Back to Journals » International Journal of Women's Health » Volume 13
Effect of Low-Dose Aspirin in Preventing Early-Onset Preeclampsia in the Taiwanese Population—A Retrospective Cohort Study
Received 26 July 2021
Accepted for publication 8 October 2021
Published 12 November 2021 Volume 2021:13 Pages 1095—1101
DOI https://doi.org/10.2147/IJWH.S331213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Kim-Seng Law,1– 3 Tien-Yung Wei1
1Department of Obstetrics and Gynecology, Tung’s Taichung Metro Harbor Hospital, Taichung, Taiwan; 2Department of Nursing, Jenteh Junior College of Medicine, Nursing and Management, Miaoli, Taiwan; 3Department of Life Science, National Chung Hsing University, Taichung, Taiwan
Correspondence: Kim-Seng Law
Tung’s Taichung Metro Harbor Hospital, 699 Taiwan Boulevard, Section 8, Wuchi, Taichung City, 435, Taiwan
Tel +886 916120758
Fax +886 26582000
Email [email protected]
Purpose: To evaluate the effect of low-dose aspirin on preventing early-onset preeclampsia during first-trimester screening using maternal factors and biochemistry.
Patients and Methods: This study was a retrospective cohort study of pregnant women with singleton pregnancies at a gestational age of 11– 13+6 weeks from May 2017 to August 2019. Serum pregnancy-associated plasma protein A (PAPP-A)/placental growth factor (PLGF) and maternal demographic and clinical characteristics were collected and analyzed using a logistic regression model with a preset detection rate of 74% and a 10% false-positive rate. Low-dose aspirin was initiated for those screened positive for the prevention of early-onset preeclampsia.
Results: Of the 805 women who underwent preeclampsia screening, 78 were screened positive for early-onset preeclampsia. With a preset detection rate of 74% and a 10% false-positive rate, there were a total of 28 women with preeclampsia, of which 11 developed preterm preeclampsia (< 37 GA) and three had early-onset preeclampsia with 72% and 75% sensitivity and specificity of 93% and 91%, respectively, resulting in an estimated 95% risk reduction for early-onset preeclampsia. Early-onset preeclampsia had lower serum PLGF (0.29 multiple of the median [MoM], range 0.1– 0.67) compared with preterm preeclampsia (0.74 MoM, range 0.1– 2.26). PLGF remains the only predictor of early-onset preeclampsia, while mean arterial pressure and chronic hypertension are predictors of preterm preeclampsia using multivariate regression. No variables accurately predicted the development of early-onset preeclampsia with the initiation of low-dose aspirin before the gestational age of 16 weeks.
Conclusion: A remarkable reduction in early-onset preeclampsia was observed with early initiation of low-dose aspirin in those screened positive for maternal characteristics and serum PAPP-A/PLGF.
Keywords: low-dose aspirin, placental growth factor, preeclampsia, pregnancy-associated plasma, protein
Introduction
Preeclampsia, especially early onset, is a major contributor to maternal and perinatal morbidity and mortality, affecting 2% of pregnancies.1 Impaired placentation with reduced placental perfusion and oxidative stress triggers generalized endothelial dysfunction and an exaggerated endothelial inflammatory response, causing an imbalance between angiogenic and anti-angiogenic factors, leading to the clinical manifestation of preeclampsia.2–5 Extensive studies with the purpose of timely identification of early-onset preeclampsia by incorporating maternal demographic and clinical characteristics with serum biomarkers and/or biophysical profiles reported various detection rates.6–10
Early (gestational age <16 weeks) initiation with low-dose aspirin (<300 mg) effectively reduced early-onset preeclampsia by transforming the spiral arterioles during early placentation and reducing blood resistance.11–15 Contrary to most studies, a recent randomized controlled trial did not find a reduction in preeclampsia in a Chinese cohort,19 which might be attributed to a wider range of initiation of low-dose aspirin (12–20 gestational age). The other proven benefits of low-dose aspirin administered in early pregnancy are the prevention of intrauterine growth restriction and preterm birth, which has been a routine practice in most institutions.20
This study aimed to evaluate the effect of low-dose aspirin (100 mg daily until delivery) on early and preterm preeclampsia in a Taiwanese population screened positive for maternal demographic and clinical characteristics and serum pregnancy-associated plasma protein-A (PAPP-A)/placental growth factor (PLGF) during the first trimester in a single institution.
