Back to Journals » International Journal of Nanomedicine » Volume 13
Effect of melanin on gold nanoparticle-induced hepatotoxicity and lipid peroxidation in rats
Authors Abdelhalim MAK , Moussa SAA , Qaid HAY, Al-Ayed MS
Received 11 April 2018
Accepted for publication 5 May 2018
Published 6 September 2018 Volume 2018:13 Pages 5207—5213
DOI https://doi.org/10.2147/IJN.S170758
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Mohamed Anwar K Abdelhalim,1 Sherif A Abdelmottaleb Moussa,2,3 Huda AY Qaid,1 Mohammed Suliman Al-Ayed1
1Department of Physics and Astronomy, College of Science, King Saud University, Riyadh, Saudi Arabia; 2Committee of Radiation and Environmental Pollution Protection (CREPP), Department of Physics, College of Science, Al-Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh, Saudi Arabia; 3Biophysics Group, Biochemistry Department, Genetic Engineering and Biotechnology Division, National Research Center, Giza, Egypt
Introduction: Melanin pigments are produced by melanocytes and are believed to act as antioxidants based on the belief that melanin can suppress electronically stirred states and scavenge the free radicals.
Materials and methods: The study was aimed to verify and prove the toxicity induced by administration of gold nanoparticles (GNPs) and to characterize the role of melanin as an antioxidant against inflammatory liver damage, oxidative stress, and lipid peroxidation induced intraperitoneally by GNPs in vivo.
Results: The findings from this study confirmed that administration of GNPs intraperitoneally caused liver damage in addition to producing oxidative stress and fatty acid peroxidation. The treatment of rats with melanin along with GNPs induced dramatic changes in all the measured biochemical parameters. Our data demonstrated that melanin completely inhibited inflammatory liver damage, oxidative stress, and lipid peroxidation, which was confirmed by the histological investigation of different liver sections stained by H&E.
Conclusion: These results suggest the beneficial use of melanin together with GNPs for alleviating its toxicity. Other studies should be implemented taking into consideration the role of melanin in comparison with other natural antioxidants.
Keywords: liver, gold nanoparticles, hepatotoxicity, oxidative stress, lipid peroxidation, histological investigation
Introduction
Several studies have reported on the production of numerous inflammatory and cytotoxic responses resulting from contact with smaller-sized gold nanoparticles (GNPs) in comparison to contact with larger-sized GNPs with the same mass concentration due to their highly reactive role with biological constituents, and have stressed on the harmful effects produced by a large number of nanoparticles.1–6 The toxic nature of GNPs is related to its variations, surface functional affinity, appearance, and diameter.1–6 The intraperitoneal administration of GNPs induced inflammation, fatty acid change and hypergenesis, hydropic deterioration, and death of most cells.1,3
Exposure to GNPs has been known to cause cardiac tissue destruction, inflammation of the airspaces in the lungs, connective tissue deposition, and chronic inflammatory cell infiltration that solely depends on the amount and length of exposure.3 These changes could be as a result of the damage to the tissues, resulting from poisoning by GNPs, which interfere with the antioxidant defense structure, thereby probably giving rise to reactive oxygen species (ROS) formation, which may ultimately lead to cell death.2,4
Recently, herbal melanin has been extracted from various sources, and the immunoprotective role of melanin has been reported in plants and skin.7–9 Melanin can be found in different organs and tissues and in the blood of living organisms, but its defining role is still unclear. Many diseases are accompanied by an increase or decrease in melanin production. Melanin plays a vital role in preventing, absorbing, and shielding the cell’s DNA in the nucleus against injury due to ultraviolet radiation (UV). Melanin shows various attractive properties, such as being a nonoxidative agent as well as its scavenging activities on free radicals.10–12
Chen et al,13 have reported that toxicity is generated by nanoparticles due to the reduction in their size. Furthermore, as there are many applications for nanoparticles, especially in drug delivery and imaging, it becomes necessary to prevent toxicity of GNPs when used in medical applications. When GNPs are injected into mice, it enters hippocampus and induces cognitive impairment, in addition to other different complications. Pretreatment of tea melanin significantly prevented the deposition of GNPs in mouse brains, especially in the hippocampus.13
The ability of melanin to act as a forager of free radicals in vitro and in vivo is well established.4–16 Previous studies on melanin revealed that melanin combines with the substances containing oxygen such as oxygen,17,18 hydroxyl radical,19 and superoxide negatively charged ion.19,20 Melanin’s ability to function as an antioxidant has been established from its ability to prevent lipid peroxidation.21–23
To make use of GNPs in drug delivery and cancer treatment, knowledge about inhibiting toxicity is essential. Prevention of toxicity stimulated by the injection of small-sized GNPs has not been well confirmed and established in rat liver. Thus, the current study aims to reinforce and prove the toxicity induced by administration GNPs in rats in vivo and also to investigate the role of melanin as an antioxidant against hepatotoxicity, oxidative stress, and lipid peroxidation.
