017, nerve stimulator-guided transperineal PNB with the sciatic spine as a marker decreased both the incidence (64% vs 90%) and intensity of CRBD and reduced postoperative pain after trans-urethral resection of prostate and trans-urethral resection of bladder tumor.32 Nonetheless, despite the statistically significant difference, it was less effective than our study which reduced the rate of CRBD from 74.5% to 23.6%. And, that study resulted in one patient with levator ani weakness after surgery. Lean et al33 performed PNB with the ischial spine as a marker in patients with interstitial cystitis who did not respond well to surgery and analgesics. The symptoms of bladder pain, frequent urination, and nocturia were significantly reduced. They32,33 all used the sciatic spine as a marker to perform PNB. However, it is easy to block the sciatic nerve and damage the pudendal artery and vein adjacent to the PN.20 Also at this level, the inferior rectal nerve may branch, resulting in incomplete block.
The difference between our study and other similar studies32,33 is the use of ultrasound to block the PN in the posterior gluteal region at the entrance to the pudendal canal.20 The degree of block that can be achieved in this area is relatively high, and the PN and its branches can all be blocked. Moreover, the blocking effect is more comprehensive with fewer adverse reactions.20 Furthermore, our study also used ultrasound to visualize the anatomy and spread of local anesthesia in real-time to void intravascular injections and prevent rectal or urethral injury. PNB-related complications,20 such as hematoma, sciatic nerve block, and local anesthetic systemic toxicity, were not observed in our study. Although the pathogenesis of BS and CRBD is similar, the present study found that their manifestations were inconsistent. BS often manifested as severe pain and darkening of the drainage fluid, while CRBD often manifested as urgency or attempts to remove the catheter. Moreover, although PNB significantly reduced the incidence of BS and CRBD, there was little change in hemoglobin and hematocrit, which may be because we dealt with BS and CRBD in time, or because the sample size was small and the difference was not significant.
Several limitations of this study should be acknowledged. First, a surgical extension can influence BS and the grade of CRBD. However, we did not consider the surgical characteristics, such as a degree of BPH, amount of prostate resected. Second, the same group of surgeons did not perform all procedures. The technique and level of skill differed between surgeons, which may have affected the results. Third, it was difficult to differentiate between severe CRBD with behavioral responses versus postoperative delirium because patients did not use standardized tools to assess postoperative delirium. In future studies, a validated tool may be needed to accurately distinguish severe CRBD from postoperative delirium.
Conclusion
Ultrasound-guided PNB at the entrance of the pudendal canal bilaterally can successfully reduce BS and CRBD and the severity of CRBD in man undergoing HoLEP, as well as providing pain relief. These results suggest that PNB is an effective and safe option to prevent BS and CRBD in male patients undergoing HoLEP who require a large-diameter urinary catheter.
Abbreviation
BS, bladder spasm; CRBD, catheter-related bladder discomfort; HoLEP, transurethral holmium laser enucleation of the prostate; PN, pudendal nerve; PNB, pudendal nerve block; BPH, benign prostatic hyperplasia; HR, heart rate; NIBP, non-invasive blood pressure; PS, physiological saline; VAS, visual analog scale.
A Data Sharing Statement Indicating
The datasets generated or analyzed during this study are available from the corresponding author named Xu Shen on reasonable request.
