Back to Journals » Journal of Experimental Pharmacology » Volume 12
Effect of Kaempferol on Tacrolimus-Induced Nephrotoxicity and Calcineurin B1 Expression Level in Animal Model
Authors Ali AS , Almalki AS , Alharthy BT
Received 28 June 2020
Accepted for publication 29 September 2020
Published 28 October 2020 Volume 2020:12 Pages 397—407
DOI https://doi.org/10.2147/JEP.S265359
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bal Lokeshwar
Ahmed Shaker Ali,1,2 Abdullah Saddah Almalki,1,3 Basma Tarek Alharthy1
1Department of Pharmacology, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 2Department of Pharmaceutics, Faculty of Pharmacy, Assiut University, Assiut, Egypt; 3Department of Pharmacy, Ajyad Hospital, Ministry of Health, Riyadh, Saudi Arabia
Correspondence: Abdullah Saddah Almalki Makkah 24268 – 9382, Kingdom of Saudi Arabia
Tel +966 126401000 - Ext 20151
Fax +966 126400855
Email [email protected]
Background: The kidneys are considered one of the most susceptible organs for adverse drug effects, particularly in post-transplant conditions. Tacrolimus (FK506), a calcineurin inhibitor immunosuppressant, is an essential component in the transplantation regimen. Despite that, nephrotoxicity is a severe drawback for its chronic utilization, where oxidative stress might be implicated. Kaempferol (KMF) is a natural flavonoid that has many adaptable biological activities, including antioxidant action.
Objective: Exploring the KMF protective effect on FK506-induced nephrotoxicity and the underlying role of calcineurin B1.
Methods: Twenty-four male albino-Wistar rats were randomly divided into three equal groups. The control group received solvents: propylene glycol, i.p. and 0.5% carboxymethyl cellulose, PO; FK506 group was injected with FK506 (0.6 mg/kg, i.p.), and FK506+KMF group was given FK506 (0.6 mg/kg, i.p.) and KMF (10 mg/kg, PO). The treatment regimen for all groups was once daily for 30 days. ELISA technique applied for measuring FK506 trough level and nephrotoxicity biomarkers in serum (cystatin C and urea) on days 15 and 30, and in kidney tissue homogenate (MDA and calcineurin B1) on day 30.
Results: In FK506-treated rats, the FK506 trough level was 7.84 ± 1.31 ug/l on day 15 and 9.54 ± 1.45 ug/l on day 30. FK506 use has significantly (P< 0.01) increased biomarkers levels of cystatin C (325% and 477%), urea (177% and 245%), MDA (1253%), except calcineurin B1 that has decreased (97%). The KMF combination has resulted in a significant reduction in the FK506 trough level by day 30 (6.79 ± 1.35 ug/l, P< 0.01). KMF has significantly ameliorated the levels of cystatin C (46% and 73%, P< 0.001), urea (38% and 68%, P< 0.001), MDA (75%, P< 0.001), and calcineurin B1 (1833%, P< 0.05).
Conclusion: Oxidative stress and calcineurin B1 are contributing factors in FK506-induced nephrotoxicity. Hence, inhibition of calcineurin enzyme is not limited to the immune cells. KMF could be a novel nephroprotective antioxidant.
Keywords: FK506, kaempferol, calcineurin B1, nephrotoxicity, biomarkers
Corrigendum for this paper has been published.