Materials and methods
Preparation of melanin
Melanin was prepared based on calibrated, standardized, and established known techniques.7 The purity of the prepared melanin was almost about 99%.24,25 The botanical name of the plant from which the black seeds were obtained is “Nigella sativa.” N. sativa seed coats were dissolved in NaOH at pH 12.5 for 3 h, giving rise to the formation of a brown solution. The solution formed was dark, clear, and had no suspension. Centrifugation was done to produce a stock solution and filtered, followed by melanin extraction using concentrated hydrochloric acid with a pH of 2.7
To obtain melanin with higher purity, alkali–acid treatment was done 2–3 times. The precipitate was washed with distilled water, filtered, and desiccated at a temperature of 80°C. The dried powder obtained was kept and later used for the preparation of other solutions at pH 7 for biological examination by dissolving the preferred volume of melanin powder (in w/w ratio) in NaOH at pH 12.5 using concentrated hydrochloric acid to correct the pH and to obtain the preferred concentration. To avoid photochemical and photophysical alterations, the herbal melanin was kept refrigerated at a temperature of −20°C.24
GNPs and dosing
Ten-nanometer GNPs with spherical morphology (products MKN-Au-010; M K IMPEX Corp; Divn MK Nano, Mississauga, Canada) were used. Doses of 50 μL of 10 nm GNPs were administered intraperitoneally to the rats. The size and morphology of different gold nanoparticles were calculated from the images taken by the transmission electron microscope. Ten-nanometer GNPs show spherical shape, while the 50 nm GNPs presented hexagonal shape.1–4
Animals
Eighteen (18) male Wistar Kyoto rats, aged 12 weeks, and weighing between 220 and 240 gm were obtained from the College of Pharmacy, King Saud University (KSU) Animal care house. The rats were kept in cages under customary environmental conditions (22°C±5°C temperature, 55%±5% humidity, and 12 h light/dark cycle). The rats had normal supply of water and were fed with pelleted standard rat chew diet. The KSU Animal Use Committee approved the animal experiments, and all experiments were conducted in accordance with the guidelines approved by KSU local animal care.
Experimental design
The acclimatization period for the animals was one week, and then rats were fasted for 24 h before injection and were divided into three sets of 6 animals each. The 1st group (G1) was the normal healthy rats that received no GNPs and served as the control group. The 2nd group (G2) were injected daily with a dose of 50 µL GNPs intraperitoneally for 7 consecutive days. The 3rd group (G3) were GNP-intoxicated rats that were injected with melanin (100 mg/kg BW/d) for 7 successive days.
Blood sampling and tissue preparation
After the final dose, the rats were starved for 12–14 h, and then euthanized; blood samples from each animal were collected into disinfected tubes for serum separation. Serum separation was done by using a centrifuge at 3,000 rpm for 10 min and preserved at a temperature of −80°C for various biochemical assessments. Midline incision method was used to collect and harvest liver and kidney tissues, which were then rinsed with icy isosmotic saline, homogenized, and frozen at a temperature of −80°C for biochemical tissue analysis (such as reduced glutathione [GSH] level estimation).25
Serum liver function markers
To evaluate the liver inflammatory damage, serum liver biomarkers such as γ-glutamyl transferase (GGT), aspartate aminotransferase (AST), total bilirubin (TBIL), alanine aminotransferase (ALT), total protein, and alkaline phosphatase (ALP) were determined using kits from Salucea (Etten-Leur, the Netherlands).
Oxidative stress biomarkers
Determination of GSH level
To verify the oxidative stress induced by intraperitoneal administration of GNPs, GSH in liver tissues of rats was determined enzymatically by the revised procedure.26
Determination of malondialdehyde (MDA)
To verify the lipid peroxidation induced by GNPs, the level of malondialdehyde (MDA) in rat liver tissues was determined using a spectrophotometer as described by Utley and Hochstein (1967).27
Histological investigation of liver tissues
Samples of liver tissues were collected and fixed in 4% formaldehyde for 24 h, dehydrated in ethyl alcohol, and cleared in xylene. Then it was embedded in paraffin. Paraffin blocks were cut using a microtone at 4 μM and then fixed on glass slides for staining. Sections were stained with hematoxylin to stain the nuclei and then stained with eosin to stain the cytoplasm.
