Back to Journals » Journal of Pain Research » Volume 15
Effect of Perioperative Intravenous Lidocaine on Postoperative Recovery in Patients Undergoing Ileostomy Closure: Study Protocol for a Randomized Controlled Trial
Authors Liu J, Liu K, Wang H, Hu H, Sun G, Ye X, Lou Z, Bian J, Bo L
Received 17 February 2022
Accepted for publication 17 June 2022
Published 2 July 2022 Volume 2022:15 Pages 1863—1872
DOI https://doi.org/10.2147/JPR.S362911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ellen M Soffin
Jia Liu,1,* Kun Liu,1,* Huixian Wang,1,* Hongli Hu,1 Guolin Sun,1 Xiaofei Ye,2 Zheng Lou,3 Jinjun Bian,1 Lulong Bo1
1Faculty of Anesthesiology, Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China; 2Department of Health Statistics, Naval Medical University, Shanghai, People’s Republic of China; 3Department of Colorectal Surgery, Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lulong Bo; Jinjun Bian, Faculty of Anaesthesiology, Changhai Hospital, Naval Medical University, Shanghai, 200433, People’s Republic of China, Tel +86-2131161839, Email [email protected]; [email protected]
Introduction: Opioids have been widely used clinically as the first choice for pain management. Ileostomy closure usually leads to temporary intestinal paralysis, which manifests as abdominal distension and pain, delayed defecation, nausea, and vomiting. Intraoperative and postoperative use of opioids inhibit gastrointestinal function and aggravate intestinal paralysis, and are notoriously addictive. Thus, reducing perioperative opioid use is important for patients undergoing ileostomy closure to restore the continuity and integrity of the intestine. Intravenous lidocaine has been shown to have anti-inflammatory properties and analgesic effects. We consider minimizing the use of opioids for such patients, and perioperative intravenous injection of lidocaine may be beneficial to the recovery of intestinal function in patients with ileostomy closure.
Methods and Analysis: This is a randomized double-blind placebo-controlled trial to investigate the effectiveness and safety of intravenous lidocaine in patients undergoing ileostomy closure. The time of first postoperative anal venting, postoperative opioids use, postoperative recovery, intraoperative adverse effects and postoperative complications will be collected and analyzed.
Keywords: anesthesia, intravenous, lidocaine, ileostomy, recovery of function
Introduction
In recent years, the incidence of colorectal cancer has been rising worldwide.1 Surgical resection, the primary treatment of choice for colorectal cancer, is widely performed.2 Rapid developments in laparoscopic rectal surgery have made anus-preserving procedures available for patients with rectal cancer.3 Prophylactic ileostomy reduces the risk and severity of complications arising from anastomotic leakage, and can improve the anorectal function of patients undergoing radical rectal cancer resection.4 However, these patients need to undergo ileostomy closure to restore the continuity and integrity of the intestine. With the concept of enhanced recovery after surgery (ERAS), anesthesiologists are concerned about how to effectively reduce the intraoperative and postoperative opioid use to restore patients’ bowel function earlier.
Recent evidence suggests that many malignancies have high levels of μ-opioid receptor expression. Activation by opioids may lead to tumor growth and spread, making it imperative to reduce the use of opioids perioperatively.5,6 Intravenous lidocaine has been shown to have anti-inflammatory properties and analgesic effects,7–9 including inhibition of leukocyte adhesion, activation, and migration; blocking of neutrophil activation; reducing the release of pro-inflammatory factors;10,11 and reducing postoperative pain and analgesic requirements.12–14 Recent clinical trials have shown that laparoscopic radical left hemicolectomy with perioperative intravenous lidocaine facilitates postoperative pain control and results in faster recovery and shorter hospital stays.15 However, a recent randomized, double-blinded, placebo-controlled trial by Herzog et al indicated that intravenous lidocaine had no significant benefits for patients undergoing robot-assisted colorectal surgery after considering multiple outcomes including cumulative morphine consumption postoperatively, time until first flatus or defecation, use of antiemetics, and time until discharge.16 Thus, whether lidocaine improves bowel function and accelerates intestinal recovery in patients undergoing abdominal surgeries remains inconclusive.