Introduction
Homeostasis of many physiological parameters depends, to a substantial degree, on vital functions of the renal system.1 Indeed, a lot of therapeutic and diagnostic substances can induce considerable nephrotoxic effects that could progress to acute kidney injury (AKI) and advanced state of chronic kidney disease (CKD), particularly renal fibrosis.2,3 Nephrotoxic drugs participate in 6% of the community and 20% of hospital-acquired AKI episodes.4 Among the elderly patients, drug-induced nephrotoxicity contributed to up to 66% of hospitals developed AKI.5
Tacrolimus, also known as FK506, is a lipophilic macrolide compound. It was isolated from Streptomyces tsukubaensis bacteria and classified as a calcineurin inhibitor (CNI) immunosuppressant.6 For its use as anti-rejection, the first official approval of FK506 was in Japan in 1993.7 Even though CNIs have contributed to transplantation advancement, but they can cause severe complications. The FK506-based therapeutic regimen has a variety of side effects, including acute and chronic nephrotoxicity. Acute toxicity usually results from afferent arteriolar vasoconstriction and rarely from thrombotic microangiopathy. In contrast, chronic toxicity occurs after long-term exposure to FK506 that eventually causes interstitial fibrosis, tubular atrophy, and allograft rejection.8
Optimization of FK506-based therapy through minimizing adverse effects and exploring the relevant pathophysiological factors are our primary goals in this study. Flavonoids are of leading botanical secondary metabolites and a common constituent in fruits, vegetables, and plant-derived foods. Due to their low toxicity and considerable health benefits in minimizing the risk of chronic diseases, Flavonoids received increased attention in pharmaceutical research and development.9
Kaempferol (KMF) is a natural flavonol metabolite, a class of flavonoids, named for German naturalist called Engelbert Kaempfer (Figure 1). It is a yellow crystalline substance with a molecular weight of 286.239 g/mol. KMF has a diphenylpropane structure (C15H10O6), responsible for its lipophilic property, and IUPAC name 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one.10 It has been identified in conventional and medicinal plants, fruits, and vegetables such as saffron, capers, chard, chives, collards, cress, chia seeds, cabbage, kale, radish, onions, arugula, broccoli, endive, mustard, spinach, and dill. In the regular human diet, the average intake of flavonols and flavones estimated 23 mg/day, of which KMF contributed approximately to 17%.11–13
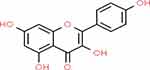 |
Figure 1 Chemical structure of KMF.14 |
According to preclinical and epidemiological studies, KMF has various health benefits and biological activities. It may prevent, reduce the risk, and treat various diseases through its actions as anti-inflammatory, antimicrobial, anticancer, neuroprotective, cardioprotective, anti-osteoporosis, anxiolytic, analgesic, antiallergic, and antidiabetic.15–17
For an in-depth comprehension of nephrotoxicity, calcineurin enzyme has been specified as a potential pathogenic factor. Calcineurin is a calcium/calmodulin-dependent heterodimer protein phosphatase consisting of a catalytic A subunit (α, β, γ isoforms) and a regulatory B subunit (B1 and B2 isoforms). Although predominantly found in neural tissues, calcineurin localized with different expression levels in many tissues and organs, including kidneys, and involved in many biological and signal transduction pathways. The calcineurin B1 isoform (CnB1) is associated with α and β isoforms of calcineurin A (CnA) and encoded by the PPP3R1 gene located on chromosome 2. The cytosolic complex of FK506/FKBP12 can bind to the calcineurin B subunit and resulting in noncompetitive inhibition of its phosphatase activity.18–20
Last but not least, this experimental study aimed at KMF selection to attenuate the nephrotoxic effect of FK506 and to explore the underlying role of CnB1 in nephrotoxicity.
Materials and Methods
Animals
This experiment has conducted on 24 male albino-Wistar rats weighing 180–230 g and aged eight weeks. The lab rats were brought from animal housing at King Fahd medical research center, Saudi Arabia. The animal welfare practices were following the National Committee of Bioethics (NCBE) and WSAVA guidelines. Throughout the experiment period, rats have ad libitum access to water and feed on a standard rodent chow diet. All animals were housed under ideal laboratory conditions (12 hours dark/light cycle at constant temperature 22–25°C, and 45–55% relative humidity). This study has conducted after getting the initial approval of the experiment protocol (Reference No. 349–18) from the local unit of biomedical ethics (Research Ethics Committee) in the Faculty of Medicine, King Abdulaziz University.
Chemicals
FK506 powder (HuiChem company, China; CAS No.104987–11-3); KMF powder (Natural Field company, China; CAS No.520–18-3); Carboxymethylcellulose (CMC) sodium powder (Sigma Aldrich, USA; CAS No. 9004–32-4); Propylene glycol (PG) (Sigma Aldrich, USA; CAS No.57–55-6); Distilled water.
Instruments
Tissue Homogenizer (Polytron PT10/35, Kinematica, Switzerland), Absorbance Microplate Reader (ELx808, BioTek, USA), Microplate Washer (ELx50, BioTek, USA), Lab Incubator (MIR-162, SANYO, Japan), Refrigerated Centrifuge (2–16KL, SIGMA, Germany), Digital Analytical Balance (PW254, Adam Equipment, UK), Lab Freezer (−80°C), Lab Refrigerator (2–8°C), Single/Multi-channel pipettes (Diamond Pipettors, Globe Scientific, USA), Laboratory consumables.
