Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Effect of Pleurotus fossulatus Aqueous Extract on Biochemical Properties of Liver and Kidney in Streptozotocin-Induced Diabetic Rat
Authors Dubey S, Yadav C, Bajpeyee A, Singh MP
Received 5 June 2020
Accepted for publication 4 August 2020
Published 24 August 2020 Volume 2020:13 Pages 3035—3046
DOI https://doi.org/10.2147/DMSO.S265798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Video abstract presented by Mohan P Singh.
Views: 180
Sushil Dubey, Chandrabhan Yadav, Anand Bajpeyee, Mohan P Singh
Centre of Biotechnology, University of Allahabad, Prayagraj, Uttar Pradesh, India
Correspondence: Mohan P Singh
Coordinator, Centre of Biotechnology Institute of Interdisciplinary Studies (IIDS), University of Allahabad, Prayagraj 211002, India
Email [email protected]
Background: The antidiabetic effects of the Pleurotus fossulatus on liver and kidney were unexplored. The present study assessed the vital effects of P. fossulatus aqueous extract (PFA-extract) on liver and kidney function in diabetic rats model.
Methods: The albino Wistar rats were divided into five groups with six animals in each. Group, I, II, and III were normal, diabetic, and drug control, respectively, and groups IV and V were test groups. As treatment dose, group II received saline, and group III (drug control) received metformin in 100 mg/kg of body weight as a standard drug. Whereas, group IV (D1) and V (D2) were test groups, received PFA-extract in 250 mg/kg, and 500 mg/kg of body weight, respectively. The changes in body weight, blood glucose level (BGL), lipid profile, liver, and kidney biochemical parameters were evaluated.
Results: The PFA-extract dose at 500 mg/kg in D2 groups showed very good effects. The body weight was recovered by 97.9% and blood glucose level (BGL) was reduced to 53% in the D2 group. For the lipid profile, total cholesterol (TC), triglyceride (TG), and high-density lipoprotein (HDL) were estimated in blood plasma and their values were 92.31± 3.788, 80.85± 8.962, and 31.35± 1.781, respectively. PFA-extract improved the liver function for which aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were evaluated and recorded to their normal range in D2 group which were 132.01± 5.553, 58.63± 4.390, and 143.26± 2.423, respectively. For the kidney function test, creatinine (CRT), blood urea nitrogen (BUN), and uric acid were evaluated and in the D2 group, it was significantly reduced ie, 0.656± 0.0707, 15.13± 1.463, and 6.27± 0.325, respectively.
Conclusion: These attributes of PFA-extract make it a potential natural agent to provide protection to the liver and kidney and also reduce the BGL and control the total lipid in type 1 diabetes condition.
Keywords: Pleurotus fossulatus, protection of liver and kidney, streptozotocin, antidiabetic
Introduction
Diabetes mellitus type 1 (insulin-dependent diabetes mellitus) is classified under metabolic disorder caused by the autoimmune destruction of pancreatic β-cells. Insulin is secreted by pancreatic β-cells which help to reduce the blood glucose level. Patients with type 1 diabetes require taking the exogenous insulin by injections on an interval of time in a day. About 5–10% (5–50 million) of all the diabetes patients are type 1 diabetes which is a huge number world-wide. Generally, type 1 diabetes is found in adolescence or childhood, but it can appear at any stage of age. People, who are at risk of developing type 1, can be identified by genetic markers and auto-antibodies which target the β-cells, insulin, glutamic acid decarboxylase (GAD), tyrosine phosphate IA-2, IA-2β, and ZnT8. These auto-antibodies are found in either one or more than one individual, who suffer from type 1 diabetes.11 An individual suffering from type 1 diabetes may suffer from weight loss, polyuria, blurred vision, polyphagia, and polydipsia. It may also cause stupor, coma in uncontrolled condition and if not treated then it may lead to death because of ketoacidosis or by non-ketotic hyperosmolar syndrome.13
During the diabetes condition, the most affected organs are liver and kidney. The liver plays a very important role in the maintenance of glucose levels in the body. It regulates the metabolism of glucose by using glucose molecules as an energy source. It also has a tendency to synthesize glycogen from non-carbohydrate compounds. Diabetes mellitus may lead to liver associated disease as Non-alcoholic fatty liver disease (NAFLD). The abnormal level of liver enzymes, ie, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) may be symptoms of NAFLD. ALT has been considered as the marker for NAFLD and AST along with ALP are the indicators of hepatocellular injury.14,18 ALP is released in the blood and plasma due to the damage of the liver and its normal range falls between 44–147 IU/L.9
A kidney is the most common organ to get affected and the disease caused by diabetes is called Diabetic Nephropathy (DN). DN is a clinical condition in diabetes where renal function and structure of the kidney gets disrupted. To monitor the kidney function test, the easiest way is to analyze the Blood Urea Nitrogen (BUN) and creatinine level in blood. These are the waste products produced due to the metabolic breakdown of protein and creatine and excreted by the kidney. During kidney disease, these materials including others, accumulate in the body which result in the elevation of urea and creatinine level. In normal condition the BUN level should range between 7–20 mg/dL and creatinine level should be 0.8–1.4 mg/dL.4 Uric acid is a waste material which is filtered by the kidney. If a kidney is not working properly then uric acid accumulates in the body and causes gout disease.