Based on the conflicting results from several studies, we designed this randomized double-blind placebo-controlled clinical trial to evaluate the efficacy and safety of lidocaine on postoperative recovery of patients with ileostomy closure. This study protocol will provide the basis for further development of perioperative intravenous lidocaine usage and optimize perioperative management for patients with ileostomy closure.
Methods and Analysis
This is a single-center, randomized, double-blind, placebo-controlled trial. Our objective is to determine whether lidocaine could reduce the total amount of perioperative opioids and promote recovery of intestinal function in patients undergoing ileostomy closure surgeries, by administering lidocaine slowly during induction of anesthesia and with a continuous intraoperative infusion. The trial was approved by the ethics committee of Shanghai Changhai Hospital on December 10, 2020, with an approval number CHEC2020-154 and will be conducted in accordance with the Declaration of Helsinki. It is registered at the China Clinical Trials Registry with registration number ChiCTR2100042532. The general flow chart of the trial is shown in Figure 1. The schedule of the trial is shown in Table 1.
 |
Table 1 Trial Schedule of Enrolment, Interventions, and Assessments for Effect of Perioperative Intravenous Lidocaine on Postoperative Recovery in Patients Undergoing Ileostomy Closure |
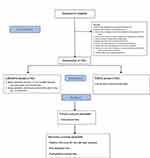 |
Figure 1 Flow diagram for effect of perioperative intravenous lidocaine on postoperative recovery in patients undergoing ileostomy closure. |
Study Population
All patients scheduled for elective ileostomy closure surgery in Changhai Hospital, Naval Medical University will be screened and recruited for the study. Patient enrolment began in January 2021. It is estimated that this trial will take 18–24 months to enroll 166 patients. The estimated completion date is November 2022.
Patients will be fully informed about the objectives, risks, and benefits of this study before surgery. Written informed consent will be obtained from patients or their legal representatives. The investigators will complete the case report form (CRF) according to the items listed in the CRF, including age (years), sex (male or female), height, weight, and American Society of Anesthesiologists (ASA) classification. If a patient does not meet the inclusion criteria, he/she will be excluded. In addition, enrolled patients have the right to discontinue their participation and withdraw from the trial at any time. They must provide a true medical history and answer the investigator’s questions. Patients’ personal data will be protected and stored by the investigator in a secure cabinet.
Inclusion Criteria
- Age 18–75 years.
- Patients proposed for elective ileostomy closure surgery after rectal cancer surgery.
- Body mass index (BMI) 18–30 kg·m−2.
- Gave written informed consent.
Exclusion Criteria
- Patients with preoperative gastrointestinal dysfunction.
- Patients who are allergic to the drugs used in the study protocol and are using drugs that are contraindicated with lidocaine.
- Patients with a history of alcohol or drug abuse, or long-term opioid use.
- Severe cardiopulmonary abnormalities, such as severe arrhythmias, atrioventricular block, heart failure, hypertrophic cardiomyopathy, dilated cardiomyopathy, pulmonary heart disease, and severe valvular heart disease, as well as respiratory failure.
- Patients with severe liver and kidney abnormalities.
- Patients with a history of previous gastrointestinal surgery.
- Patients who are unable to cooperate with the study for any reason.
- Patients who have used other trial drugs in the 3 months prior to enrolment or are participating in other clinical trials.
- Patients with severe surgical complications.
Randomization and Blinding
Randomization and allocation concealment to avoid selection bias will be performed by a medical statistician prior to starting the trial. Using a computer-generated random number table, patients are randomly assigned in a 1:1 ratio to the lidocaine group (trial group) and the saline group (control group). The group information will also be sealed in 166 opaque envelopes, which will be opened by the investigator according to the grouping after the enrolment is completed. The subject information will be written on the envelope. The pharmacist on duty on the day of the surgical procedure will be notified to prepare the corresponding trial drug.
On the morning of the surgery, a clinical pharmacist will give the test syringes to the anesthesiologist. Two syringes will be prepared for each patient as follows: 5 mL syringe for intravenous injection prior to induction of anesthesia and 50 mL syringe for continuous intravenous intraoperative infusion. In the control group, the syringes will both contain 0.9% saline injection; in the trial group, the 5 mL syringe will contain 100 mg of 2% lidocaine hydrochloride solution and the 50 mL syringe contained 200 mg of lidocaine at 4 mg/mL. All syringes will be appropriately labelled.
