Back to Journals » Journal of Pain Research » Volume 9
Effect of pedicle fixation combined with 125I seed implantation for metastatic thoracolumbar tumors
Authors Qian J, Bao Z, Zou J, Yang H
Received 28 January 2016
Accepted for publication 17 March 2016
Published 10 May 2016 Volume 2016:9 Pages 271—278
DOI https://doi.org/10.2147/JPR.S105284
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Jiale Qian, Zhaohua Bao, Jun Zou, Huilin Yang
Department of Orthopedic Surgery, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China
Purpose: The aim of this study was to investigate the clinical efficacy of pedicle fixation combined with 125I brachytherapy in treating metastatic thoracolumbar tumors.
Patients and methods: A retrospective analysis of the clinical data of seven metastatic thoracolumbar tumor patients who received pedicle fixation combined with radioactive 125I seed implantation brachytherapy in our department between January 2009 and December 2013 was performed. The visual analog scale (VAS) for pain and the Karnofsky performance status (KPS) score before the operation and 1, 6, and 12 months after the operation were observed and recorded. The changes in the scores at each time point were compared.
Results: All the patients underwent a successful operation, without any complications during their hospitalization. All the patients received postoperative follow-up, and the duration of follow-up was 15–50 months, with an average of 32.2 months. One pancreatic cancer patient died of liver failure and hypoproteinemia 28 months post surgery. The VAS scores of patients before the operation and 1, 6, and 12 months after the operation were 7.43±0.98, 2.71±0.49, 3.00±0.82, and 4.29±0.98, respectively; the KPS scores were 52.9±9.5, 84.3±5.3, 75.7±5.3, and 72.9±4.9, respectively. These results suggest that the VAS score at each time point was significantly decreased compared with that before the operation, while the KPS score was significantly increased compared with that before the operation. Both differences had statistical significance (P<0.05).
Conclusion: As a therapy for advanced malignant tumors with thoracolumbar metastasis, pedicle fixation combined with 125I brachytherapy can effectively relieve short-term pain and improve patient’s quality of life.
Keywords: metastatic spinal tumor, pedicle fixation, radioactive particles, iodine-125
Introduction
The incidence of malignant tumors has been steadily increasing with the gradually accumulating environmental pollution. In the recent years, tumor treatment has made great progress, which has significantly improved the survival rate of tumor patients. However, the existing medical technology still cannot completely eradicate tumor cells or control the distant metastasis of tumor cells. Bones are the most common locations (along with the lungs and liver) of malignant metastatic tumors, and ∼70%–80% of cancer patients will eventually develop bone metastasis. By analyzing 2,000 patients who died of metastatic bone tumors, Clain1 found that patients with tumors involving the spinal cord accounted for 69%, followed by the pelvis (41%), femur (25%), and skull (14%). This result indicated that the spinal cord is the most vulnerable location for malignant tumors with bone metastasis. Moreover, some literature sources have confirmed that the prevalence of metastatic spinal tumors is gradually increasing.2 When vertebral metastases occur in tumor patients, the major symptom is biological and mechanical pain caused by tumor tissues, followed by neurological symptoms when tumor tissues compress the spinal cord or nerve root. The vertebral metastases in tumor patients often suggest that the tumors are in their advanced stages. When treating metastatic spinal tumors, we should focus on relieving pain, protecting nerve function, reconstructing spinal stability, and improving patient’s quality of life. In terms of the surgical treatment of metastatic spinal tumors, percutaneous vertebroplasty or kyphoplasty is the most commonly used method.3 However, posterior fixation can also be used jointly to further enhance spinal stability. In terms of nonsurgical therapeutic measures, local radiation treatment can effectively relieve pain.4 Compared with the existing single radiotherapy, chemotherapy, or surgery, our department now combines surgical treatment with radiation therapy, using posterior tumor resection, pedicle fixation, and 125I brachytherapy, to treat advanced metastatic spinal tumors. This strategy not only relieves the mechanical pain caused by tumor compression and stabilizes spinal alignment but also relieves the biological pain caused by the tumor tissue. This method has achieved a certain efficacy, which is reported as follows.