Inspite of many drugs and strategies for type 1 diabetes mellitus, they have some side effects and limitations, so these drugs require perpetual improvement. Natural medicines are being used since ancient times for the treatment of diabetes mellitus.27 The natural compounds may help to restore and maintain the numbers of pancreatic β-cells. These natural compounds can be found in some plants and in some edible mushrooms which are macrofungi. People in Asian countries like India and China were well versed with the culinary effects of these mushrooms. Mushrooms have many species, some of them are poisonous and some are edible. Oyster mushroom (Pleurotus) is one of the edible mushrooms, contains vitamins (A, B, C, D, K) and dietary fibers like α/β-glucans and chitin.19 They are very good in Reactive Oxygen Species (ROS) scavenging activity, which is also a cause of diabetes mellitus.
Pleurotus mushroom has many species that have been tested for their medicinal and nutritional value. Among them, Pleurotus ostreatus has been tested for anti-cancerous and anti-diabetic properties in the in vitro cell lines and on the animal model.24,26 Pleurotus abalones, Pleurotus sajor-caju, Pleurotus citrinopileatus, Pleurotus pulmonarius, Pleurotus eryngii5 have been used to explore their anti-diabetic properties, all of these have shown to lower the blood glucose level but not very efficiently, so the searching of other species with greater potential to lower the blood glucose level was required.
The present study determined the properties of P. fossulatus aqueous extract to reduce not only the blood glucose level but also provides the protection to liver and kidney from harmful effects during diabetic conditions.
Materials and Methods
Chemicals Required
Streptozotocin (STZ) was used to induce diabetes and metformin was used as a drug control. All the chemicals and solvent used were analytical grade and purchased from Himedia and Merck. The biochemical assays were performed by the Star 21 Plus Semiautomatic Biochemistry Analyzer.
Mushroom Extract Preparation
Pleurotus fossulatus was cultivated in Mushroom Biotechnology lab, Centre of Biotechnology, University of Allahabad, India. The fruiting body was air-dried under shade for five days, then prepared the powder by using a grinder.
The magnetic-stirrer-based extraction of P. fossulatus was performed. For the extraction, we took 100 gm powder of P. fossulatus and mixed with 1000 mL milli q water and kept at 60°C temperature for 72 hours. During this time continuous stirring by magnetic bead was allowed. The extract was filtered twice by Whatman no.1 filter paper and dried by using water-bath at 60°C. The total percentage yield of extract was 25% w/w.
Animal Experiments Design
Albino Wistar rats weighing 175 ± 25 gm were used for all experiments in the present study. The sex of animals used in the study was male, collected at random from the animal house of the University of Allahabad. Animals were housed in separate cages under controlled conditions of temperature (22 ± 2°C). All animals were given a standard diet (Golden feed, New Delhi, India) and water ad libitum. Animals were kept at 12:12 hours, light: dark cycle. Animals were further divided into five groups with six animals in each group.