Acknowledgments
We thank Dr. Yibing Yao and Dr. Jing Cao for their contributions to the previous research on pudendal nerve block.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gudmundsson J, Sigurdsson JK, Stefansdottir L, et al. Genome-wide associations for benign prostatic hyperplasia reveal a genetic correlation with serum levels of PSA. Nat Commun. 2018;9(1):4568. doi:10.1038/s41467-018-06920-9
2. Zeng XT, Jin YH, Liu TZ, et al. Clinical practice guideline for transurethral plasmakinetic resection of prostate for benign prostatic hyperplasia (2021 edition). Mil Med Res. 2022;9(1):14. doi:10.1186/s40779-022-00371-6
3. Cornu JN, Ahyai S, Bachmann A, et al. A systematic review and meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic obstruction: an update. Eur Urol. 2015;67(6):1066–1096. doi:10.1016/j.eururo.2014.06.017
4. Ma ZZ, Han YX, Wang WZ, et al. The use of a homemade rate adjustment card in patients with continuous bladder irrigation after transurethral resection of the prostate. Transl Androl Urol. 2020;9(5):2227–2234. doi:10.21037/tau-20-1288
5. Zhang J, Wang Y, Li S, et al. Efficacy and safety evaluation of transurethral resection of the prostate versus plasmakinetic enucleation of the prostate in the treatment of massive benign prostatic hyperplasia. Urol Int. 2021;105(9–10):735–742. doi:10.1159/000511116
6. Young MJ, Osman NI, Phillips L, et al. Another therapeutic role for intravesical botulinum toxin: patients with long-stay catheters and refractory bladder pain and catheter bypass leakage. Eur Urol Focus. 2020;6(2):339–343. doi:10.1016/j.euf.2018.10.011
7. Jang E, Hong SH, Kim KS, et al. Catheter-related bladder discomfort: how can we manage it? Int Neurourol J. 2020;24(4):324–331. doi:10.5213/inj.2040108.054
8. Koning MV, Vlieger RD, Teunissen A, et al. The effect of intrathecal bupivacaine/morphine on quality of recovery in robot‐assisted radical prostatectomy: a randomised controlled trial. Anaesthesia. 2020;75(5):599–608. doi:10.1111/anae.14922
9. Gger YE, Zkent MS, Gger E, et al. A randomized‐controlled, prospective study on the effect of dorsal penile nerve block after TURP on catheter‐related bladder discomfort and pain. Int J Clin Pract. 2021;75(5):e13963. doi:10.1111/ijcp.13963
10. Park JY, Hong JH, Yu J, et al. Effect of ketorolac on the prevention of postoperative catheter-related bladder discomfort in patients undergoing robot-assisted laparoscopic radical prostatectomy: a randomized, double-blinded, placebo-controlled study. J Clin Med. 2019;8(6):759. doi:10.3390/jcm8060759
11. Li S, Li P, Wang R, Li H. Different interventions for preventing postoperative catheter-related bladder discomfort: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022;78(6):897–906. doi:10.1007/s00228-021-03251-5
12. Kim D-H, Park J-Y, Yu J, et al. Intravenous lidocaine for the prevention of postoperative catheter-related bladder discomfort in male patients undergoing transurethral resection of bladder tumors: a randomized, double-blind, controlled trial. Anesth Analg. 2020;131(1):220–227. doi:10.1213/ANE.0000000000004405
13. De E, Gomery P, Rosenberg LB. Palliation of bladder spasms #337. J Palliat Med. 2017;20(10):1158–1159. doi:10.1089/jpm.2017.0353
14. Yu SS, Doo AR, Park HJ. The novel method to reduce catheter-related bladder discomfort after transurethral prostate surgery. World J Mens Health. 2020;38(1):137–138. doi:10.5534/wjmh.190069
15. Sun S, Wang C, Zhang J, et al. Occurrence and severity of catheter-related bladder discomfort of general anesthesia plus epidural anesthesia vs. general anesthesia in abdominal operation with urinary catheterization: a randomized, controlled study. Front Surg. 2021;8:658598. doi:10.3389/fsurg.2021.658598
