Back to Journals » ClinicoEconomics and Outcomes Research » Volume 6
Effect of occasional epoetin use in combination with a stable darbepoetin dosage on anemia management in hemodialysis patients
Authors Shimamatsu K
Received 30 August 2014
Accepted for publication 20 October 2014
Published 9 December 2014 Volume 2014:6 Pages 531—535
DOI https://doi.org/10.2147/CEOR.S73473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Giorgio L Colombo
Kazumasa Shimamatsu
Shimamatsu Naika Iin (Clinic), Shiseikai Medical Corporation, Chikushino City, Japan
Aim: Taking advantage of the characteristics of both darbepoetin (DA) and epoetin (EPO) might be a reasonable option for stabilizing hemoglobin (Hb) control in hemodialysis (HD) patients. The effect of DA assisted by EPO (DA/EPO) on Hb control was evaluated retrospectively in comparison with that of EPO monotherapy.
Methods: Twenty-six HD patients whose annual mean Hb values were available for both an EPO monotherapy period and a DA/EPO period were selected for analysis. During the DA/EPO period, DA was given on the second HD day of a week, and EPO was given if needed on the first and third HD days. Under stable DA dosage, when Hb rose >12 g/dL, EPO was eliminated. When Hb decreased <10 g/dL, EPO was added again. The variability of annual mean Hb values from the 26 HD patients during the DA/EPO period was compared with that during the EPO period. Additionally, the distance in Hb (d-Hb; absolute value of difference) between the annual mean Hb values and the target Hb (11 g/dL) during the DA/EPO period was compared with that during the EPO period.
Results: The variability of annual mean Hb values during the DA/EPO period was significantly smaller than that during the EPO period (11.2±0.25 g/dL versus [vs] 11.0±0.50 g/dL; the F-test for equality of variance, P<0.001). Additionally, the d-Hb during the DA/EPO period was significantly smaller than that during the EPO period (0.22±0.21 g/dL vs 0.38±0.31 g/dL, P<0.03). The total doses (as EPO equivalents) of DA with EPO were reduced to 82.2% of the baseline EPO dose during the EPO monotherapy period. The expenditure for the DA/EPO period was significantly reduced to 80.9% of that for the EPO monotherapy. Also, the annual total amount of intravenous iron supplementation during the DA/EPO period was significantly reduced compared with that during the EPO period (546±304 mg/year vs 684±314 mg/year, P<0.05).
Conclusion: The occasional use of EPO in combination with a stable DA dosage may be useful for Hb control within a narrow range of the target level.
Keywords: combination therapy, erythropoiesis-stimulating agents, hemoglobin cycling, hemoglobin SD
Introduction
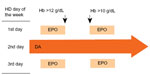
The use of both long- and short-acting erythropoiesis-stimulating agents (ESAs) may be a reasonable option for anemia management to avoid high hemoglobin (Hb) levels and to stabilize Hb cycling. After a smooth introduction of darbepoetin (DA) as a long-acting ESA in hemodialysis (HD) patients on epoetin (EPO) monotherapy in 2007,1 a stable DA dose was intermittently combined with EPO depending on Hb levels (Figure 1). In this study, the effect of DA assisted by EPO (DA/EPO) on Hb control was retrospectively evaluated in comparison with that of EPO monotherapy.