Drugs Preparation
FK506 and KMF powder have dissolved in PG and 0.5% CMC, respectively, as five days stock solution to ensure physiochemical stability. The preparations were kept in light protected bottles and stored between 2–8 C. According to rat weight, the daily doses of FK506 and KMF have been calculated to ensure dose accuracy.
Study Design
As in vivo animal model, this experiment carried out on 24 rats for 30 days. The rats have randomly divided into three equal groups (Figure 2). The control group (8 rats) received the solvents (0.1 mL of PG, i.p.) and (0.5 mL of 0.5% CMC, PO) once a day; the FK506 group (8 rats) was injected with the FK506 drug (0.6 mg/kg, i.p.) once a day; FK506 + KMF group (8 rats) was given both of the FK506 (0.6 mg/kg, i.p.) and KMF (10 mg/kg, PO) once a day. The animal equivalent dose of FK506 has been calculated based on post-transplants human prophylaxis dose (0.1 mg/kg/day). The concept of dose extrapolation between species was considered.21
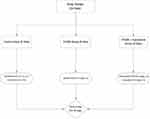 |
Figure 2 Design of nephrotoxicity study on rats. |
Preparation of Serum Samples
After anesthesia of rats by diethyl ether, blood sampling has drawn via the retro-orbital puncture (4 mL) on day 15 and from the abdominal aorta artery (10 mL) on day 30. The collected blood in serum separator tubes kept at room temperature for 15 minutes to allow for clotting. By centrifugation at 4000 × g for 10 minutes, the serum has separated and then stored at −80 °C until assay time.
Preparation of Renal Homogenate
From each rat, on day 30, a kidney was removed following euthanasia of the anesthetized rats by aortic exsanguination. About 0.1 g of the renal tissue immediately frozen in the dry ice (the solid form of CO2) and then kept at −80°C. After the addition of 1% cold PBS (10x mL/g), the tissue samples mechanically homogenized for five seconds and promptly kept in the iced water and then at −80°C. On the assay day, to separate the supernatant for analysis, the renal homogenates were transferred into the Eppendorf tubes (1.5 mL) and centrifuged at 4000 × g (at 4 °C) for 30 minutes.
Measurement of FK506 Trough Level
In rats serum, on days 15 and 30, the trough Level of FK506 was measured by competitive enzyme-linked immune-sorbent assay (ELISA) technique according to catalog No. MBS288390, MyBioSource, USA. The minimum detection level of the assay kit is 0.069 ng/mL, with an intra-assay coefficient of variation (≤ 4.9%). The optical density (OD) of samples have spectrophotometrically determined at a wavelength of 450 nm. Based on the standard curve, the FK506 concentration was calculated.
Measurement of Nephrotoxicity Biomarkers
Serum Cystatin C and Urea Levels
Serum levels of cystatin C and urea were determined on days 15 and 30 by sandwich ELISA technique and according to manual No. MBS763996 and MBS2600001, MyBioSource, USA. The minimum detection levels of assay kits are 18.75 pg/mL and 0.6 mmol/L, respectively, with an intra-assay coefficient of variation (< 8%). At a wavelength of 450 nm, the OD measured to calculate analytes concentration in the samples based on the standard curve.
Renal MDA and CnB1 Levels
In the renal homogenate, by the thirtieth day, MDA and CnB1 concentrations were determined by the competitive and sandwich ELISA technique according to manual No. MBS738685 and MBS450043, MyBioSource, USA. The OD of the samples was measured at a wavelength of 450 nm. The minimum detection levels of assay kits are 1.0 ng/mL and 0.104 ng/mL, respectively, with an intra-assay coefficient of variation less than 10%.
Total Protein Quantitation
For accurate data, total protein content in the rats’ kidney was quantified by colorimetric BCA assay according to catalog No. 23,227, ThermoFisher, USA. The sample absorbance was measured spectrophotometrically at a wavelength of 562 nm with an assay detection level down to 5 µg/mL (coefficient of variation 14.7%).