Statistical analysis
Data are presented as mean ± SE. Analysis of data was performed using one-way analysis of variance at a confidence level of *p<0.05 compared with the control group and compared with the GNP group.
Results
Figure 1 shows a disturbance in the liver ALP activity after the administration of GNPs. A significant elevation (p<0.05) to 538.17±34.71 U/L in the GNPs group was observed compared with 370.67±11.17 U/L in the control group. Rats that were treated with melanin simultaneously for up to 7 days showed significantly decreased activity of liver ALP, to 375.22±11.17 U/L, almost near to that seen in the control group.
Figure 2 demonstrates the elevation of liver ALT activity, which increased (p<0.05) to 118±3.71 U/L in GNP rats as compared to 97.70±5.88 U/L in control rats. The coadministration of melanin with GNPs significantly reduced this elevation to 98.50±5.88 U/L.
Figure 3 shows a significant elevation in the liver enzyme GGT in the GNP group (2.83±0.18 U/L) in comparison to the control group (1.15±0.30 U/L). Coadministration of melanin with GNPs significantly reduced the GGT level to 1.72±0.30 µ/L.
Figure 4 illustrates a notable increase (p<0.05) in the serum level of serum protein in the GNP rats (7.33±0.33 g/dL) compared to the control rats (5.95±0.25 g/dL). The coadministration of melanin with GNPs induced a significant decrease in the serum protein level (6.08±0.25 g/dL) compared to the GNP-administrated rats.
  | Figure 4 Effect of GNPs and melanin on total protein level in rats. |
Figure 5 shows a significant reduction in the liver GSH levels in the GNP group; it was 20±0.99 μg/mL compared with the control group, 65±2.89 μg/mL. The coadministration of melanin with GNPs induced a significant elevation in the GSH level to 65.10±2.99 μg/mL compared to the GNPs group.
Figure 6 shows an obvious significant elevation on MDA activity in GNP rats (**p<0.01) when compared to control rats (0.33±0.03 vs 0.16±0.01 μmol/mL). The coadministration of melanin with GNPs induced a significant reduction in MDA level to 0.15±0.01 μmol/mL compared to the GNPs group.
  | Figure 6 Effect of GNPs and melanin on liver MDA level in rats. |
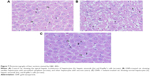
Hepatoxicity was confirmed and verified through histological investigation of the liver tissues, stained with H&E.
Figure 7A shows a normal liver section. Each hepatic lobule consists of a central vein lined with simple squamous epithelium, surrounded by polygonal hepatic cells, and contains quite clear blood sinusoids in-between. The cytoplasm of the hepatocytes appeared homogenous and contained various uniformly distributed coarse basophilic granules. The hepatocytes are usually uni-or binucleated. Numerous spindle-shaped Kupffer’s cells arise between the hepatocytes.
Figure 7B shows liver sections obtained from rats treated with 100 μL of 10 nm GNPs for 7 days revealed different histological lesions compared with the control group; these lesions also showed signs of vacuolar degeneration and necrosis. Necrosis refers to severe morphological changes accompanying the cell death, and it indicates to the presence of potentially irreversible cellular injuries. The necrotic cells showed nuclear alterations including pyknosis, karyorrhexis, and karyolysis.
Figure 7C shows the liver of rats treated with melanin (200 mg/kg/d) along with 100 μL of 10 nm GNPs for 7 days showed protection against hepatocyte vacuolar degeneration and necrosis when compared to the GNP-treated rats, and these cells appeared like those of normal liver cells.
Discussion
This study showed that GNPs caused damage to hepatocytes, as supported by the substantial elevation of serum liver markers in injected rats compared with control rats. Our finding was supported by previous studies,28–31 as these results revealed that excessive zinc administration could lead to liver damage. Coadministration of melanin to GNP-treated rats significantly reduced the levels of all the serum liver markers (p<0.001) when compared with the injected rats. This shows that the melanin could act as a defense against the liver dysfunction triggered by GNPs’ hepatotoxicity.
Some studies have reported that the pathogenic mechanism instigated by in vivo administration of metal oxide particles is dominated by oxidative stress, cell changes and death, and DNA destruction.32,33 Thus, a protective plan geared toward the reduction of the generation of inflammatory precursors could ameliorate liver damage and prevent or improve organ dysfunction. In our study, the ingestion of melanin immediately along with GNPs was beneficial in preventing the GNP-induced inflammatory liver injury.