Postoperative follow-up and data entry will be completed by a dedicated trial staff. This protocol ensures that all aspects of the trial—from patient grouping, to drug dispensing, and until postoperative follow-up—are handled by trained personnel. Neither the patients, nor the anesthesiologists, nursing staff, or investigators at postoperative follow-up are aware of the patient grouping or the contents of the syringe.
Intervention
In the lidocaine group, 1.5 mg/kg predicted body weight (PBW) of lidocaine (with a total dose not to exceeding 100 mg) will be administered by slow intravenous push approximately 30s before induction of anesthesia. This will be followed by continuous intravenous infusion of lidocaine at a rate of 2 mg· kg−1·h−1 until the end of the operation. In the control group, normal saline will be administered in the same manner. Both groups will have intravenous patient-controlled analgesia for 48 hours postoperatively. The same analgesic formulation, ie, dexmedetomidine 2 ug·kg−1, fentanyl 0.01 mg·kg−1, butorphanol tartrate 0.06mg·kg−1, and metoclopramide 20 mg, diluting to 100 mL with 0.9% saline, will be given to both groups. PBW is calculated as follows, male 50 + (0.91 × [height −152.4]), female 45.5 + (0.91 × [height - 152.4]).
Anesthesia Management
A standardized anesthesia management and care process is uniformly implemented for all patients. Bowel preparation will be strictly implemented according to surgical routine.
Induction of Anesthesia
On the day of surgery, ECG, non-invasive arterial pressure, saturation of pulse oximetry, and bispectral index will be routinely monitored upon admission to the operating room. A peripheral intravenous access will be established. In the trial group, 1.5 mg·kg−1 (PBW) of lidocaine (with a total dose not exceeding 100 mg) will be given slowly and intermittently before induction of anesthesia, followed by continuous infusion of lidocaine at a rate of 2 mg·kg−1·h−1 until the end of the operation. The same volume of normal saline will be given to the control group. Oxygen will be preadministered (8 L·min−1) before induction, and the concentration of inhaled oxygen will be 100%. Patients in both groups will be induced with propofol 1.5–2.0 mg/kg, sufentanil 0.3 μg·kg−1, dexamethasone 10 mg, and rocuronium 0.6 mg·kg−1 successively. After the patient loses consciousness and adequate muscle relaxation is achieved, the same attending anesthesiologist will perform tracheal intubation and connect the anesthesia machine for mechanical ventilation. Subsequently, a unilateral transversus abdominal plane (TAP) block on the surgical side will be performed with 20 mL of 0.375% ropivacaine.
Maintenance of Aesthesia
After tracheal intubation, the patient will be routinely ventilated with pulmonary mechanical ventilation, and the respiratory parameters will be adjusted as follows: oxygen concentration 100%, tidal volume 6–8 mL·kg−1, and respiratory rate 12–14 breaths·min−1 to maintain end-expiratory carbon dioxide pressure between 30 and 35 mmHg. Continuous pumping of remifentanil 0.1–0.5 μg·kg−1·min−1 and propofol 4–6 mg·kg−1·h−1 will be performed to maintain intraoperative the bispectral index (BIS) between 40 and 60. Hemodynamic stability will be maintained. Intraoperatively, when there are changes in blood pressure or heart rate beyond 20% from the baseline value, 5 ug of sufentanil or appropriate vasoactive drugs (eg, ephedrine, phenylephrine, and esmolol) will be given. Rocuronium will be supplemented with 0.2 mg·kg−1 as needed, and all pumping will be stopped at the end of the procedure. At the end of the surgery, neuromuscular blockade will be antagonized with neostigmine 1 mg and atropine 0.5 mg, if necessary. After the operation, patients will remain intubated and will be transferred to the postanesthesia care unit (PACU). Indications for tracheal tube removal are as follows: the patient could open the eyes on expiration and move the limbs as instructed, hemodynamic stability, recovery of spontaneous breathing, respiratory rate of 12–20 breaths·min−1, tidal volume > 8 mL·kg−1, end-expiratory carbon dioxide ≤ 45 mmHg, and oxygen saturation ≥ 95%. The time taken from the end of the procedure to tracheal extubation will be recorded. Patients will be discharged from the PACU once with a modified Aldrete score ≥9.