Patients and Methods
This retrospective cohort study analyzed the electronic data of pregnant women at our institution between May 2017 and August 2019. Women with singleton and gestational age of 11–13+6 weeks were screened based on serum PAPP-A/PLGF and maternal demographic and clinical characteristics (medical history and mean arterial pressure [MAP]) at their first antenatal visit. The screening was performed using an algorithm developed by the Fetal Medicine Foundation8 with a cut-off reading <1:200 as a high risk of developing early-onset preeclampsia and a preset detection rate of 74% with a 10% false-positive rate (Genetics Generation Advancement Corp. Taipei Taiwan).
Maternal demographics including ethnicity, parity, body mass index (BMI), smoking history, diabetes mellitus, chronic hypertension, previous history of preeclampsia were included in the analysis. Fetal gestational age was calculated according to the last menstrual period and/or determined by ultrasonography with fetal crown-rump length measured if the last menstruation could not be recalled or if the patient had a history of irregular menstruation.
Maternal serum biochemistry with PAPP-A and PLGF [converted to multiples of the median (MoM)] using an automated biochemical analyzer (BRAHMS KRYPTOR; Thermo Fisher Scientific, Hennigsdorf, Germany) was carried out by Genetic Generation Advancement Corp. Taipei Taiwan. MAP was measured in duplicates using a validated automated electronic BP device according to the standard guideline.16 Most of the low-dose aspirin (Bokey) of 100 mg was administered daily before week 16 in those screened positive until delivery. Mothers who experienced abortion or stillbirth due to congenital anomalies and those who did not show signs of preeclampsia at the same time were excluded.
Early-onset preeclampsia was defined as hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) plus proteinuria before 34 gestational age, preterm preeclampsia before 37, and term preeclampsia after 37 gestational age. The Institutional Review Board of Tung’s Taichung Metro Harbor Hospital (IRB #108031 and protocol number TTMHH-109C0005 was approved before the commencement of this study. An IRB with the number 108031 and the Trial number TTMHH-109C0005 was obtained from Tung’s Taichung MetroHarbour Hospital with a waiver of medical records consent from the patients due to confidentiality required by the boards of IRB board, and this was in compliance in accordance with the Declaration of Helsinki.
Maternal demographics data were presented using descriptive statistics. Continuous variables were represented as mean plus range and compared using the Student’s t-test. Categorical variables were expressed numerically as percentages and compared using the chi-square test or Fisher’s exact test, as appropriate. With the outcome of preterm preeclampsia as the responsive variable, a multivariate logistic regression model was built for each variable. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Statistical significance was set at P < 0.05. All statistical analyses were performed using R (R Core Team 2019), a language and environment for statistical computing developed by the R Foundation for Statistical Computing, Vienna, Austria. URL, https://www.R-project.org/).
Results
There were 823 women with singletons who underwent first-trimester preeclampsia screening during the study period; 18 were excluded because they did not meet the gestational age criterion. Among the remaining 805 patients, 78 were positive (with a cut-off <1:200), of which 28 had preeclampsia with 11 preterm preeclampsia and three early-onset preeclampsia. Among the 727 women who tested negative, 29 developed preeclampsia with seven preterm preeclampsia and one early-onset preeclampsia (Figure 1). The overall preeclampsia rate was 7%, with early-onset preeclampsia (0.5%), preterm preeclampsia (2.1%), and term preeclampsia (4.9%). The average delivery ages for early-onset and preterm preeclampsia were 30.32 and 33.8 weeks, respectively.
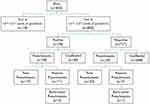 |
Figure 1 Flow chart. |
With a preset detection rate of 74% and a false-positive rate of 10% used in this model, the reduction of risk with the initiation of low-dose aspirin to prevent early-onset preeclampsia was 95%.
Demographic data for those who developed preterm preeclampsia and those not affected are shown in Table 1, with BMI 27.39 vs 23.71 kg/m2, PAPP-A/PLGF, 1.2 MoM vs 1.57 MoM, and 0.74 MoM vs 1 MoM, respectively. MAP was higher in those who developed preterm preeclampsia, and more women with a history of chronic hypertension subsequently developed preterm preeclampsia. Similar demographic and clinical characteristics were observed in those who developed early-onset preeclampsia and those who were not affected. Serum PAPP-A, PLGF, MAP, and chronic hypertension had a linear correlation with early-onset preeclampsia, except for BMI (Table 2). A lower serum PLGF was observed in those who later on developed early-onset preeclampsia compared to those who had preterm preeclampsia (0.29 MoM, range 0.1–0.67 vs 0.74 MoM, range, 0.1–2.26).