Patients and methods
January–March 2008 was a period of transition from the period of EPO monotherapy to the introduction of DA in our clinic. Out of 81 HD patients who existed on April 1, 2008, only 26 patients who had a history of HD treatment both in the EPO monotherapy period (April 1, 2006–March 31, 2007) and in the DA/EPO period (April 1, 2008–March 31, 2009) throughout with no episodes of bleeding complications and/or hospital admission and no change in other comorbidities were retrospectively selected for analysis. HD consisted of 5-hour treatments performed three times per week. No patients used catheters for vascular access for HD. The mean patient age (15 male, 11 female) at the beginning of the DA/EPO period was 60.6±13.9 years (mean ± standard deviation [SD]). The mean HD duration was 9.1±7.1 years. The original diagnoses were chronic glomerulonephritis in 16 patients, diabetic nephropathy in four patients, hypertensive nephrosclerosis in four patients, polycystic kidney disease in one patient, and Alport syndrome in one patient. The preparations (10 μg or 20 μg) of DA (Nesp®; Kyowa Hakko Kirin Co Ltd, Tokyo, Japan) were administered on the second HD day of a week. The preparations (750 IU or 1,500 IU) of EPO (Epogin®; Chugai Pharmaceutical Co Ltd, Tokyo, Japan) could be administered (as 750 IU or 1,500 IU of EPO preparation × two times per week) on the first and third HD days of a week. The target Hb value was 11 g/dL. The Hb level was measured twice a month (24 measurements per patient per year). As shown in Figure 1, EPO was eliminated after the introduction of DA therapy when the Hb levels were >12 g/dL. EPO was reintroduced when the Hb levels were <10 g/dL after the elimination of EPO. Stop or restart of EPO was done on first and third HD days of the week simultaneously. When the DA/EPO combination continued without elimination of EPO for more than 2 months, the fixed DA dosage was considered to be increased by 10 μg (the average duration that DA/EPO patients were receiving EPO was 11.5±10.5 weeks). In terms of iron supplementation, according to our previously reported method,2–4 40 mg of intravenous (iv) iron (saccharated ferric oxide, Fesin®; Nichi-Iko Co Ltd, Toyama, Japan) was administered once a week when serum ferritin levels were <100 ng/mL. Once serum ferritin levels were >100 ng/mL, 40 mg of iv iron was administered twice a month and adjusted quarterly according to serum ferritin measurements. When the quarterly serum ferritin values exceeded 300 ng/mL, the frequency of iv iron was decreased. When serum ferritin values reached 500 ng/mL, iv iron supplementation was discontinued. The serum ferritin concentration was determined quarterly. The variability of the annual mean Hb from 26 HD patients during the DA/EPO period was compared with that during the EPO period. In addition, the distance in Hb (d-Hb; absolute value of difference) between the 26 patients’ annual mean Hb and the target Hb (11 g/dL) during the DA/EPO period was compared with that during the EPO period. All the data were normally distributed. Coefficient of variance (CV) was calculated as SD divided by mean. The F-test for equality of variance and the paired t-test were applied for analysis. A P-value <0.05 was considered to represent statistical significance.
This study was done by Institutional Review Board Approval (Clinical Research Network Fukuoka: IRB#11001122; Registration #13-E16).
Results
There was no significant difference between the mean SD values from individual HD patients during the EPO period and during the DA/EPO period (0.58±0.18 vs 0.58±0.19, Table 1). As shown in Figure 2, the variability of the annual mean Hb of the 26 HD patients during the DA/EPO period was significantly less than that during the EPO period (11.2±0.25 g/dL vs 11.0±0.50 g/dL, the F-test for equality of variance, P<0.001). The CV of annual mean Hb levels decreased (narrowed) during the DA/EPO period compared with that during the EPO period (0.022 vs 0.046, respectively). In addition, the d-Hb of the annual mean Hb during the DA/EPO period was significantly less than that during the EPO period (0.22±0.21 g/dL vs 0.38±0.31 g/dL, P<0.03, Figure 3).