Statistical Analysis
It has done this through the use of statistical and graphing software SPSS v25.0 and GraphPad Prism v8.0.1. The results were presented as means ± standard error of the mean (SEM). A P-value of less than 0.05 has been considered statistically significant. The one-way ANOVA test, followed by appropriate post hoc tools (Tukey-HSD or Games-Howell), was used to identify statistically significant differences among groups.
Results
As shown in (Table 1), the mean values of FK506 trough level and nephrotoxicity biomarkers have been statistically analyzed and compared between groups to make inferences about FK506 and KMF effects as illustrated below:
 |
Table 1 Effect of FK506 and KMF on FK506 Trough Level and Nephrotoxicity Biomarkers |
Effect of KMF on FK506 Trough Level
The presented data in (Table 1) and (Figure 3) showed that KMF combined use with FK506 for 30 days has led to a statistically significant decrease in FK506 trough level (P<0.01; 29%) while by day 15 its effect was non-significant.
Effect of FK506 and KMF on Serum Cystatin C and Urea Levels
The sole use of the FK506 led to a significant (P<0.001) increase in cystatin C and urea levels by day 15 (177% and 325%) and day 30 (245% and 477%) in comparison to the control group (Table 1). Upon KMF combination with FK506, it resulted in a significant (P<0.001) decline in cystatin C and urea levels, whether day 15 (38% 46%) or day 30 (68% and 73%) compared to the FK506 group; however, cystatin C and urea levels remained higher than those in the control group (Figures 4 and 5).
Effect of FK506 and KMF on Renal MDA and CnB1 Levels
The data in (Table 1) and (Figures 6 and 7) showed that 30 days of FK506 injection has significantly increased MDA concentration (P<0.001, 1253%) accompanied by a decrease in the CnB1 level (P<0.01, 97%). The inclusion of KMF resulted in a significant decline in the MDA concentration (P<0.001, 75%) with a considerable improvement in the CnB1 levels (P<0.05, 1833%).
Discussion
FK506 is one of the outstanding immunosuppressants that was and still used to prevent allogeneic transplant rejection. Unfortunately, FK506-based regimens have a variety of significant limitations and side effects, including nephrotoxicity. As a narrow therapeutic window drug, pharmacokinetic variability can affect FK506 trough level, efficacy, and side effects. Whether in rat or human, the FK506 metabolism mainly occurs in the liver in addition to the gut and kidney. More than 90% of FK506 dosage metabolizes by the CYP3A subfamily (CYP3A4 and CYP3A5 in humans and CYP3A2 in rats).22,23 P-gp, a cellular efflux transporter, also plays a significant role in FK506 pharmacokinetic. Both CYP3A and Pgp activity significantly influenced by a complex interplay between the genetic polymorphisms and inhibitory or inductive effects of many drugs, herbs, food constituents, and endogenous substances that can affect FK506 blood level.24,25
Many drugs and phytochemicals are substrates for both CYP3A4 and P-gp. In the present study, to avoid any influence on bioavailability, the FK506 was injected intraperitoneal, while KMF was given orally despite having low oral bioavailability (13%) and short half-life (1.5 hours).26 We found that KMF combination with FK506 drug can significantly reduce its trough level by day 30 (Table 1) (Figure 3). According to this experiment and other studies, the increase in total body clearance due to KMF is the fundamental reason for the decline in the FK506 level. The prolonged exposure to KMF and its Quercetin metabolite can induce P-gp and CYP3A4 activity and therefore resulting in high drug clearance. In this context, an in vitro study conducted on human Caco-2 cells has shown that ten days utilization of KMF significantly increased the P-gp mRNA expression by twofold. In contrast, Quercetin metabolites increased the mRNA expression for both P-gp and CYP3A4 by a factor of 3.6 and 2.2, respectively.27 Another in vitro study on Caco-2 cells confirmed that three consecutive days of KMF and Quercetin treatment could induce a considerable increase in the mRNA expression for both P-gp and CYP3A4.28
The duration of body exposure to KMF appears to be critical for its interaction with drugs. According to a study on rats, a single oral dose of KMF (12 mg/kg) could significantly enhance some etoposide pharmacokinetic parameters after oral or intravenous administration.29 Inhibition of P-gp and CYP3A4 is more rapid and can produce an effect within a short time, especially if competitive inhibition, while induction of functional expression of such proteins is a slow process and may require a more extended period dependent on the rate of protein synthesis.30 Therefore, KMF can act as an inhibitor or inducer of P-gp and CYP3A4, depending on the length of exposure (Table 1) (Figure 3).