 |
Table 1 Demographic Characteristics |
 |
Table 2 Demographics of the Study Population |
Multivariate regression analysis showed that only serum PLGF was relevant for the development of early-onset preeclampsia (Table 3). Chronic hypertension and high MAP were the two predictors of preterm preeclampsia (Table 4). Table 5 reveals that those screened positive and prescribed low-dose aspirin before week 16 had no single variable predicting early-onset preeclampsia, while serum PLGF and MAP were correlated with the development of preterm preeclampsia (Table 6).
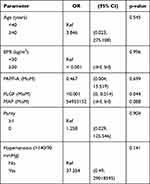 |
Table 3 Results of Multivariate Logistic Regression |
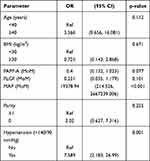 |
Table 4 Adjusted Odds Ratio Between Preterm Preeclampsia and Maternal Factors/Biochemistry |
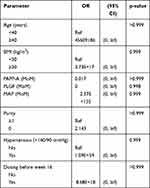 |
Table 5 Results of the Subgroup Analysis Subjects Tested Positive (n = 78) |
 |
Table 6 Correlation of Tested Positive (78) with the Development of Preterm Preeclampsia |
Table 7 shows the distribution of low-dose aspirin initiation between different gestational ages in those who were screened positive.
 |
Table 7 Distribution of Bokey Initiated in Different Gestational Weeks |
Discussion
Early initiation of low-dose aspirin reduces 95% of presumed early-onset preeclampsia in the present analysis by using maternal characteristics and biomarkers in first-trimester preeclampsia screening.
Although the preventive role of low-dose aspirin has been extensively studied in western countries,11–15 there have been no data reporting the risk reduction of low-dose aspirin in the Taiwanese population to date.
Predictive performance for early-onset and preterm preeclampsia by combining maternal demographic characteristics with serum biomarkers (PAPP-A/PLGF) was 75% and 72% with a specificity of 90.6% and 93%, respectively, similar to previous findings.7,8,10 Serum PLGF remained the only predictor of preeclampsia in the present multivariate analysis and was found to be much lower in those who later developed early-onset preeclampsia than in those who had preterm preeclampsia. The effect of low-dose aspirin administered before week 16 precludes a sensitive marker for predicting early-onset preeclampsia, which may be replaced with the ratio of soluble Fms-like tyrosine kinase-1 to PLGF in the second trimester, as used in current practice for a more accurate prediction of eminent preeclampsia.7,12,17
In the screening for preeclampsia that required emergent delivery before week 34, the detection rate, at a false positive rate of 10%, was approximately 50% using maternal characteristics alone. This was improved to approximately 90% with the addition of biophysical markers and approximately 75% with biochemical markers. The detection rate increased to more than 95% with a combination of maternal factors, biophysical markers, and biochemical markers.18
The mechanisms that underlie early- and late-onset preeclampsia were different, with low-dose aspirin exerting its effect by reversing abnormal vascularity formation in early gestation with no effect observed in those with abnormal vasculature in chronic hypertension,14–16 which was also demonstrated in this study. There were no single markers that could reliably predict which women would subsequently develop early-onset preeclampsia among those screened positive and who had received prophylactic low-dose aspirin compared to those who developed preterm preeclampsia with lower serum PLGF and higher MAP that were not deterred by the effect of early initiation of low-dose aspirin.
The mode of conception was another issue that should be addressed while screening for preeclampsia, as some authors21 have found higher odds of developing preeclampsia in those conceived by IVF; however, we are unable to distinguish those who conceived through IVF due to incomplete data registration.
The initiation of low-dose aspirin should be started at 16 weeks or earlier, as demonstrated by Bujold et al,20 with a significant reduction in preeclampsia (RR, 0.47; 95% CI, 0.34–0.65). Only three of the 78 patients who tested positive were administered with low-dose aspirin after 16 weeks in our study, with a trivial effect on our final analysis.