Group I received normal saline P.O. (by opening/orally) only and served as vehicle control, Group II, III, IV, and V were diabetic, induced by streptozotocin (STZ). Group II received saline only for 21 days. Group III received metformin, 100 mg/kg/day P.O., group IV received extract 250 mg/kg/day P.O., and group V received extract 500 mg/kg/day P.O. All dosing of the test sample was done P.O. throughout the experiment.
Induction of Diabetes
Diabetes was induced in rats by intraperitoneal injection (i.p.) of STZ at a dose of 60 mg/kg body weight. STZ was dissolved in ice-cold 0.1M citrate buffer. The animals were allowed to drink 5% glucose solution overnight to overcome STZ-induced hypoglycemia. The animals were considered diabetic if their blood glucose value was above 200 mg/dl on 3rd day of STZ injection. The treatment was continued till 21 days; blood glucose level and body weights were measured on 0, 4, 8, 12, 16, and 21 days of post-treatment.
Separation of Plasma from the Blood
For the separation of plasma, 5 mL of blood was collected in an anticoagulant (EDTA) containing a centrifuge tube. The tube was centrifuged at 3000 rpm for 20 minutes. RBCs and plasma were separated in pallets and supernatant, respectively. The plasma was collected in a separate vial.
Effects of PFA-Extract on Plasma Lipid Profiling
Plasma was used to test the total cholesterol (TC), and high-density lipoprotein (HDL) (catalog number- CHOD-PAP, 71LS200-75) and triglyceride (TG) (catalog number- GPO-PAP, 72LS100-40) were estimated by using kits purchased from Arkray Healthcare Pvt. Ltd, Mumbai, India. All the samples and standards were prepared according to the instructions, given in the catalogue.
Effects of PFA-Extract on Liver Function
Plasma was used to determine the alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) by using kits with catalog no. 75DP200-20, 76LS200-60, 77LS200-60, respectively, purchased from Arkray Healthcare Pvt. Ltd, Mumbai, India. All the samples and standards were prepared according to the instructions, given in the catalogue.
Effects of PFA-Extract on Kidney Function
Creatinine (CRT), blood urea nitrogen (BUN), and uric acid were estimated in plasma by kit-based method, purchased from Erba Mannheim Pvt. Ltd with catalog no. 120,246, 120,214, and 120,185, respectively. All the samples and standards were prepared according to the instructions, given in the catalogue.
Histology of Liver
The liver from each group was removed, washed with saline and kept in 10% formalin solution. After washing the liver was transferred in mixture of absolute alcohol and xylene (1:1) for 15–20 minutes. The tissues were put in xylene for 30 minutes. The paraffin blocks were prepared and the sections of 5 μm thickness were cut by a microtome. The sections were transferred to slides and de-waxed with xylene. The sections were stained with hematoxylin solution for 30 minutes and subsequently counterstained with eosin and then mounted.
Statistical Analysis
The data of body-weight and blood glucose level were analyzed by two-way ANOVA and rest other data were analyzed by one-way ANOVA followed by the Bonferroni test. P<0.05 was considered as a level of significance. All data are presented in Mean ± SD. The graphs were plotted by using GraphPad Prism5 software.
Results
The Effect of PFA-Extract on Body Weight
The effect of P. fossulatus aqueous extract (PFA-extract) on the body weight of rats is given in Table 1 and statistical comparison is shown in Figure 1. There was a significant difference (P<0.05) in the body weight of diabetic control, D1, and D2 groups as compared to normal control before the commencement of the experiment. But after 4 days of the experiment, there was no significant difference in the body weight of these groups. The diabetic control and drug control groups showed a significant difference (P<0.05) at 12th and 16th days but the test groups, ie, D1 and D2 had no significant difference. However, the D2 group showed significant (P<0.05) improvement in body weight after 21 days of dosing and recovered by 97.9% whereas, drug control recovered 98.6%.