Drug-induced nephrotoxicity is one of the most common incidents in clinical practice that directly or indirectly result in renal injury. In this study, FK506-induced nephrotoxicity has been assessed by a variety of biomarkers in serum and renal homogenate (Table 1). Measurement of the FK506 level in blood is variable and may not always give a good indication for overall body exposure, especially early after transplantation. Local kidney exposure to FK506 could be more realistic and serious. It can induce reversible and irreversible adverse effects on many renal compartments, including glomerular sclerosis, tubular atrophy, arteriolar hyalinosis, and interstitial fibrosis. Therefore, the assessment of physiological effects with therapeutic drug monitoring may help in ensuring effective and safe FK506 use.31
Reducing nephrotoxicity incidence requires understanding the underlying pathogenic factors and mechanisms. It is a multifactorial process that begins with acute local inflammation, oxidative stress, renal vasoconstriction, and ends with a chronic fibrogenic response after prolonged FK506 use.32 Deleteriously, FK506 repeated injection affected renal biomarkers in serum and tissue homogenate (Table 1). The results of this study are consistent with the previous in that FK506-induced nephrotoxicity can be characterized by increased levels of cystatin C and urea (Figures 4 and 5).33,34 It is well recognized that cystatin C and urea concentrations in serum depend on the glomerular filtration rate (GFR), which could decline due to renal injury.35 In renal homogenate, the significant increase in the MDA level (Figure 6) is an index for oxidative stress, subsequent cytotoxicity, and renal injury. Oxidative stress is a common pathogenic pathway in CNI-induced renal injury and CKD patients.36,37 The high MDA level is potentially due to enhanced production of reactive oxygen species (ROS), including superoxide radicals, hydrogen peroxide, and hydroxyl radicals. Preclinical studies have confirmed that FK506 use can boost ROS generation that ultimately leads to cell apoptosis.38–41
On the other hand, renal CnB1 concentration has markedly reduced in FK506-treated rats (Figure 7). In a multitude of organs, including the kidneys, calcineurin enzyme expresses at high levels and performs critical processes in various cells and tissues, such as T cell activation, angiogenesis, cardiac hypertrophy, muscle, and neural development.19 Since inhibition of the calcineurin/NFAT pathway is not specific to the immune cells, CNIs can cause sweeping harmful changes involved in a diverse array of pathological conditions, including nephrotoxicity.42,43 Sirolimus (Rapamycin) is a structural analog of FK506 but has less nephrotoxicity. Although it binds to the same cytosolic FKBP-12, Sirolimus/FKBP-12 complex inhibits the mTOR pathway other than calcineurin/NFAT.44,45 The calcineurin subunits and isoforms might contribute to distinct aspects of calcineurin activity in the setting of immune function and adverse drug effects.46 For calcineurin being a heterodimer protein, the CnB subunit noncovalently attaches to the binding domain of the CnA subunit. In an in vivo study, the transgenic mice lacking α isoform of CnA (CnAα) reproduced histological features similar to CNI related nephrotoxicity, whereas loss of the β isoform did no.47 To our knowledge, the pathophysiological roles of renal CnB1 are still not extensively investigated. Most studies have measured the effect of FK506 and other CNIs on calcineurin activity but not on its expression level in the kidney and other organs. Accordingly, the relation between nephrotoxicity and low CnB1 expression level is rationalize by high accumulation of FK506 in the kidney and negative feedback regulation. This study may offer valuable insight into this novel pathway.