The mean EPO dose was 3,656±2,107 IU/week during the EPO period; during the DA/EPO period, the mean DA dose was 13.6±7.5 μg/week, with a mean EPO dose of 284±436 IU/week. The total doses (as EPO equivalents; EPO dose, IU =200× DA dose, μg1,5) of DA with EPO were significantly reduced to 82.2% of the baseline EPO dose during the EPO monotherapy period. The expenditure for the DA/EPO period, calculated using the published prices for 2008 when DA was introduced to our clinic, was significantly reduced to 80.9% of that for the EPO monotherapy period (n=26, ¥229×103 Yen/year vs ¥283×103 Yen/year, P<0.03). The annual total amount of iv iron supplementation during the DA/EPO period was significantly reduced compared with that during the EPO period (n=26, 546±304 mg/year vs 684±314 mg/year, P<0.05). The mean serum ferritin concentration during the DA/EPO period was significantly reduced compared with that during the EPO period (199±49 ng/mL vs 231±98 ng/mL, P<0.05). There was no change in transferrin saturation between DA/EPO and EPO periods (27.2%±5.3% vs 26.7%±6.9%, ns).
Discussion
The combination of short- and long-acting ESAs, such as EPO and DA described in this study, takes advantage of the differences in the withdrawal times of each ESA. The results of this study suggest that by maintaining a relatively stable DA dosage with transient EPO assistance, we may more precisely adjust Hb levels to the target Hb level (Figures 2 and 3). In the study reported by the European/Australian Novel Erythropoietin Stimulating Protein Study Group, DA and EPO for anemia management were assessed in patients with chronic renal failure who were not yet on dialysis. This study indicated that high Hb levels (>14 g/dL) developed in 24% of patients treated with DA and in 35% of patients treated with EPO.5 In addition, recent large-scale randomized controlled trials and reviews have strongly suggested harmful effects of higher ESA doses and higher Hb levels close to normal.6–10 Under these circumstances, the Kidney Disease Improving Global Outcomes (KDIGO) guideline for anemia recommends a narrower target Hb (10–11.5 g/dL)11 compared with the target given in past major guidelines.12,13 The DA/EPO combination in the present study may indicate one of the solutions for both the reduction (by approximately 18%) of ESA doses and the achievement of narrower Hb control ranges.
The Dialysis Outcomes and Practice Patterns Study (DOPPS) reported that facility-level interpatient Hb variability, measured as the SD of Hb levels, was strongly and positively associated with patient mortality.14 Facility-level Hb SDs varied more than fivefold across facilities (range, 0.51–2.72 g/dL; median, 1.27 g/dL). Overall, an 8% higher mortality rate was observed for every 0.5 g/dL greater facility-level Hb SD in the DOPPS after adjustment for numerous patient characteristics and facility practices.14 In addition, during DOPPS, a strong correlation was observed between the facility mean within-patient Hb SD and the interpatient Hb SD across facilities. Also, Yang et al demonstrated a 33% increase in the adjusted rate of death for each 1 g/dL increase in Hb variability (described as residual SD) in HD patients.15 There was no significant difference in the within-patient Hb SD between the EPO and the DA/EPO period in the present study. This might be caused by, in addition to small number of patients, the relatively less Hb variability (SD of 0.58±0.18) even in EPO monotherapy period compared with reports mentioned earlier.14,15 However, the interpatient Hb SD significantly decreased (narrowed) by almost half the range during the DA/EPO period compared with the EPO period (Figure 2). The DA/EPO combination method employed in this study may become one approach to anemia management to improve HD patient survival rates.
The economic disadvantages of the DA/EPO combination may be a concern. In the present study, the total doses (as EPO equivalents) of DA/EPO were reduced to approximately 82% of the baseline EPO dose during the EPO monotherapy period resulting in reduced ESA expenditures. The combination of DA/EPO may cost higher compared with each monotherapy. However, as EPO was used as temporal assist of 11.5 weeks in average, the cost of DA/EPO on annual basis became lower than that of EPO monotherapy. Also the Japanese published price of DA is cheaper than that of EPO when compared as EPO equivalent dose. Further, a stable (fixed) dosage of 10 μg or 20 μg of DA of a longer-acting ESA might be favorable to cost-saving. In addition, actual DA requirement was less than expected (DA:EPO =1:220 vs the original rate of 1:200).1 The annual total amount of iv iron supplementation in this study was significantly reduced during the DA/EPO period compared with that during the EPO period. Despite the fact that no change in transfferin saturation between the two periods was found, the annual mean serum ferritin concentration significantly decreased during the DA/EPO period compared with the EPO monotherapy period. The DA/EPO therapy used in this study may utilize iron more effectively for erythropoiesis to maintain the same target Hb level as EPO monotherapy, possibly by mobilizing iron smoothly into the blood stream from iron-storage sites. The present study also indicated that the small doses of 10 μg and 20 μg of DA and 750 IU and 1,500 IU of EPO were sufficient for the DA/EPO combination therapy. Furthermore, by designating the days of the week to apply the preparations (the second HD day of a week for DA and the first and third HD days of a week for EPO), the medical staff did not make any injection errors during the study period.