Due to the recognized relationship between nanoparticles and oxidative stress, fatty peroxidation can be mentioned as one of the probable reasons for DNA destruction triggered by GNPs.34 ROS are known to combine with DNA molecules, leading to the destruction of nitrogenous bases together with DNA support.32
MDA, the main producer of fatty acid peroxidation, is a mutagenic and carcinogenic substance that can combine with DNA to form chemical compounds with adenosine nucleosides (deoxyguanosine and deoxyadenosine) and deoxyribonucleosides (deoxycytidine).35,36 DNA damage may trigger signal transduction pathways that may result in programmed cell death.37
Eumelanin, which is the essential form of melanin found in humans, can protect against UV radiation, and owing to its antioxidant ability, it reduces DNA damage by creating a cap cover round the nucleus.38,39 Our data were consistent with those published earlier that melanin acts as an antioxidant, confirmed by its ability to stop fatty acid peroxidation.22,23 Together with reducing oxidative stress, melanin also reduces the inflammatory reaction of hepatocytes by preventing the creation of proinflammatory cytokines from permeating monocytes, and possibly lymphocytes also.
Our data might suggest or support the ability to use melanin to significantly reduce the inflammatory liver damage caused by administration of GNPs, as observed by the considerable alterations in the liver function markers as well as liver tissues biomarkers observed in this study. Moreover, new experiments should be performed to test the possibility of using melanin as a protective agent against the nephrotoxicity induced by GNPs.
Conclusion
This study underlines the hepatotoxic effects caused by intraperitoneal administration of GNPs that was confirmed by a significant rise in the levels of serum liver function markers such as ALT, ALP, GGT, TBIL, and total protein; this was in addition to the significant elevation in the MDA level and the decrease in the oxidative stress biomarker GSH level. The treatment with melanin successively alleviated ALT, ALP, GGT, TBIL, total protein, and MDA, while it elevated the GSH, which in turn efficiently ameliorated the lipid peroxidation and inflammatory liver damage induced in GNPs intoxicated rats. Our data demonstrated that melanin is a highly potent antioxidant that can protect the hepatocytes from damage produced by oxidative stress and other associated vascular complexities caused by GNP generation, due to the creation of ROS.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the research Group Project No RGP-285.
Disclosure
The authors report no conflicts of interest in this work.
References
Abdelhalim MAK, Jarrar BM. Histological alterations in the liver of rats induced by different gold nanoparticles size, dose and exposure duration. J Nanobiotechnology. 2012;10:5. | ||
Abdelhalim MAK, Jarrar BM. Renal tissue alterations were size-dependent with smaller ones induced more effects and related with the time exposure of gold nanoparticles. Lipids Health Dis. 2011;10:163. | ||
Abdelhalim MAK, Jarrar BM. Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver. Lipids Health Dis. 2011;10:166. | ||
Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nano level. Science. 2006;311(5761):622–627. | ||
Abdelhalim MAK. Exposure to gold nanoparticles produces cardiac tissue damage that depends on the size and duration of exposure. Lipids Health Dis. 2011;10:205. | ||
Abdelhalim MAK. Gold nanoparticles administration induces disarray of heart muscle, hemorrhagic, chronic inflammatory cells infiltrated by small lymphocytes, cytoplasmic vacuolization and congested and dilated blood vessels. Lipids Health Dis. 2011;10:233. | ||
Haseeb A, Elhag H, inventor; Haseeb A, Elhag H, assignee. Process for producing melanin using cultures of the genus Nigella. WIPO patent WO 2012125091A1. 2012 Sept 20. Available from: www.google.com/patents/WO2012125091A1. Accessed June 08, 2018. | ||
El-Obeid A, Al-Harbi S, Al-Jomah N, Hassib A. Herbal melanin modulates tumour necrosis factor alpha (TNF-α), interleukin 6 (IL-6) and vascular endothelial growth factor (VEGF) production. Phytomedicine. 2006;13(5):324–333. | ||
Sava VM, Hung YC, Blagodarsky VA, Hong MY, Huang GS. The liver-protecting activity of melanin-like pigment derived from black tea. Food Res Int. 2003;6:505–511. | ||