Postoperative Analgesia
At 30 min before the end of the procedure, 5 μg sufentanil will be administered for analgesia transition. The patient’s self-controlled intravenous analgesia pump will then be connected. Both groups will receive the same previously described solution via their analgesia pump. After the endotracheal tube will be removed, 5 ug sufentanil will be given every 10 mins if the visual analog scale (VAS) score is greater than 3 (VAS: 0 = no pain, 10 = unbearable severe pain). The corresponding VAS scores and postoperative Riker Sedation-Agitation Score will be recorded immediately after the patient will be discharged from the PACU. The patients’ VAS scores will be also followed up at 24- and 48-h postoperatively, and the amount of opioid and other analgesics will be recorded in the first 48 h postoperatively.
Follow-Up Visits
All patients will receive dedicated follow-up visits after surgery. The personnel in charge of follow-up will be blinded to grouping and medication use, and completed follow-up and data entry of nodal time observation indicators based on the CRF form only. Patients will be excluded from the study for any of the following: serious allergic reaction to lidocaine, serious adverse events such as cardiovascular and cerebrovascular accidents, withdrawal of consent to participate while trial is underway, or missed visits.
Outcomes
Baseline Data
The patient’s general condition, including gender, age, BMI, history of comorbid diseases, and the time interval between the ileostomy and the current ileostomy closure procedure will be recorded. The following intraoperative information will be recorded: heart rate, mean artery pressure (MAP), and oxygen saturation values at four time points (admission, 0.5 h after the start of the surgery, end of the procedure, and off the PACU); duration of the surgery; degree of intraoperative bowel adhesions (mild, moderate, severe); intraoperative blood loss; intraoperative fluid volume; intraoperative sufentanil and remifentanil doses; intraoperative vasoactive drug type and doses; The time from the end of surgery to extubation; resting VAS score immediately off the PACU and postoperative Riker Sedation-Agitation Scale.
Primary Outcome
The time of first postoperative anal venting is defined as the interval from the completion of surgical suturing until the patient’s first act of anal venting.
Secondary Outcome
Secondary observations will be total amount of opioids used over the first 48 postoperative hours, resting VAS score at 24- and 48 h postoperatively, time to first bowel movement, time to first drink of water, length of postoperative hospitalization, length of total hospital stay, and the occurrence of intraoperative adverse effects and postoperative complications.
Sample Size Calculation
The study is a randomized controlled study, with the trial group being the lidocaine group and the control group being the normal saline group. According to relevant literature, the time of first postoperative anal venting before the study was 40±14 h, and it is proposed to be 32±14 h after the lidocaine given. According to the criteria of Power=0.90 and α=0.05, the sample size required for each of the test and control groups is 66 cases as computed on SPSS version 23.0 (IBM, Chicago, USA). After considering a 20% attrition rate, the total sample size required for the study protocol is 166 cases according.
Data Analysis
SPSS 23.0 statistical software (IBM, Chicago, USA) will be used for analysis, and the measurement data such as opioid dosage, VAS score, and length of stay will be expressed as mean ± standard deviation, and the trends of oxygen saturation, MAP, and heart rate changes over time and within-group comparisons will be analysed by ANOVA with general linear model repeated measures; the comparison of the count data such as ASA classification and surgical modality will be performed by chi- square test, and a P<0.05 indicates a statistically significant difference. Indicators of circulating parameters at different time points between the two groups will be tested using the independent samples t test, and when the data will be not normally distributed, the rank sum test will be used instead of the t-test.
Assessment of Safety
First, the patient will be operated in a well-supervised operating room where the anesthesiologist will routinely monitor ECG, non-invasive arterial pressure, and pulse oximetry. If a patient experiences an adverse reaction related to the test drug, the administration of the drug will be withdrawn immediately and appropriate treatment will be given to maintain the patient’s respiratory and circulatory stability. Then, in the PACU and postoperative wards, the patients will be closely monitored such as in the operating theater. The serious adverse event will be reported to the chief investigator, and relevant expert consultations will be held to clarify the cause, severity, and consequences of the adverse event. These information will be entered into the hospital’s electronic information system for archiving.