There are limitations to the present study. Out of 81 HD patients who existed on April 1, 2008, only 26 patients who had a history of HD treatment both in the EPO monotherapy period (April 1, 2006–March 31, 2007) and in the DA/EPO period (April 1, 2008–March 31, 2009) throughout with no episodes of bleeding complications and/or hospital admission were retrospectively selected for analysis. As a result, the present study became a retrospective one in a small group of patients. However, some positive results from this pilot study may be indicative of justification of further prospective large-scale controlled trial.
Conclusion
The occasional use of EPO in combination with a stable DA dosage may be useful for controlling Hb levels within a narrow range of the target level.
Disclosure
The author (Kazumasa Shimamatsu, MD, PhD) has no conflicts of interest regarding the content of this article. The author is also responsible for the final content of the paper. The author was financially supported by Shiseikai Medical Corporation.
References
Shimamatsu K, Inamasu H. A safe and easy introduction of darbepoetin- alpha in patients receiving maintenance hemodialysis and epoetin monotherapy: a “half-and-half” combination therapy. Curr Ther Res. 2013;74:5–8. | |
Shimamatsu K. Experience with iv iron chondroitin-sulphate colloid in Japanese haemodialysis patients. Nephrol Dial Transplant. 1998;13:1053. | |
Shimamatsu K. Low-dose maintenance supplementation of intravenous iron chondroitin-sulfate colloid in hemodialysis patients: a 3-year follow-up. Dial Transplant. 2003;32:595–598. | |
Mitsuiki K, Harada A, Miyata Y. Reduction of recombinant human erythropoietin maintenance dose by supplementation with a low-dose iron preparation, given intravenously. Clin Exp Nephrol. 2001;5:228–233. | |
Locatelli F, Olivares J, Walker R, et al; European/Australian NESP 980202 Study Group. Novel erythropoiesis stimulating protein for treatment of anemia in chronic renal insufficiency. Kidney Int. 2001;60: | |
Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. | |
Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2085. | |
Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin-alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. | |
Pfeffer MA, Burdmann EA, Chen C-Y, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361: 2019–2032. | |
Koulouris I, Alfayez M, Trikalinos TA, Balk EM, Jaber BL. Dose of erythropoiesis-stimulating agents and adverse outcomes in CKD: | |
McMurray JJV, Parfrey PS, Adamson JW, et al. KDIGO Clinical Practice Guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. | |
National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(Suppl 3):S11–S146. | |
Locatelli F, Aljama P, Bárány P, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Section II. Targets for anaemia treatment. Nephrol Dial Transplant. 2004;19(Suppl 2):ii6–ii15. | |
Pisoni RL, Bragg-Gresham JL, Fuller DS, et al. Facility-level interpatients hemoglobin variability in hemodialysis centers participating in the Dialysis Outcomes and Practice Patterns Study (DOPPS): associations with mortality, patient characteristics, and facility practices. Am J Kidney Dis. 2011;57:266–275. | |
Yang W, Israni RK, Brunelli SM, et al. Hemoglobin variability and mortality in ESRA. J Am Soc Nephrol. 2007;18:3164–3170. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.