Patients and methods
Patients included in this study
Seven metastatic thoracolumbar tumor patients, who were admitted to our department for treatment between January 2009 and December 2013, were selected. The inclusion criteria were: 1) clear diagnosis of a primary tumor; 2) chest and back pain and the restriction of spinal cord activity, with or without neurological symptoms; 3) preoperative magnetic resonance imaging, electroconvulsive therapy, or positron emission tomography–computed tomography (CT) suggesting metastatic thoracolumbar cancer with pathological fracture complications; 4) good general condition and suitability for surgery; and 5) predicted survival time (as determined by the preoperative Tokuhashi score) >6 months and a Tomita score <8 points. Of the seven patients, there were two males and five females. Their ages were between 34 years and 59 years, with an average of 48 years. Among them, four patients had lung cancer, with one patient having T12 and L3 metastases; one patient having T9, T10, T11, and L3 multiple metastases; one patient having T9 metastasis; and one patient having T9, L1, and L2 metastases. One patient had ovarian cancer with T3 metastasis, one patient had pancreatic cancer with L3 metastasis, and one patient had gastric cancer with L3 metastasis.
The study was approved by the Ethics Committee of The First Affiliated Hospital of Soochow University, and follow up information was obtained with consent of the patients. All aspects of the study were conducted in accordance with the principles of the Declaration of Helsinki.
Particle dosage plan
One week preoperatively, the patient underwent a CT scan and obtained images 5 mm thick with a layer spacing of 5 mm. Focal lines were measured, and total activity or image data were calculated into the source of the radioactive particle implantation treatment planning system. Therapeutic dose recommendations for the particles were as follows: if the patient has a history of 125I particle radiation therapy, use 90–110 Gy; if there is no such history, use an 125I particle treatment dose of 110–120 Gy. The particle treatment activity of the 125I particles was 0.5–0.7 mCi. The exact tolerance dose for the spinal cord is not yet clear; generally, 125I particle implantation treatment of the spinal cord involves doses <70 Gy.
Operation procedures
After successful general anesthesia and endotracheal intubation, the patient was placed in the prone position. Routine disinfection and draping of the surgical area was performed. C-arm fluoroscopy was performed to locate the inflicted vertebra. With the inflicted vertebra in the center, incisions were made in the middle of the vertebra above and below the inflicted vertebra. The skin, subcutaneous tissue, and thoracodorsal fascia were subsequently stripped. A periosteum detacher was used to strip the paraspinal muscles outward on both sides to the outside of the zygapophyseal joints to expose the lamina of the inflicted vertebra. Two pedicle screws were inserted at the pedicles of the adjacent vertebra above and below the inflicted vertebra. Then, a spinous rongeur was used to bite off the ligaments on the spinous process and between the spinous processes. A gun-type rongeur was used to bite off the lamina of the inflicted vertebra all the way to the bilateral pedicles. Intravertebral palliative tumor resection was conducted. Two 18G puncture needles were directly inserted from the back of the inflicted vertebra to the front. After C-arm fluoroscopy confirmed that the guide needle was in a good position, the particles were placed in the gun, which was then connected to the puncture needle. Radioactive 125I seeds were gradually implanted into the tumor. At the very end, C-arm fluoroscopy was used again to confirm that the positions of both pedicle screws and the radioactive particles were suitable. Then, the connecting rod was installed and the end caps were tightened. After adequate hemostasis, one negative pressure drainage tube was placed under the incision. After the surgery equipment was counted, the incision was sutured layer by layer.
Postoperative treatment
The postoperative sensations of the patient’s torso and changes in lower limb feeling and movement were closely monitored. Conventional oxygen and electrocardiogram monitoring were provided for 12 hours. Cefonicid sodium was given for 3 days as a preventive anti-infection therapy, while appropriate symptomatic treatment was applied to reduce swelling and relieve pain. For patients who had preoperative thoracic spinal stenosis symptoms, 1,000 mg of methylprednisolone was administered to prevent edema and 40 mg of omeprazole–sodium bicarbonate was given to protect the stomach. Six hours after the operation, nurses would urge the patients to turn their body regularly to prevent deep vein thrombosis in the lower limbs. One day after surgery, a bladder function exercise was conducted and the Foley catheter was removed to prevent a urinary tract infection. Two days after surgery, the drainage tube was removed according to the conditions (eg, drainage <50 mL) and the occurrence of cerebrospinal fluid leakage was closely monitored. If there was cerebrospinal fluid leakage, the patient would assume a Trendelenburg position for 3–5 days. In all, 3–5 days after the operation, the patient was encouraged to get out of bed and engage in an appropriate amount of activity with waist protection. Two weeks after surgery, the stitches were removed. Patients who met certain criteria were transferred to the oncology department for the follow-up treatment.