To protect the non-immune tissues from CNI toxicity, we have selected KMF as a natural antioxidant flavonoid.48 KMF can work as a cellular antioxidant combating FK506 generated ROS and oxidative stress.49,50 Combining KMF with FK506 drug has brought about a significant decline in the levels of cystatin C (Figure 4), urea (Figure 5), and MDA (Figure 6) that might be due to improving the glomerular filtration. Moreover, KMF has significantly improved renal CnB1 concentration (Figure 7). It reported that oxidants and oxidative processes could inactivate the calcineurin phosphatase enzyme, and antioxidants protect it.51,52
In contrast, in a study about natural CNI compounds, KMF and Quercetin have exhibited a noncompetitive inhibitory effect on calcineurin phosphatase activity in purified enzymes and Jurkat T-cells models. The CnA subunit might be mediating the mechanism of inhibition that enhancing in the presence of calmodulin and calcineurin B subunit. Furthermore, KMF and Quercetin have shown inhibitory activity against IL-2 gene expression.53,54 A meta-analysis of independent gene expression datasets with computational drug repurposing analysis has predicted that KMF is among the top-ranked novel compounds with anti-fibrotic effects against interstitial fibrosis and tubular atrophy.55 Accordingly, KMF could be a CNI with nephroprotective activities when combined with FK506 drug and nephrotoxic CNIs.
Conclusion
FK506 inhibition of calcineurin enzyme is not specific to the immune cells and includes the kidneys. The low renal concentration of CnB1 following FK506 use could justify the pathogenesis of nephrotoxicity. Prolonged KMF exposure has reduced the FK506 trough level in serum. Independently, the KMF combination has lessened the levels of urea, cystatin C, MDA, with a considerable increase in the CnB1. It can work as cellular antioxidant combating FK506-generated oxidative stress. Consequently, KMF could be a novel nephroprotective antioxidant when combined with FK506 and similar CNIs. Further studies are needed to explore the KMF impact on pharmacokinetic parameters and immunosuppressive activity.
Acknowledgments
The authors acknowledge the Deanship of Scientific Research, King Abdulaziz University, Saudi Arabia, for funding this project (Grant number 20476/1439 H). We gratefully acknowledge Dr. Huda Mohammed Alkreathy for her significant assistance throughout our study and manuscript writing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jameson J, Loscalzo J. Harrison’s Nephrology and Acid-Base Disorders, 2e. McGraw-Hill Education; 2013.
2. Naughton CA. Drug-induced nephrotoxicity. Am Fam Physician. 2008;78(6):743–750.
3. Choudhury D, Ahmed Z. Drug-associated renal dysfunction and injury. Nat Rev Nephrol. 2006;2(2):80.
4. Schissler MM, Zaidi S, Kumar H, Deo D, Brier ME, McLeish KR. Characteristics and outcomes in community-acquired versus hospital-acquired acute kidney injury. Nephrology. 2013;18(3):183–187. doi:10.1111/nep.12036
5. Khan S, Loi V, Rosner MH. Drug-induced kidney injury in the elderly. Drugs Aging. 2017;34(10):729–741. doi:10.1007/s40266-017-0484-4
6. Kino T, Hatanaka H, Hashimoto M, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot (Tokyo). 1987;40(9):1249–1255. doi:10.7164/antibiotics.40.1249
7. Fung JJ. Tacrolimus and transplantation: a decade in review. Transplantation. 2004;77(9 Suppl):S41–S43. doi:10.1097/01.TP.0000126926.61434.A5
8. Nankivell BJ, P’Ng CH, O’Connell PJ, Chapman JR. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology: comparison of cyclosporine and tacrolimus eras. Transplantation. 2016;100(8):1723–1731. doi:10.1097/TP.0000000000001243
9. Liu YJ, Zhan J, Liu XL, Wang Y, Ji J, He QQ. Dietary flavonoids intake and risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Clin Nutr. 2014;33(1):59–63. doi:10.1016/j.clnu.2013.03.011
10. Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–1246. doi:10.3390/nu2121231
11. Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4(3):384S–392S. doi:10.3945/an.112.003517
12. Yang R-Y, Lin S, Kuo G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac J Clin Nutr. 2008;17(S1):275–279.
13. Haytowitz DB, Wu X, Bhagwat S USDA database for the flavonoid content of selected foods, release 3.3. 2018.
14. Kaempferol:Chemical-Structure.4444395. Royal society of chemistry. 2020. Available from: http://www.chemspider.com/Chemical-Structure.4444395.html.