 |
Table 1 The Effects of PFA-Extract on Body Weight in Rats |
The Effect of PFA-Extract on Blood Glucose Level (BGL)
The effect of PFA-extract on rats is shown in Table 2 and statistical comparison is given in Figure 2. The blood glucose level of diabetic control was slightly higher than the normal group before the commencement of the experiment. There was a sharp increase of BGL in all groups observed except normal control on 4th day. On the 8th and 12th day of the drug control experiment, D1, and D2 groups started to reduce significantly (P<0.05) as compared to normal and diabetic control groups. After 21 days of the treatment, the BGL reduced by 35.7%, 53.0%, and 55.3% of D1, D2, and drug control groups, respectively, and the D2 group showed no significant difference (P<0.05) as compared to the normal group.
 |
Table 2 The Effect of PFA-Extract on BGL in Rats |
The Effect of PFA-Extract on Lipid Profiling
The effect of PFA-extract on lipid profile is depicted in Table 3 and the statistical comparison is shown in Figure 3. In this study, we analyzed total cholesterol (TC), triglyceride (TG), and high-density lipoprotein. The D2 group showed a significant reduction (P<0.0001) of TC and TG levels when compared to the diabetic control group and there was a slight difference (P<0.05) as compared to the normal control group. The HDL level was highest (P<0.0001) in the D2 group than normal and drug control groups.
 |
Table 3 The Effect of PFA-Extract on Lipid Profile in Rats |
The Effect of PFA-Extract on Liver Function
The effect of PFA-extract on liver function is given in Table 4 and the statistical comparison is depicted in Figure 4. Three enzymes were examined, ie, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatases (ALP). The AST level was significantly (P<0.0001) reduced to normal level in the D2 group whereas, the D1 group had a low significant difference (P<0.001) as compared with the diabetic control group and there was no significant difference with the normal control group. The ALT and ALP levels reduced significantly (P<0.05), (P<0.0001) in the D2 group as compared with the normal control group.
 |
Table 4 The Effect of PFA-Extract on Liver Function in Rats |
The Effect of PFA-Extract on Kidney Function
The effect of PFA-extract on creatinine (CRT), blood urea nitrogen (BUN), and uric acid is mentioned in Table 5 and statistical comparison is shown in Figure 5. After 21 days of dosing, the CRT level in D1 and D2 group was found normal and had no significant difference with the normal control group. There was a significant difference (P<0.0001) (P<0.0001) of BUN and uric acid level was observed, respectively, in D1, and D2 groups when compared with the normal control group.
 |
Table 5 The Effect of PFA-Extract on Kidney Function in Rats |
Observations of Liver Histology
As shown in Figure 6, all the images of liver histo-slides were taken at 400X from Olympus microscope (modal; CKX53, camera model; Megcam-MU2A). Normal control group had normal structure of hepatocytes with regular radial morphology of hepatic architecture with normal sinusoids. Diabetic group had many pathological changes, characterized as increased number of kuffer cells, monocytes, dilatation of sinusoids, and narcosis. Whereas, drug control group had improved dilatation of sinusoids, normal kuffer cells, and improved hepatic architecture compared with normal control. D1 and D2 group showed significant improvement, characterized as improved hepatic architecture, reduced kuffer cells, and improved sinusoids, as compared with normal control and drug control groups.
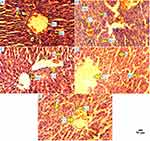 |
Figure 6 Histopathology of rat liver. Different groups of rat liver (400X) stained with hematoxylin and eosin. |
Discussion
DM is a complex and serious metabolic disorder which can affect the major vital functions of the body. There are about 390 million people suffering from DM, world-wide. These numbers are expected to rise by 500 million by 2030.15 There is no permanent treatment for the DM till now, but few oral/injection-based therapies are used to control the blood glucose level (BGL) and protect from adverse effects. The therapies are; insulin, metformin, biguanides, α-amylase, and α-glucosidase inhibitors, sulfonylureas.25 Due to the possibility of adverse/side effects of these synthetic medicines, these are less preferred than natural hypoglycemic agents, which can have less side-effect. Edible mushrooms are used as hypoglycemic agents from ancient time.5 P. fossulatus is a species of oyster mushroom, cultivated in Microbial and Mushroom Biotechnology Lab of Centre of Biotechnology, University of Allahabad, India. For the first time, we are reporting its antidiabetic property along with protective effect on the liver, kidney, and pancreas. This study was carried to determine the effects of PFA-extract on BGL, body weight, lipid profile, kidney function and liver function on the STZ-induced diabetic rat model.