Patient and Public Involvement
Patients and the public will not be involved in the development of the research or the design of the study protocol. Results of the trial will be disseminated by publication in a medical journal and poster presentation at an anesthesia conference.
Discussion
This study protocol is a single-center, randomized, double-blind, placebo-controlled study. We aim to evaluate the influence of perioperative intravenous lidocaine on the postoperative recovery in patients undergoing ileostomy closure. It is known that ileostomy closure often leads to temporary intestinal paralysis, which manifests as abdominal distension and pain, delayed defecation, nausea, and vomiting. These cause pain to patients, and prolong their hospital stay and increase medical costs. Due to the concept of ERAS, a rational and optimized anesthesia management program for ileostomy closure is on the agenda.
It has been shown that intestinal injury from surgical operations cause sympathetic hyperexcitability and abdominal pain. Opioids are the first choice for postoperative treatment of moderate to severe pain. However, early clinical trials have demonstrated that morphine and other opioids inhibit the release of acetylcholine in the mesenteric plexus, resulting in increased colonic muscle tone and decreased gastrointestinal motility.17,18 Therefore, to reduce the side effects of opioids, multiple analgesics, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and local anesthetics, can be used as part of a multimodal analgesic plan in the perioperative period.
Local injection of lidocaine inhibits the opening of sodium channels and reduces the depolarization of cell membranes caused by sodium inward flow, thus providing analgesia.19 In recent years, lidocaine has been shown to have analgesic and anti-inflammatory properties that make it useful as an adjunct to general anesthesia. Ates et al found that after perioperative sedation of lidocaine in patients undergoing nasal septal surgery, pain scores at 30 min, 1-, 2-, 4-, 8-, 12- and 24-h postoperatively were significantly alleviated compared to the control group (saline group).20 Intravenous lidocaine has been reported to reduce the use of intraoperative opioids and the occurrence of postoperative nausea and vomiting (PONV) in patients undergoing upper airway surgery.21 Available clinical evidence suggests that lidocaine not only improves postoperative pain and reduces opioid use in ear, nose, and throat (ENT) patients. Patients undergoing abdominal surgery can also benefit from it. Six recent guidelines in ERAS mention the importance of perioperative intravenous lidocaine in multimodal analgesia. These guidelines are covering the following procedures: pancreaticoduodenectomy,22 elective rectal/pelvic surgery,23 partial gastrectomy,24 gastrointestinal surgery,25 radical cystectomy,26 and routine gynecologic/oncologic surgery.25 According to the study of Moeen et al, intravenous lidocaine in open radical cystectomy improves the postoperative analgesic effect and promotes bowel function recovery.27
It is well-known that postoperative intestinal paralysis is associated with the suppression of gastrointestinal reflexes, sympathetic hyperexcitability, systemic immune response, and opioid use. Local and systemic inflammatory responses caused by the surgery also play a key role.28 First, tissue trauma caused by surgical operations causes local accumulation of large amounts of cytokines and inflammatory transmitters in the intestine, resulting in edema of the intestinal wall, which directly affects gastrointestinal motility.29 Second, increased permeability of the intestinal epithelial barrier also leads to translocation of bacteria and endotoxins, exacerbating the inflammatory response.30 In contrast, local anesthetics, especially lidocaine, can exert anti-inflammatory effects by modulating the release or signaling of inflammatory mediators. Both in vitro and in vivo studies have shown that lidocaine reduces the release of pro-inflammatory cytokines by inhibiting the activation of neutrophils.31 There is also evidence that lidocaine has the potential to attenuate the inflammatory response of endothelial cells and thus maintain the integrity of the endothelial barrier.32 Thus, perioperative intravenous push and pump lidocaine could theoretically reduce the release of inflammatory factors, attenuate the inflammatory response, maintain the integrity of the intestinal barrier, and promote the early recovery of postoperative bowel function.