The limitation of our study is its retrospective nature and the inherent loss of data in some fields. Moreover, the uterine arterial pulsatility index was not incorporated into the prediction model, which could have a lower sensitivity than the current standard triple test. In addition, no external validation of the prediction model applied in this study has been carried out, which could raise the debate on its accuracy. The dosage of 100 mg aspirin administered in this study is another issue that should be addressed, as the American College of Obstetrics and Gynecology/USPSTF and Maternal-Fetal Medicine recommend a higher dosage of aspirin (150 mg) in current practice. However, this is the first study involving a cohort of Taiwanese women with detailed maternal demographic characteristics documented and consistently administered prophylactic low-dose aspirin with follow-up at the same institution. The effect of early initiation of low-dose aspirin in the study population has been very encouraging, which merits more in-depth investigation to verify the benefits in our population.
Conclusions
A remarkable reduction was observed in early-onset preeclampsia with early initiation of low-dose aspirin in those screened positive for maternal characteristics and serum PAPP-A/PLGF. The incorporation of uterine artery pulsatility index along with maternal biophysical and biochemistries should be explored in future studies to better suit our population.
Acknowledgments
The authors would like to thank Pei-Jhen Wu for performing the statistical analyses in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137. doi:10.1053/j.semperi.2009.02.010
2. Chaiworapongsa T, Chaemsaithonh P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10:466–480. doi:10.1038/nrneph.2014.102
3. Roberts JM, Taylor RN, Musci TJ, et al. Pre-eclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi:10.1016/0002-9378(89)90665-0
4. Redman CW, Sacks GP, Sargent IL. Pre-eclampsia: an excessive maternal inflammatory response to pregnancy. AM J Obstet Gynecol. 1999;180:499–506. doi:10.1016/S0002-9378(99)70239-5
5. Sibai B, Dekker G, Kupferminc M. Preeclampsia. Lancet. 2005;365:785–799. doi:10.1016/S0140-6736(05)17987-2
6. Wright D, Syngelaki A, Akolekar R, et al. Competing risks model in screening for preeclampsia by maternal characteristics and maternal history. Am J Obstet Gynecol. 2015;213:
7. Poon LC, Nicolaides KH. Early prediction of preeclampsia. Obstet Gynecol Int. 2014;2014:29739. doi:10.1155/2014/297397
8. Akolekar R, Syngelaki A, Poon L, et al. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33:8–15. doi:10.1159/000341264
9. Poon LC, Akolekar R, Beta J, et al. Hypertensive disorder in pregnancy: screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound Obstet Gynecol. 2010;35:662–670.
10. Tan MY, Syngelaki A, Poon LC, et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol. 2018;52:186–195. doi:10.1002/uog.19112
11. Roberge S, Nocolaides KH, Demers S, et al. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41:491–499. doi:10.1002/uog.12421
12. O’Gorman N, Wright D, Rolnik DL, et al. Study protocol for the randomized controlled trial: combined multiple markers screening and randomized patient treatment with aspirin for evidence-based PREeclampsia prevention (ASPRE). BMJ Open. 2016;6:e011801. doi:10.1136/bmjopen-2016-011801
13. Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622. doi:10.1056/NEJMoa1704559
14. ACOG Committee Opinion. No 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44–e52. doi:10.1097/AOG.0000000000002708
15. Seidler AL, Askie L, Ray JG. Optimal aspirin dosing for preeclampsia prevention. Am J Obstet Gynecol. 2018;219:117–118. doi:10.1016/j.ajog.2018.03.018
16. Li YY, Gu QR, Chen GR, et al. Preventing preterm preeclampsia. Am J Obstet Gynecol. 2018;219:633–634. doi:10.1016/j.ajog.2018.08.027
17. Zeisler H, Llurb E, Chantraine F, et al. Predictive value of the sFLt-1: PlGFratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. doi:10.1056/NEJMoa1414838
18. Chaemsaithong P, Sahota DS, Poon LC. First trimester preeclampsia screening and prediction. Am J Obstet Gynecol. 2020;
19. Lin L, Huai J, Li B, et al. A randomized controlled trial of low-dose aspirin for the prevention of pre-eclampsia in women at high-risk in China (the APPEC study). Am J Obstet Gynecol. 2021. doi:10.1016/j.ajog.2021.08.004
20. Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy. A meta-analysis. Obstet Gynecol. 2010;116(2):402–414. doi:10.1097/AOG.0b013e3181e9322a
21. Watanabe N, Fujiwara T, Suzuki T, et al. Is in vitro fertilization associated with preeclampsia? A propensity score matched study. BMC Pregnancy Childbirth. 2014;14:69. doi:10.1186/1471-2393-14-69
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