Bridelli MG, Ciati A, Crippa PR. Binding of chemicals to melanins re-examined: adsorption of some drugs to the surface of melanin particles. Biophys Chem. 2006;119(2):137–145. | ||
Meredith P, Sarna T. The physical and chemical properties of melanin. Pigment Cell Res. 2006;19(6):572–594. | ||
Perna G, Frassanito MC, Palazzo G, et al. Fluorescence spectroscopy of synthetic melanin in solution. J Lumin. 2009;129(1):44–49. | ||
Chen YS, Hung YC, Hong MY, et al. Control of in vivo transport and toxicity of nanoparticles by tea melanin. J Nanomater. 2012;2012:9. | ||
Prota G. Melanins and Melanogenesis. San Diego, CA: Academic Press; 1992. | ||
Herrling T, Jung K, Fuchs J. The role of melanin as protector against free radicals in skin and its role as free radical indicator in hair. Spectrochim Acta A Mol Biomol Spectrosc. 2008;69(5):1429–1435. | ||
Rozanowska M, Sarna T, Land EJ, Truscott TG. Free radical scavenging properties of melanin interaction of eu- and pheomelanin models with reducing and oxidising radicals. Free Radic Biol Med. 1999;26(5–6):518–525. | ||
Sarna T, Menon IA, Sealy RC. Photosensitization of melanins: a comparative study. Photochem Photobiol. 1985;42(5):529–532. | ||
Sealy RC, Sarna T, Wanner EJ, Reszka K. Photosensitization of melanin: an electron spin resonance study of sensitized radical production and oxygen consumption. Photochem Photobiol. 1984;40(4):453–459. | ||
Sarna T, Pilas B, Land EJ, Truscott TG. Interaction of radicals from water radiolysis with melanin. Biochim Biophys Acta. 1986;883(1):162–167. | ||
Korytowski W, Kalyanaraman B, Menon IA, Sarna T, Sealy RC. Reaction of superoxide anions with melanins: electron spin resonance and spin trapping studies. Biochim Biophys Acta. 1986;882(2):145–153. | ||
Bustamante J, Bredeston L, Malanga G, Mordoh J. Role of melanin as a scavenger of active oxygen species. Pigment Cell Res. 1993;6(5):348–353. | ||
Ezzahir A. The influence of melanins on the photoperoxidation of lipids. J Photochem Photobiol B. 1989;3(3):341–349. | ||
Krol ES, Liebler DC. Photoprotective actions of natural and synthetic melanins. Chem Res Toxicol. 1998;11(12):1434–1440. | ||
Al-Tayib OA, Haseeb AM, El-Tahir KE, Idriss MH. The aqueous extracts of the Nigella sativa melanin: experimental in vivo test and in vitro HEp-2 cell lines cytotoxicity effects. IOSR J Agric Vet Sci. 2016;9(6):84–90. | ||
LoPachin RM, Rudy TA, Yaksh TL. An improved method for chronic catheterization of the rat spinal subarachnoid space. Physiol Behav. 1981;27(3):559–561. | ||
Gurr JR, Wang AS, Chen CH, Jan KY. Ultrafine titanium dioxide particles in the absence of phopactivation can induce oxidative DNA damage to human bronchial epithelial cells. Toxicol. 2005;213(1–2):66–73. | ||
Utley HG, Bernheim F, Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch Biochem Biophys. 1967;118(1):29–32. | ||
Wilms LC, Hollman PC, Boots AW, Kleinjans JC. Protection by quercetin and Quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutat Res. 2005;582(1–2):155–162. | ||
Lass A, Suessenbacher A, Wolkart G, Mayer B, Brunner F. Functional and analytical evidence for scavenging of oxygen radicals by L-arginine. Mol Pharmacol. 2002;61(5):1081–1088. | ||
Piacenza L, Peluffo G, Radi R. L-arginine-dependent suppression of apoptosis in Trypanosoma cruzi: contribution of the nitric oxide and polyamine path ways. Proc Natl Acad Sci U S A. 2001;98(13):7301–7306. | ||
Zhang XJ, Chinkes DL, Wu Z, Herndon DN. Enternal arginine supplementation stimulates DNA synthesis in skin donor wound. Clin Nutr. 2011;30(3):391–396. | ||
Borm PJ, Robbins D, Haubold S, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3:11. | ||
Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2011;60(11):1307–1315. | ||
Xiong D, Fang T, Yu L, Sima X, Zhu W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ. 2011;409(8):1444–1452. | ||
Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181–182:219–222. | ||
Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278(33):31426–31433. | ||
Sharma V, Shukla RK, Saxena N, Parmar D, Das M, Dhawan A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol Lett. 2009;185(3):211–218. | ||
Kobayashi N, Nakagawa A, Muramatsu T, et al. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J Invest Dermatol. 1998;110(5):806–810. | ||
Montagna W, Carlisle K. The architecture of black and white facial skin. J Am Acad Dermatol. 1991;24(6 Pt 1):929–937. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.