Our protocol has several limitations. First, patients with ileostomy closure will be recruited from several surgeon groups, and the diameter of the anastomoses will likely vary due to the differences in anatomical site, surgical technique, and types of closures used, all of which will influence the recovery of bowel function after surgery. Second, the time between the surgical resection and ileostomy closure intervals and the degree of intraoperative bowel adhesions in patients are also important surgical factors that need to be considered.
Summary
In summary, the manuscript presents a protocol to study the efficacy and safety of intravenous lidocaine in patients undergoing ileostomy closure. If the result of this study protocol is positive, it will provide evidence for optimal perioperative management to improve the recovery of patients undergoing ileostomy closure.
Dissemination
The results of this study will be disseminated through presentations at anesthesia conferences and publication in scientific journals.
Audits
The data monitoring committee will conduct audits through regular interviews, letters, or telephone. The data monitoring committee reserves the right to audit the recruitment of patients at any time. The auditing process will be independent from the investigators.
Amendments to the Protocol
Any deviations from the protocol will be fully documented in a report to be submitted all relevant regulatory bodies, and thoroughly recorded in a protocol deviation log. The PI will determine the protocol amendments. Protocol amendments will be sent as updated protocols to investigators. A copy of each revised protocol will be added to the investigator site file. The protocol will also be updated in the clinical trials registry website.
Strategies to Improve the Adherence to Protocols
The anesthesiologist who administers anesthesia in this study will be trained to follow the standardized procedure. The investigator staff will be well-trained to perform preoperative recruitment, assessment, and postoperative follow-up. We will train the whole study team to standardize the use of assessment scales in this study. Moreover, the investigator who will do the assessments will be blind to the intervention.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chu L, Wang H, Qiu S, et al. Risk factors of delayed recovery of gastrointestinal function after ileostomy reversal for rectal cancer patients. Cancer Manag Res. 2021;13:5127–5133. doi:10.2147/cmar.S311715
2. Aljorfi AA, Alkhamis AH, Systematic A. Review of early versus late closure of loop ileostomy. Surg Res Pract. 2020;2020:9876527. doi:10.1155/2020/9876527
3. Celayir MF, Tanal M, Besler E, et al. Protective loop ileostomy closure techniques: comparison of three different surgical techniques. Cureus. 2020;12(10):e10977. doi:10.7759/cureus.10977
4. Sauri F, Sakr A, Kim HS, et al. Does the timing of protective ileostomy closure post-low anterior resection have an impact on the outcome? A retrospective study. Asian J Surg. 2021;44(1):374–379. doi:10.1016/j.asjsur.2020.10.007
5. Bortsov AV, Millikan RC, Belfer I, et al. μ-Opioid receptor gene A118G polymorphism predicts survival in patients with breast cancer. Anesthesiology. 2012;116(4):896–902. doi:10.1097/ALN.0b013e31824b96a1
6. Lennon FE, Mirzapoiazova T, Mambetsariev B, et al. The Mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and Epithelial Mesenchymal Transition (EMT) in human lung cancer. PLoS One. 2014;9(3):e91577. doi:10.1371/journal.pone.0091577
7. Beloeil H, Mazoit JX. [Effect of local anesthetics on the postoperative inflammatory response]. Ann Fr Anesth Reanim. 2009;28(3):231–237. French. doi:10.1016/j.annfar.2008.12.021
8. Garutti I, Rancan L, Simón C, et al. Intravenous lidocaine decreases tumor necrosis factor alpha expression both locally and systemically in pigs undergoing lung resection surgery. Anesth Analg. 2014;119(4):815–828. doi:10.1213/ane.0000000000000360
9. Sridhar P, Sistla SC, Ali SM, et al. Effect of intravenous lignocaine on perioperative stress response and post-surgical ileus in elective open abdominal surgeries: a double-blind randomized controlled trial. ANZ J Surg. 2015;85(6):425–429. doi:10.1111/ans.12783
10. Beaussier M, Delbos A, Maurice-Szamburski A, et al. Perioperative use of intravenous lidocaine. Drugs. 2018;78(12):1229–1246. doi:10.1007/s40265-018-0955-x
11. Dai Y, Jiang R, Su W, et al. Impact of perioperative intravenous lidocaine infusion on postoperative pain and rapid recovery of patients undergoing gastrointestinal tumor surgery: a randomized, double-blind trial. J Gastrointest Oncol. 2020;11(6):1274–1282. doi:10.21037/jgo-20-505
12. Marret E, Rolin M, Beaussier M, et al. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg. 2008;95(11):1331–1338. doi:10.1002/bjs.6375
13. McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs. 2010;70(9):1149–1163. doi:10.2165/10898560-000000000-00000
14. Vigneault L, Turgeon AF, Côté D, et al. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anaesth. 2011;58(1):22–37. doi:10.1007/s12630-010-9407-0
15. Gu X, Pre- XC. Peri-, and postoperative intravenous ropivacaine versus that of lidocaine for analgesia after hand-assisted laparoscopic surgery of left colon cancer: a retrospective analysis. J Invest Surg. 2021;34(12):1322–1328. doi:10.1080/08941939.2020.1801913
16. Herzog J, Schou M, Jensen KM, et al. A randomised controlled trial of lidocaine infusion on post-operative opioid consumption in patients undergoing robotic colorectal surgery. Dan Med J. 2020;67(1):A06190342.