Therapeutic efficacy assessment
The visual analog scale (VAS) score was used to assess the pain condition before the operation and 1 month, 6 months, and 12 months after the operation: 0 point represented no pain and 10 points represented the most severe pain. The Karnofsky performance status (KPS) score5 (Table 1) was used to assess the general condition of the patients.
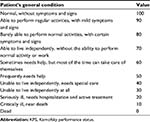  | Table 1 KPS scoring system |
Data analysis
The SPSS 17.0 statistical analysis software (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Variance analysis was used for the VAS and KPS scores, which were repeatedly measured before the operation and 1 month, 6 months, and 12 months after the operation.
Results
All the patients underwent a successful operation. There were no complications during the hospitalization. The patients all received postoperative follow-up, and the duration of the follow-up was 15–50 months, with an average of 32.2 months. There was one death involving a pancreatic cancer patient who died of liver failure and hypoproteinemia 28 months after the operation. The evaluations at 1 month, 6 months, and 12 months after surgery did not reveal detachment of the radioactive particles; the position of the pedicle screws was satisfactory, without broken screws.
VAS score changes
The VAS scores of the seven tumor patients before surgery and 1 month, 6 months, and 12 months after the operation were 7.43±0.98, 2.71±0.49, 3.00±0.82, and 4.29±0.98, respectively, suggesting that the VAS score at each time point was significantly lower compared with that before the operation; this difference was statistically significant (P<0.05; Figure 1).
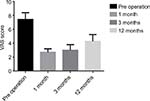  | Figure 1 VAS at each stage. |
KPS score changes
The KPS scores at the preoperative stage and at the 1-month, 6-month, and 12-month follow-ups of the patients were 52.9±9.5, 84.3±5.3, 75.7±5.3, and 72.9±4.9, respectively, suggesting that the postoperative performance status scores were significantly increased compared with those before the operation; this difference was statistically significant (P<0.05; Figure 2)
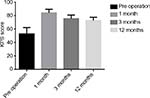  | Figure 2 KPS at each stage. |
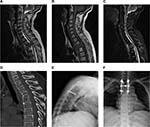
A typical case
In a female patient, 56 years old, T3 vertebral bone metastasis was found after 5 years of the ovarian cancer surgery. There was obvious chest and back pain without significant neurological symptoms. The pathological examination showed epithelial ovarian cancer. Six cycles of chemotherapy with a strategy of cis-diamminedichloroplatinum + epidoxorubicin + cyclophosphamide were conducted. The preoperative VAS score was 8, and the KPS score was 50. The VAS scores by telephone follow-up at 1 month, 6 months, and 12 months after surgery were 2 points, 3 points, and 3 points, respectively; the KPS scores were 90 points, 80 points, and 80 points, respectively (Figure 3).
Discussion
In recent years, the treatment of tumors has made major breakthroughs; in particular, the radiation therapy field has achieved satisfactory clinical efficacy. As a precision radiation therapy, radioactive particle brachytherapy has a history of ∼100 years. By the 1970s, with the continuous improvement in the preparation, application, and protection of radioactive particles, the permanent implantation of 125I seeds opened a new avenue for the radiation therapy of tumors. Initially, this method was used to treat prostate cancer in clinical practice and achieved satisfactory efficacy.6 To date, this method has been used to treat tumors in multiple systems, including lung cancer and pancreatic cancer. Literature sources have reported that this method can relieve some symptoms and elongate patient survival with satisfactory efficacy.7,8
125I seeds are a type of sealed, solid radiation source that can be used for brachytherapy. The housing material, a titanium tube, is 4.5 mm long and 0.8 mm in diameter, and the thickness of the wall is 0.05 mm. The internal core material is a 0.5 mm ×3 mm silver thread, which is coated with 125I isotopes. As an example of a low-dose-rate irradiation material, 125I radioactive particles have a half-life of 59.6 days and mainly emit X-rays with photon energies of 27.4 keV and 31.4 keV and γ rays with an energy level of 35.5 keV. The half-value layer of lead is 0.025 mm, while the half-value layer of tissue is 20 mm. In principle, 125I seeds can relieve pain after they are implanted into the body because they release a large amount of X-radiation and γ-radiation into human tissue. On the one hand, these rays can kill tumor cells by generating oxygen-free radicals and on the other hand, these rays can kill tumors by destroying the DNA inside the nuclei of tumor cells and inhibiting mitosis. Continuous irradiation can also generate cumulative damage effects, thereby relieving the biological pain caused by tumor tissues.