15. Calderon-Montano JM, Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11(4):298–344. doi:10.2174/138955711795305335
16. Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138(4):2099–2107. doi:10.1016/j.foodchem.2012.11.139
17. Devi KP, Malar DS, Nabavi SF, et al. Kaempferol and inflammation: from chemistry to medicine. Pharmacol Res. 2015;99:1–10. doi:10.1016/j.phrs.2015.05.002
18. Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80(4):1483–1521. doi:10.1152/physrev.2000.80.4.1483
19. Musson RE, Smit NP. Regulatory mechanisms of calcineurin phosphatase activity. Curr Med Chem. 2011;18(2):301–315. doi:10.2174/092986711794088407
20. Klee CB, Yang S-A. Chapter 90 - calcineurin. In: Bradshaw RA, Dennis EA, editors. Handbook of Cell Signaling (Second Edition). San Diego: Academic Press; 2010:705–710.
21. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27. doi:10.4103/0976-0105.177703
22. Iwasaki K. Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics. Drug Metab Pharmacokinet. 2007;22(5):328–335. doi:10.2133/dmpk.22.328
23. Shiraga T, Matsuda H, Nagase K, et al. Metabolism of FK506, a potent immunosuppressive agent, by cytochrome P450 3A enzymes in rat, dog and human liver microsomes. Biochem Pharmacol. 1994;47(4):727–735. doi:10.1016/0006-2952(94)90136-8
24. Schutte-Nutgen K, Tholking G, Suwelack B, Reuter S. Tacrolimus - pharmacokinetic considerations for clinicians. Curr Drug Metab. 2018;19(4):342–350. doi:10.2174/1389200219666180101104159
25. Vanhove T, Annaert P, Kuypers DRJ. Clinical determinants of calcineurin inhibitor disposition: a mechanistic review. Drug Metab Rev. 2016;48(1):88–112. doi:10.3109/03602532.2016.1151037
26. Qian YS, Ramamurthy S, Candasamy M, Shadab M, Kumar RH, Meka VS. Production, characterization and evaluation of kaempferol nanosuspension for improving oral bioavailability. Curr Pharm Biotechnol. 2016;17(6):549–555. doi:10.2174/1389201017666160127110609
27. Patel J, Buddha B, Dey S, Pal D, Mitra AK. In vitro interaction of the HIV protease inhibitor ritonavir with herbal constituents: changes in P-gp and CYP3A4 activity. Am J Ther. 2004;11(4):262–277. doi:10.1097/01.mjt.0000101827.94820.22
28. Chae YJ, Cho KH, Yoon IS, et al. Vitamin D receptor-mediated upregulation of CYP3A4 and MDR1 by quercetin in caco-2 cells. Planta Med. 2016;82(1–2):121–130.
29. Li C, Li X, Choi J-S. Enhanced bioavailability of etoposide after oral or intravenous administration of etoposide with kaempferol in rats. Arch Pharm Res. 2009;32(1):133–138. doi:10.1007/s12272-009-1127-z
30. Pal D, Mitra AK. MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci. 2006;78(18):2131–2145. doi:10.1016/j.lfs.2005.12.010
31. Sikma MA, van Maarseveen EM, van de Graaf EA, et al. Pharmacokinetics and toxicity of tacrolimus early after heart and lung transplantation. Am J Transplant. 2015;15(9):2301–2313.
32. Vandenbussche C, Van der Hauwaert C, Dewaeles E, et al. Tacrolimus-induced nephrotoxicity in mice is associated with microRNA deregulation. Arch Toxicol. 2018;92(4):1539–1550.