Streptozotocin (STZ) is a diabetogenic agent, causes irreversible damage to the pancreatic β-cells, resulting in impaired secretion of insulin.22 STZ-induced diabetes rat generally considered as diabetes type 1 models because animals start to lose body weight drastically, which have already been reported by the researchers.7,10 The loss of body weight is caused by the degradation of structural protein, which participates in the building of body weight.12 Previous reports suggest that protein synthesis decreased because of a low level of ATP formation in insulin-deficient animals.21 The present study suggests that the PFA-extract at 500 gm dose improves the body weight by 97.9% in the STZ-induced diabetic rat model (Table 1; Figure 1). The possible mechanism behind this, as it improves the secretion of insulin and promotes ATP formation which further promotes the synthesis of structural proteins. So, P. fossulatus can be very helpful in controlling diabetes if it is given with other definite medicines.
The elevated level of blood glucose is always a serious concern because it could hamper the vital functions of many organs like kidney function, liver function and cause eye-related problems. So, it is necessary to reduce the blood glucose level and improve the complications associated with diabetes.2,3,24 In the present study, the PFA-extract reduced the blood glucose level significantly in STZ-induced diabetic rats. The D2 group which received 500 mg/kg of body weight dose was found the most effective than the D1 group which received the lowest dose, ie, 250 mg/kg of body weight. The possible mechanism behind the PFA-extract’s hypoglycemic activity is because of its insulin-releaser action and recovery of β-cells.16 Ng et al (2015)19 also reported hypoglycemic and antidiabetic activity of P. sajor-caju aqueous extract on STZ-induced diabetic rats which further confirms the relevance of our work. Due to its efficacy in controlling the blood glucose levels, P. fossulatus could be a potential natural antidiabetic agent.
Destruction of β-cells causes the reduction of insulin secretion which leads to low lipoprotein lipase activity and elevated level of free fatty acids from fat tissues, this results in hyperlipidemia condition, which is associated with uncontrolled diabetes type 1. From studies, it has been reported that TC, TG level in blood increases whereas, HDL level decreases.1,6,17 Our results showed the same pattern, ie, TC and TG level elevated and HDL level decreased. After treatment with the PFA-extract, at 500 mg dosing, TC and TG levels were found significantly low whereas, HDL level increased maximum (Figure 3). The results showed that a 500 mg dose can reduce the risk of cardiovascular disease and help in the removal of cholesterol. The improved hyperlipidemia condition may be due to the improvement of insulin secretion because insulin controls the biosynthesis of cholesterol and reduces the level of lipolysis.8
The high blood glucose level in diabetic patients can impair liver function. The elevated level of serum AST, ALT, and ALP in a diabetic patient can be a screening marker of liver damage.23 Whenever the organs get injured, their tissues release AST, ALT, and ALP into the bloodstream whereas the increased number of kuffer cells and monocytes can be seen in tissues of liver (Figure 6), this is why the histopathology and these enzymes have been estimated.9 The effect of PFA-extract on AST, ALT, and ALP was also checked and we found that our 500 mg concentration of dose showed to lower these enzymes effectively (Figure 4), as compared with normal and diabetic control groups.
High blood glucose levels could damage the kidney and hamper its filtration rate. In diabetic conditions, the kidney grows large, and glomerular filtration rate (GFR) gets impaired.4 BUN is a clinical diagnostic marker for acute and chronic kidney disease. The excess level of creatinine in blood is an indicator of impaired GFR which is a sign of renal injury. In another study, the researcher found that the estimated glomerular filtration rate (eGFR) is independently associated with serum uric acid, which is an indicator of the damaged kidney.20 In our study, we found that the 500 mg concentration of PFA-extract can reduce the creatinine and BUN level effectively (no significant difference was found as compared with the drug control group) whereas uric acid level reduced significantly as compared with normal and diabetic control (but less effective than the drug control group) (Figure 5).