125I seeds are radioactive. To reduce the irradiation effect to the minimal level, we should pay attention to the following points. 1) Operations with sealed 125I seeds should be performed behind adequate shielding. The half-value layer of lead to shield 125I is 0.025 mm; therefore, 0.025 mm thick lead can reduce >99.9% of the radiation. When operating with forceps, the operator should keep a certain distance away from the particle source and gently pick up the particle source so that it will not be damaged. The particle source should not be picked up by hand. After the surgery is finished, the number of particle sources should be counted and they should not be left in the operation room. If the particle sources are lost, detectors that can detect radioactive rays of 30 keV should be used to search for the sources. 2) All patients and family members should be informed regarding 125I seed-related protection. Family members should routinely keep a safe distance of 1 m away from the patient; it should be emphasized that pregnant woman and children should not visit the patient in the ward at a close distance. 3) With the gradual shrinkage of the tumor during the treatment, one or more capsules of 125I seeds may become detached; therefore, it is recommended that the patients have regular checkups.
For metastatic spinal tumors, simple local radiation therapy was previously used. Although a good pain-relieving effect was achieved,4 some studies have suggested that compared with simple radiation therapy, radiation therapy combined with spinal decompression and spinal fixation can relieve pain more effectively and improve the quality of life.9 Vertebroplasty and vertebral column decompression fixation are common surgical methods of treating metastatic spinal tumors. In recent years, 125I seed implantation combined with vertebroplasty and vertebral column decompression fixation has become a new therapeutic technique for metastatic thoracolumbar tumors. Compared with the individual treatment approach, this combination method has a more satisfactory clinical efficacy. There are more reports on vertebroplasty combined with 125I seed implantation. For example, Yang et al10 used 125I seeds combined with percutaneous kyphoplasty to treat metastatic spinal tumors. The preoperative average VAS score was 8.4 points, and one day after surgery, the average score dropped to 2.9 points. One week after the surgery, the average score dropped to 2.0 points, and the average score dropped to 1.5 points 6 months after the surgery. Huang et al also reported that vertebroplasty combined with 125I seed implantation had excellent therapeutic effects in the treatment of metastatic thoracolumbar tumors.11 The numerical rating scale pain score at the time of follow-up after 2 months of surgery dropped from the preoperative score of 7.12±1.48 to 2.26±1.07, and the pain relief effect was significant. Rogers et al12 reported that the permanent implantation of 125I seeds wrapped in resorbable materials to treat metastatic paraspinal tumors had significant pain-relieving effects; in addition, particle implantation could enhance the local lesion control.
Although simple radiation therapy can partially relieve pain, this therapy cannot effectively relieve the mechanical pain caused by the compression of tumor tissues. In addition, this therapy cannot solve the problem of spinal cord instability caused by pathological vertebral fracture and cannot relieve neurological symptoms. Simple radiation therapy is mainly suitable in the following cases: 1) inability to tolerate surgery or a predicted survival time of <6 months; 2) no pathological fractures or a low incidence of pathological fractures; 3) absence of significant spinal cord instability symptoms or neurological symptoms; and 4) presence of tumors that are sensitive to radiation therapy. However, we should note that simple radiation therapy cannot control pain for the long term. Some studies have shown that the pain relief duration of most patients who have received simple radiation therapy is shorter than their survival time.13 However, as a noninvasive therapy, this method can relieve pain to a certain degree for tumor patients who cannot tolerate surgery, which has some advantages over invasive surgery.
Vertebroplasty combined with 125I seed implantation can relieve most of the pain, thus improving the quality of life. According to literature reports, the probability of this surgery inducing the complications of bone cement leakage or pulmonary embolism is not high, but its applicable conditions are limited. This method is mainly suitable when: 1) there are osteolytic lesions; 2) the posterior edge of the vertebra is complete; and 3) there are no significant neurological symptoms caused by tumor compression. This noninvasive surgery method also poses certain advantages, which are mainly as follows: 1) the surgical incision is smaller, and the postoperative recovery time is shorter than that of open surgery and 2) due to the filling by bone cement, the probability of radioactive particle shifting is low.