33. Al-Harbi NO, Imam F, Al-Harbi MM, et al. Treatment with aliskiren ameliorates tacrolimus-induced nephrotoxicity in rats. J Renin-Angio Aldo Syst. 2015;16(4):1329–1336. doi:10.1177/1470320314530178
34. Randhawa PS, Starzl TE, Demetris AJ. Tacrolimus (FK506)-associated renal pathology. Adv Anat Pathol. 1997;4(4):265–276. doi:10.1097/00125480-199707000-00032
35. Naesens M, Kuypers DRJ, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481. doi:10.2215/CJN.04800908
36. Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, antioxidant therapies and chronic kidney disease. Nephrology. 2012;17(4):311–321. doi:10.1111/j.1440-1797.2012.01572.x
37. Wu Q, Wang X, Nepovimova E, Wang Y, Yang H, Kuca K. Mechanism of cyclosporine A nephrotoxicity: oxidative stress, autophagy, and signalings. Food Chem Toxicol. 2018;118:889–907. doi:10.1016/j.fct.2018.06.054
38. Jeon SH, Park HM, Kim SJ, et al. Taurine reduces FK506-induced generation of ROS and activation of JNK and bax in madin darby canine kidney cells. Hum Exp Toxicol. 2010;29(8):627–633. doi:10.1177/0960327109359019
39. Al-Harbi NO, Imam F, Al-Harbi MM, et al. Olmesartan attenuates tacrolimus-induced biochemical and ultrastructural changes in rat kidney tissue. Biomed Res Int. 2014;2014:1–7. doi:10.1155/2014/607246
40. Zhou X, Yang G, Davis CA, et al. Hydrogen peroxide mediates FK506-induced cytotoxicity in renal cells. Kidney Int. 2004;65(1):139–147. doi:10.1111/j.1523-1755.2004.00380.x
41. Han SY, Mun KC, Choi HJ, et al. Effects of cyclosporine and tacrolimus on the oxidative stress in cultured mesangial cells. Transplant Proc. 2006;38(7):2240–2241. doi:10.1016/j.transproceed.2006.06.078
42. Yu DY, Luo J, Bu F, Zhang W, Wei Q. Effects of cyclosporin A, FK506 and rapamycin on calcineurin phosphatase activity in mouse brain. IUBMB Life. 2006;58(7):429–433. doi:10.1080/15216540600791555
43. Campagne O, Mager DE, Brazeau D, Venuto RC, Tornatore KM. The impact of tacrolimus exposure on extrarenal adverse effects in adult renal transplant recipients. Br J Clin Pharmacol. 2019;85(3):516–529. doi:10.1111/bcp.13811
44. Tumlin JA. Expression and function of calcineurin in the mammalian nephron: physiological roles, receptor signaling, and ion transport. Am J Kidney Dis. 1997;30(6):884–895. doi:10.1016/S0272-6386(97)90100-1
45. DiJoseph JF, Sharma RN, Chang JY. The effect of rapamycin on kidney function in the sprague-dawley rat. Transplantation. 1992;53(3):507–513. doi:10.1097/00007890-199203000-00002
46. Musson REA, Cobbaert CM, Smit NPM. Molecular diagnostics of calcineurin-related pathologies. Clin Chem. 2012;58(3):511–522. doi:10.1373/clinchem.2011.167296
47. Gooch JL, Roberts BR, Cobbs SL, Tumlin JA. Loss of the alpha-isoform of calcineurin is sufficient to induce nephrotoxicity and altered expression of transforming growth factor-beta. Transplantation. 2007;83(4):439–447. doi:10.1097/01.tp.0000251423.78124.51
48. Damiano S, Ciarcia R, Montagnaro S, et al. Prevention of nephrotoxicity induced by cyclosporine-A: role of antioxidants. J Cell Biochem. 2015;116(3):364–369.
49. Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18(9):567–579. doi:10.1016/j.jnutbio.2006.10.007
50. Dennis JM, Witting PK. Protective role for antioxidants in acute kidney disease. Nutrients. 2017;9:7.
51. Zhou X, Mester C, Stemmer PM, Reid GE. Oxidation-induced conformational changes in calcineurin determined by covalent labeling and tandem mass spectrometry. Biochemistry. 2014;53(43):6754–6765. doi:10.1021/bi5009744
52. Sommer D, Fakata KL, Swanson SA, Stemmer PM. Modulation of the phosphatase activity of calcineurin by oxidants and antioxidants in vitro. Eur J Biochem. 2000;267(8):2312–2322. doi:10.1046/j.1432-1327.2000.01240.x
53. Wang H, Zhou CL, Lei H, Zhang SD, Zheng J, Wei Q. Kaempferol: a new immunosuppressant of calcineurin. IUBMB Life. 2008;60(8):549–554. doi:10.1002/iub.94
54. Wang H, Zhou CL, Lei H, Wei Q. Inhibition of calcineurin by quercetin in vitro and in jurkat cells. J Biochem. 2010;147(2):185–190. doi:10.1093/jb/mvp163
55. Li L, Greene I, Readhead B, et al. Novel therapeutics identification for fibrosis in renal allograft using integrative informatics approach. Sci Rep. 2017;7(1):39487. doi:10.1038/srep39487
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