17. Steinbrook RA. Epidural anesthesia and gastrointestinal motility. Anesth Analg. 1998;86(4):837–844. doi:10.1097/00000539-199804000-00029
18. Taguchi A, Sharma N, Saleem RM, et al. Selective postoperative inhibition of gastrointestinal opioid receptors. N Engl J Med. 2001;345(13):935–940. doi:10.1056/NEJMoa010564
19. Casale R, Symeonidou Z, Bartolo M. Topical treatments for localized neuropathic pain. Curr Pain Headache Rep. 2017;21(3):15. doi:10.1007/s11916-017-0615-y
20. Ates İ, Aydin ME, Ahiskalioglu A, et al. Postoperative analgesic efficacy of perioperative intravenous lidocaine infusion in patients undergoing septorhinoplasty: a prospective, randomized, double-blind study. Eur Arch Otorhinolaryngol. 2020;277(4):1095–1100. doi:10.1007/s00405-020-05801-6
21. Wang Q, Ding X, Huai D, et al. Effect of intravenous lidocaine infusion on postoperative early recovery quality in upper airway surgery. Laryngoscope. 2021;131(1):E63–e69. doi:10.1002/lary.28594
22. Lassen K, Coolsen MME, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg. 2013;37(2):240–258. doi:10.1007/s00268-012-1771-1
23. Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31(6):801–816. doi:10.1016/j.clnu.2012.08.012
24. Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. 2014;101(10):1209–1229. doi:10.1002/bjs.9582
25. Feldheiser A, Aziz O, Baldini G, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60(3):289–334. doi:10.1111/aas.12651
26. Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin Nutr. 2013;32(6):879–887. doi:10.1016/j.clnu.2013.09.014
27. Moeen SM, Moeen AM. Usage of intravenous lidocaine infusion with Enhanced recovery pathway in patients scheduled for open radical cystectomy: a randomized trial. Pain Physician. 2019;22(2):E71–e80. doi:10.36076/ppj/2019.22.E71
28. Aktaş A, Kayaalp C, Ateş M, et al. Risk factors for postoperative ileus following loop ileostomy closure. Turk J Surg. 2020;36(4):333–339. doi:10.47717/turkjsurg.2020.4911
29. Kalff JC, Schraut WH, Simmons RL, et al. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998;228(5):652–663. doi:10.1097/00000658-199811000-00004
30. Venara A, Neunlist M, Slim K, et al. Postoperative ileus: pathophysiology, incidence, and prevention. J Visc Surg. 2016;153(6):439–446. doi:10.1016/j.jviscsurg.2016.08.010
31. Estebe JP. Intravenous lidocaine. Best Pract Res Clin Anaesthesiol. 2017;31(4):513–521. doi:10.1016/j.bpa.2017.05.005
32. de Klaver MJM, Buckingham MG, Rich GF. Lidocaine attenuates cytokine-induced cell injury in endothelial and vascular smooth muscle cells. Anesth Analg. 2003;97(2):465–470. doi:10.1213/01.Ane.0000073162.27208.E9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