Conclusion
The PFA-extract showed its potential to lower the adverse effects on vital organs, ie, liver, kidney, and pancreas in the diabetes type 1 rat model. The aqueous extract had the potential to control body weight and reduce the blood glucose level. It also had shown the tendency to modulate the lipid profile which can be very helpful in prevention or mitigation of cardiovascular disease. The possible mechanism behind its protective effects may be due to the regeneration property of pancreatic β-cells which improved insulin secretion. The main bioactive molecules of P. fossulatus are still unknown; it requires further investigations. To the best of our knowledge, no one has reported such kind of attributes of P. fossulatus before.
Abbreviations
ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANOVA, analysis of variance; AST, aspartate aminotransferase; ATP, adenosine triphosphate; BGL, blood glucose level; BUN, blood urea nitrogen; CRT, creatinine; C, central vain; DM, diabetes mellitus; DN, diabetic nephropathy; D1, dosing group 1; D2, dosing group 2; EDTA, ethylenediaminetetra acetic acid; eGFR, estimated glomerular filtration rate; GAD, glutamic acid decarboxylase; GFR, glomerular filtration rate; Hc, hepatocytes; HDL, high density lipoprotein; IP, intraperitoneal injection; Kc, kuffer Cells; Mc, monocytes; NAFLD, non-alcoholic fatty liver disease; PFA-extract, pleurotus fossulatus aqueous extract; PO, by opening; Pv, portal vain RPM, revolutions per minutes; ROS, reactive oxygen species; S, sinusoid; TC, total cholesterol; TG, triglyceride; STZ, streptozotocin.
Data Sharing Statements
The data used in this study can be provided from the corresponding author upon valid reason.
Ethical Approval Statements
All the animal experiments were approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and Institutional Animal Ethics Committee (IAEC) of the University of Allahabad, Prayagraj, India (Reg. No. 839/GO/Re/04/CPCSEA, Approval no. IAEC/AU/2019(1)/29). We followed the environment, housing, and management, veterinary care guidelines in accordance with the Guide for the Care and Use of Laboratory Animals (Eighth Edition, published by National Research Council of the National Academies Press)
Consent for Publication
All the authors are agreed and have given their consent to publish this research work in a good and reputed journal.
Acknowledgments
Authors would like to thank the Centre of Biotechnology, University of Allahabad for providing chemicals and access to laboratory.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interest with respect to research, authorship, and publication of this research article.
References
1. Ahmed I, Lakhani MS, Gillett M, et al. Hypotriglyceridemic and hypocholesterolemic effects of anti-diabetic Momordica charantia (karela) fruit extract in streptozotocin-induced diabetic rats. Diab Res Clin Pract. 2001;51:155–161. doi:10.1016/S0168-8227(00)00224-2
2. Attele AS, Zhou YP, Xie JT, et al. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi:10.2337/diabetes.51.6.1851
3. Chen J, Li WL, Wu JL, et al. Hypoglycemic effects of a sesquiterpene glycoside isolated from leaves of loquat (Eriobotrya japonica (Thunb.) Lindl.). Phytomedicine. 2008;15:98–102. doi:10.1016/j.phymed.2006.12.014
4. Dabla PK. Renal function in diabetic nephropathy. World J Diab. 2010;1:48–56. doi:10.4239/wjd.v1.i2.48
5. Dubey SK, Chaturvedi VK, Mishra D, et al. Role of edible mushroom as a potent therapeutics for the diabetes and obesity. 3 Biotech. 2019;9:450–462. doi:10.1007/s13205-019-1982-3
6. Elberry AA, Harraz FM, Ghareib SA, et al. Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslipidemia in streptozotocin-induced diabetic rats. Int J Diab Mellt. 2015;3:37–44. doi:10.1016/j.ijdm.2011.01.004