For tumor patients who are in an advanced stage and have thoracolumbar metastasis, we use a surgical method of pedicle fixation combined with brachytherapy using 125I seed implantation. This method can better relieve the mechanical pain caused by tumor tissue compression and the biological pain caused by tumor cells, stabilizing spinal alignment. Additionally, for patients who currently have or will have neurological symptoms, this method can improve and prevent neurological disorders, eventually improving the patient quality of life as much as possible. This method is mainly suitable under the following circumstances: 1) patients have significant neurological symptoms caused by compression of the spinal cord or nerve root; 2) there is a pathological vertebral fracture caused by tumor damage, and there is existing or predicted spinal cord instability; 3) there is intractable pain, and nonsurgical treatment is ineffective; 4) there are multiple metastases, which are difficult to remove or cannot be completely removed; and 5) the predicted survival time is >3–6 months, and the general condition is good.
The treatment of metastatic spinal tumor patients is related to the general condition of the patients, the control of the primary lesion, and the expectations. Therefore, various treatment strategies have been developed. Some researchers have suggested that as long as an appropriate therapy is selected, the pain of the metastatic spinal tumor patients can be relieved, their quality of life can be improved, and their survival time can be extended as well.14 At present, the evaluation systems for selecting a metastatic spinal tumor treatment strategy primarily include the Tokuhashi and Tomita scales. The Tokuhashi scale is a metastatic spinal tumor patient survival time and prognosis evaluation system that was proposed by Tokuhashi et al.15 The Tokuhashi scale is a 12-point system that can be derived from six items: general health condition, number of bone metastases, number of vertebral metastases, major organ metastasis, primary tumor type, and spinal cord paralysis. Subsequently, Tomita et al16 made some revisions to this scale. The Tomita scale evaluates the malignancy of the primary tumor, visceral involvement, and number of bone metastases. This scale suggests that patients with 2–3 points should have a wide or marginal resection, patients with 4–5 points should have marginal or intralesional resection, and patients with 6–7 points should have short-term palliative treatment. We determined the size of the tumor tissue resection according to the Tomita score, and whether vertebral column decompression was performed depended on whether neurological symptoms were present. Compared with simple radiation therapy, we suggest that tumor resection and pedicle fixation combined with 125I seed implantation can more effectively relieve pain symptoms and improve functional activity. Compared with the treatment protocol of vertebroplasty combined with radioactive particles, our proposed method can relieve neurological symptoms more completely and can be applied more broadly.
In addition, compared with simple laminectomy plus external beam radiation, we consider our method to have the following advantages. 1) The particle implantation scope and the exposure dose fall rapidly and facilitate an accurate tumor and organ assessment rather than approximation. The target dose is very high and does not increase important risks such as injury to the normal spinal cord. 2) The treatment is targeted and can be matched to the tumor shape. 3) 125I seeds only have a physical half-life, with no biological half-life.
Although pedicle fixation combined with 125I seed implantation can effectively relieve pain and improve the quality of life, it still has certain limitations. For example, the operation time is long, the trauma is extensive, and there can be excessive intraoperative blood loss. We should also consider the following issues.
Distribution and location of the radioactive particles and the distance from the tumor tissues
Some researchers have suggested that a correct distribution of the radioactive particles can maximally kill tumor cells while reducing damage to the surrounding normal tissues.17 Other researchers have suggested that to prevent 125I seeds from causing radioactive damage to the spinal cord, the distance between the particles and the spinal cord should be >1.5 cm. Therefore, we must devise an implantation strategy based on the preoperative CT and magnetic resonance imaging. However, because there are many intraoperative uncertainties, proper implantation may not be possible according to the strategy designed before the operation.
Relationship between the radioactive particle dose and the surgery efficacy
Although 125I has a small irradiation radius and only a low radiation dose is applied, if the dose exceeds the safe dose range for humans, it will inevitably cause radioactive injury. However, if the implanted dose of the radioactive particles is not sufficient, it will affect the killing effect against the tumor cells. There is no uniformly agreed-upon indicator of 125I dose in the existing 125I seed implantation literature. The dose determination is contingent on the clinical experience of the operator. In addition, the correlation between a radioactive dose and its therapeutic efficacy remains unclear, requiring further investigation.