16. Pournajafian A, Ghodraty MR, Shafighnia S, et al. The effect of intravesical diluted bupivacaine on catheter-related bladder discomfort in young and middle-aged male patients during postanaesthetic recovery. Turk J Anaesthesiol Reanim. 2020;48(6):454–459. doi:10.5152/TJAR.2020.18999
17. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56(4):581–631. doi:10.1124/pr.56.4.4
18. Akkaya T, Ozkan D, Karakoyunlu N, et al. Pudendal block in transurethral prostatectomy. Eur J Anaesthesiol. 2015;32(9):656–657. doi:10.1097/EJA.0000000000000172
19. Kale A, Usta T, Basol G, et al. Comparison of ultrasound-guided transgluteal and finger-guided transvaginal pudendal nerve block techniques: which one is more effective? Int Neurourol J. 2019;23(4):310–320. doi:10.5213/inj.1938112.056
20. Bendtsen TF, Parras T, Moriggl B, et al. Ultrasound-guided pudendal nerve block at the entrance of the pudendal (Alcock) canal: description of anatomy and clinical technique. Reg Anesth Pain Med. 2016;41(2):140–145. doi:10.1097/AAP.0000000000000355
21. Waldum SH, Staff AC, Lukasse M, et al. Intrapartum pudendal nerve block analgesia and risk of postpartum urinary retention: a cohort study. Int Urogynecol J. 2021;32(9):2383–2391. doi:10.1007/s00192-021-04768-0
22. Kastler A, Puget J, Tiberghien F, et al. Dual site pudendal nerve infiltration: more than just a diagnostic test? Pain Physician. 2018;21(1):83–90. doi:10.36076/ppj.1.2018.83
23. Gaudet-Ferrand I, Arena P, Bringuier S, et al. Ultrasound‐guided pudendal nerve block in children: a new technique of ultrasound‐guided transperineal approach. Paediatr Anaesth. 2018;28(1):53–58. doi:10.1111/pan.13286
24. Rahmi A, Erbin A, Celik S, et al. Periprostatic nerve block alone versus combined with perineal pudendal nerve block or intrarectal local anesthesia during transrectal ultrasound‐guided prostate biopsy: a prospective randomized controlled trial. Int J Urol. 2019;26(8):833–838. doi:10.1111/iju.14036
25. Steen CJ, Lam D, Chandra R, et al. Pudendal nerve block for posthemorrhoidectomy pain: a prospective, single-blinded randomized control trial. Dis Colon Rectum. 2022;65(4):546–551. doi:10.1097/DCR.0000000000002293
26. Zugail AS, Pinar U, Irani J. Evaluation of pain and catheter-related bladder discomfort relative to balloon volumes of indwelling urinary catheters: a prospective study. Investig Clin Urol. 2019;60(1):35–39. doi:10.4111/icu.2019.60.1.35
27. Prajapati DJ, Patel M, Patel P, et al. Effect of caudal bupivacaine alone and with adjuvant fentanyl and nalbuphine to minimize the catheter-related bladder discomfort after tubeless percutaneous nephrolithotomy: a prospective randomized study. J Anaesthesiol Clin Pharmacol. 2020;36(4):524–530. doi:10.4103/joacp.JOACP_285_18
28. Bach H, Kaasby K, Srensen A, et al. Incidence and severity of catheter-related bladder discomfort among nonurological adult patients in a postanesthesia care unit. J Perianesth Nurs. 2020;35(1):29–33. doi:10.1016/j.jopan.2019.06.013
29. Am A, Ac A, Ew A, et al. Facteurs prédictifs de l’inconfort lié à la sonde vésicale. Prog Urol. 2020;30(16):1045–1050. doi:10.1016/j.purol.2020.09.014
30. Coupland CAC, Hill T, Dening T, et al. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084–1093. doi:10.1001/jamainternmed.2019.0677
31. Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol. 2008;212(1):218–225. doi:10.1016/j.expneurol.2008.04.010
32. Xiaoqiang L, Xuerong Z, Juan L, et al. Efficacy of pudendal nerve block for alleviation of catheter-related bladder discomfort in male patients undergoing lower urinary tract surgeries: a randomized, controlled, double-blind trial. Medicine. 2017;96(49):e8932. doi:10.1097/MD.0000000000008932
33. Lean LL, Hegarty D, Harmon D. Analgesic effect of bilateral ultrasound-guided pudendal nerve blocks in management of interstitial cystitis. J Anesth. 2012;26(1):128–129. doi:10.1007/s00540-011-1243-z
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