7. Erejuwa OO, Sulaiman SA, Wahab MS, et al. Antioxidant protective effect of glibenclamide and metformin in combination with honey in pancreas of streptozotocin-induced diabetic rats. Int J Mol Sci. 2010;11:2056–2066. doi:10.3390/ijms11052056
8. Gong F, Li F, Zhang L, et al. Hypoglycemic effects of crude polysaccharide from purslane. Int J Mol Sci. 2009;10:880–888. doi:10.3390/ijms10030880
9. Hasan KM, Tamanna N, Haque MA. Biochemical and histopathological profiling of Wistar rat treated with Brassica napus as a supplementary feed. Food Sci Human Well. 2018;7:77–82. doi:10.1016/j.fshw.2017.12.002
10. Juśkiewicz J, Zduńczyk Z, Jurgoński A, et al. Extract of green tea leaves partially attenuates streptozotocin-induced changes in antioxidant status and gastrointestinal functioning in rats. Nutr Res. 2008;28:343–349. doi:10.1016/j.nutres.2008.03.004
11. Kahanovitz L, Sluss PM, Russell SJ. Type 1 diabetes – a clinical perspective. Point Care. 2017;16:37–40. doi:10.1097/POC.0000000000000125
12. Kalaiarasi P, Kaviarasan K, Pugalendi KV. Hypolipidemic activity of 18β-glycyrrhetinic acid on streptozotocin-induced diabetic rats. Eur J Pharmacol. 2009;612:93–97. doi:10.1016/j.ejphar.2009.04.003
13. Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J Diab. 2015;6:850–867. doi:10.4239/wjd.v6.i6.850
14. Krakoff J, Clark JM, Crandall JP, et al. Diabetes Prevention Program Research Group. Effects of metformin and weight loss on serum alanine aminotransferase activity in the diabetes prevention program. Obesity. 2010;18:1762–1767. doi:10.1038/oby.2010.21
15. Lauritano C, Ianora A. Marine organisms with anti-diabetes properties. Mar Drugs. 2016;14:220–234. doi:10.3390/md14120220
16. Li F, Zhang Y, Zhong Z. Antihyperglycemic effect of Ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int J Mol Sci. 2011;12:6135–6145. doi:10.3390/ijms12096135
17. Mansi K, Amneh M, Nasr H. The hypolipidemic effects of Artemisia sieberi (A. herba-alba) in alloxan induced diabetic rats. Int J Pharmacol. 2007;3:487–491. doi:10.3923/ijp.2007.487.491
18. Mathur S, Mehta DK, Kapoor S, et al. Liver function in type-2 diabetes mellitus patients. Int J Sci Stud. 2016;3:43–47.
19. Mihailovic M, Jovanovic JA, Uskokovic A, et al. Protective effects of the mushroom lactarius deterrimus extract on systemic oxidative stress and pancreatic islets in streptozotocin-induced diabetic rats. J Diab Res. 2014;2015:1–10. doi:10.1155/2015/576726
20. Ng SH, Zain M, Shazwan M, et al. Hypoglycemic and antidiabetic effect of Pleurotus sajor-caju aqueous extract in normal and streptozotocin-induced diabetic rats. Biomed Res Int. 2015;2015:1–8.
21. Qin Q, Qian Y, Zhu G, et al. The correlation between serum uric acid and renal function in elderly Chinese diabetes with normoalbuminuria. Int J Endocrinol. 2019;2019:1–7.
22. Ramesh B, Saravanan R, Pugalendi KV. Effect of dietary substitution of groundnut oil on blood glucose, lipid profile, and redox status in streptozotocin-diabetic rats. Yale J Biol Med. 2006;79:9–17.
23. Senthilkumar GP, Subramanian S. Evaluation of antioxidant potential of Terminalia chebula. Fruits studied in streptozotocin-induced diabetic rats. Pharm Biol. 2007;45:511–518. doi:10.1080/13880200701446720
24. Tong H, Xia F, Feng K, et al. Structural characterization and in vitro antitumor activity of a novel polysaccharide isolated from the fruiting bodies of Pleurotus ostreatus. Bioresour Technol. 2009;100:1682–1686. doi:10.1016/j.biortech.2008.09.004
25. Xie JT, Wang A, Mehendale S, et al. Anti-diabetic effects of Gymnema yunnanense extract. Pharmacol Res. 2003;47:323–329. doi:10.1016/S1043-6618(02)00322-5
26. Xiong M, Huang Y, Liu Y, et al. Antidiabetic activity of ergosterol from Pleurotus ostreatus in KK‐Ay mice with spontaneous type 2 diabetes mellitus. Mol Nutr Food Res. 2018;62:1700444.
27. Ahmed AM. History of diabetes mellitus. Saudi Med J. 2002;23:373–378.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.