Postoperative supplementation of radioactive particles
The half-life of 125I is 59.6 days, which means that it takes 59.6 days for the radioactive intensity to decrease to half of its original value. With the decrease in the radioactivity of the 125I source, its damaging effect on tumor tissues will also decrease. Whether it is necessary to supplement the particle source and the method to supplement the particle source should also be considered.
Lifespan of the pedicle screws
Most metastatic thoracolumbar tumors mainly cause bone damage. Therefore, the inflicted segments may lose their spinal cord alignment-stabilizing function due to pathological fractures. However, the goal of our surgery does not involve the complete reduction of the fracture and interbody grafts. Therefore, the stability of the lesion segments will rely on the maintenance of the pedicle screw system. With time, the stress on the pedicle screws will gradually increase, causing a risk of pedicle screw breakage.
Conclusion
As a therapy for treating advanced malignant tumors with thoracolumbar metastasis, pedicle fixation combined with 125I brachytherapy can effectively relieve short-term pain, rebuild the stabilization of the spine, and improve the quality of life. However, the long-term effect requires further research.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos 81472132 and 81572183) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author contributions
HY contributed substantially to the conception and design and gave final approval of the manuscript version to be published. JQ contributed to the analysis and interpretation of all data and drafted the article. ZB and JZ critically revised the article for important intellectual content. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15–29. | ||
Paton GR, Frangou E, Fourney DR. Contemporary treatment strategy for spinal metastasis: the “LMNOP” system. Can J Neurol Sci. 2011;38(3):396–403. | ||
Lieberman I, Reirdaardt MK. Vertebroplasty and kyphoplasty for osteolytic vertebral collapse. Clin Orthop Relat Res. 2003;415 | ||
Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;415(Suppl):S158–S164. | ||
Karnofsky DA, Burchenal JH. Present status of clinical cancer chemotherapy. Am J Med. 1950;8(6):767–788. | ||
Zuber S, Weiß S, Baaske D, et al. Iodine-125 seed brachytherapy for early stage prostate cancer: a single-institution review. Radiat Oncol. 2015;10:49. | ||
Zhang L, Chen LH, Wang J, et al. CT-guided radioactive 125I seed implantation treatment of multiple pulmonary metastases of hepatocellular carcinoma. Clin Radiol. 2014;69(6):624–629. | ||
Kelleher FC. Hedgehog signaling and therapeutics in pancreatic cancer. Carcinogenesis. 2011;32(4):445–451. | ||
Thomas KC, Nosyk B, Fisher CG, et al. Cost-effectiveness of surgery plus radiotherapy versus radiotherapy alone for metastatic epidural spinal cord compression. Int J Radiat Oncol Biol Phys. 2006;66(4):1212–1218. | ||
Yang ZZ, Yang DK, Xie L, et al. Treatment of metastatic spinal tumors by percutaneous vertebroplasty versus percutaneous vertebroplasty combined with interstitial implantation of 125I seeds. Acta Radiol. 2009;50(10):1142–1148. | ||
Huang X, Xu S, Du Z, Li F, Wang L. Treatment of metastatic thoracolumbar tumors by percutaneous vertebroplasty combined with interstitial implantation of 125I seeds. Zhonghua Zhong Liu Za Zhi. 2014;36(3):228–231. | ||
Rogers CL, Theodore N, Dickman CA, et al. Surgery and permanent 125I seed paraspinal brachytherapy for malignant tumors with spinal cord compression. Int J Radiat Oncol Biol Phys. 2002;54(2): | ||
Arcangeli G, Giovinazzo G, Saraeino B, et al. Radiation therapy in the management of symptomatic bone metastases: the effect of total dose and histology on pain relief and response duration. Int J Radiat Oncol Biol Phys. 1998;42(5):1119–1126. | ||
Jónsson B, Sjöström L, Olerud C, Andréasson I, Bring J, Rauschning W. Outcome after limited posterior surgery for thoracic and lumbar spine metastases. Eur Spine J. 1996;5(1):36–44. | ||
Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 1990;15(11):1110–1113. | ||
Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001;26(3):298–306. | ||
Jiang YL, Meng N, Wang JJ, et al. CT-guided iodine-125 seed permanent implantation for recurrent head and neck cancers. Radiat Oncol. 2010;5:68. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.