Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Effect of Isoniazid Prophylaxis Therapy on the Prevention of Tuberculosis Incidence and Associated Factors Among HIV Infected Individuals in Northwest Ethiopia: Retrospective Cohort Study
Authors Beshaw MA , Balcha SA , Lakew AM
Received 10 January 2021
Accepted for publication 19 May 2021
Published 8 June 2021 Volume 2021:13 Pages 617—629
DOI https://doi.org/10.2147/HIV.S301355
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Mulat Addis Beshaw,1 Shitaye Alemu Balcha,1 Ayenew Molla Lakew2
1Department of Internal Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Mulat Addis Beshaw P.O. Box = 196 Tel +251918019749
Email [email protected]
Background: Treating latent tuberculosis (TB) infection with Isoniazid (INH) among human immune virus (HIV) infected patients reduces active TB occurrence and death by 62% and 26%, respectively. Even though other studies show aforementioned evidence, TB incidence and its associated factors among HIV-infected individuals who were on INH and never on INH is not well studied in northwest Ethiopia. Therefore, this study tried to assess the effect of INH prophylaxis in TB prevention and associated factors among HIV-infected individuals.
Methods: Data were extracted from charts of HIV-infected clients who completed INH (193) and were never on INH (198) after a simple random sampling selection was done among newly diagnosed patients on follow-up from 2008 to 2015. After data were collected, it was entered into Epi info version 7 and exported to SPSS version 22 for analysis. Cox regression model was fitted and the hazard ratio was reported.
Results: In this study, the overall TB incidence rate among HIV patients was 3.5/100 person-years (PY) [95% CI: 2.55, 4.82]. But it was 7.1/100 PY among patients who were never on INH and 0.35/100 PY among patients who completed INH. INH completed [adjusted hazard ratio (AHR) = 0.08, 95% CI: 0.02– 0.37], on anti-retroviral therapy (ART) [AHR = 0.02, 95% CI: 0.01– 0.04], baseline World Health Organization (WHO) stage I & II [AHR =0.22, 95% CI: 0.08– 0.62] and baseline CD4 ≤ 350 [AHR=3.76, 95% CI: 1.39– 10.18] were significantly associated with TB incidence.
Conclusion: Putting patients on INH for 6 months and ART were protective factors against TB. Therefore, health institutions are recommended to provide INH after ruling out active TB and contraindications for HIV-infected individuals.
Keywords: HIV, TB, incidence, isoniazid, prophylaxis, factors
Introduction
People living with HIV develop a variety of different opportunistic infections (OIs) in their life span. Tuberculosis is the most common life-threatening and leading cause of death accounting for approximately 25% of all causes of death among HIV patients.1,2
In 2018, among all TB cases, about 8.6% were HIV infected; TB/HIV co-infection was highest in African countries; more than 50% in the southern part of Africa.3 In Ethiopia, in 2014/15, among 96% of newly enrolled Pre-ART patients screened for TB; 9.2% were identified with a TB co-infection.4 Moreover, the prevalence of Pulmonary TB among Pre-ART patients was 7.5% according to a study done in Gondar University Hospital.5
The risk of developing TB is 20–37 times more among HIV infected than non-HIV infected individuals.6 The annual and the lifetime risk of developing active TB in HIV infected and uninfected individuals is 10% respectively.7
The WHO as well as the Federal Ministry of Health of Ethiopia formulated and recommend using the three TB/HIV integrated activities to decrease TB/HIV co-infection. These activities are integrating and use of TB/HIV services delivery, use of “three I’s (Intensive TB case finding, Isoniazid preventive therapy (IPT) and Infection prevention at health Institutions)” and minimize the incidence of HIV among TB patients.8
INH can prevent both reactivation of latent TB infection to active TB and reinfection with mycobacterium TB bacilli.9,10 A retrospective study in public health facilities in Addis Ababa identified that providing INH prophylaxis to HIV infected individuals prevented the occurrence of TB by 96% compared to those who did not take it.11
Although the WHO and the Joint United Nation Program recommend the use of INH prophylaxis at least for 6 months to prevent new TB incidence, the coverage remained very low according to global TB reports in Ethiopia. The IPT coverage was 5.4% among the overall enrolled patients in 2011, and it was more than two-fifths (45%) in 2018 among newly enrolled patients. This low coverage is attributable to weak active TB screening, low INH access, fear of INH resistance, and toxicities.12–15
Before providing INH prophylaxis, every HIV-infected individual should be screened for active TB and contraindications should be excluded. This is because it is not advisable to give INH to HIV-infected individuals if patients have one of the symptoms (“Cough, fever, night sweats, weight loss symptoms”) unless TB is ruled out.8,16 Researches had shown that using INH prophylaxis for HIV infected individuals did not increase the risk of developing drug resistance TB.17
One of the drawbacks of providing INH is its side effects. In a study done in Gondar town, side effects that led to poor INH adherence were “abdominal pain, vomiting, skin rash, jaundice and numbness”.18
According to TB/HIV co-infection related studies, the most common risk factors that contributed to active TB incidence were INH prophylaxis, WHO clinical staging, CD4 count, Body Mass Index (BMI), functional status, hemoglobin level, cotrimoxazole prophylaxis.11,19–25
This study was aimed to assess the effect of INH prophylaxis in the prevention of TB incidence and its associated factors among HIV infected individuals. Since it was not well studied and INH implementation varies across different health institutions in northwest Ethiopia, this study will fill knowledge, practice/implementation, and research gaps in the area. Information obtained from this research will help policy makers, health managers, health care workers and other stakeholders. In addition, it will provide additional sources of information for interested researchers to further investigate TB/HIV co-infection and the effectiveness of INH prophylaxis against TB prevention among HIV infected patients.
Methods
Study Area and Design
A retrospective cohort study was conducted in Gondar and Azezo health centers in Gondar town. Gondar is located northwest of Ethiopia. It is 180 km from the capital city of the Amhara region, Bahir Dar and also 748 km from Addis Ababa the capital city of Ethiopia.
According to the Ethiopian population projection census of 2015, the total population in Gondar administrative town was 323,900.26
In Gondar town, there are eight health centers, one referral hospital and 14 health posts.27
The town has also one private general hospital, 14 specialized clinics, 18 medium clinics, 9 first level clinics, 1 basic laboratory, 1 international laboratory and 23 pharmacies.27
Each health center is expected to serve 40,958 of the population. TB screening and treatment is given in all 8 health centers, in the referral hospital and in 12 private clinics. TB/HIV services are given in 20 health institutions. TB and HIV/AIDS services including screening and treatment of opportunistic infection, prevention of mother to child transmission (PMTCT), provision of INH, Cotrimoxazole, ART, anti-TB drugs, care and support for HIV-infected individuals are the major activities for both the health centers. Gondar and Azezo health centers provide health care services for more than 2000 and 754 HIV infected individuals respectively.27
Study Participants
HIV infected adults (≥18 years) who completed INH (took INH for 6 months) and who never took the prophylaxis from September 11, 2008 to March 8, 2015 in both Gondar and Azezo Health Centers were included in the study. These individuals were either on pre-ART or ART. All HIV infected adults who took INH prophylaxis partially, charts with incomplete data and individuals on anti-TB treatment at the beginning of the study were excluded.
Sample Size Estimation and Sampling Procedures
The minimum sample size, 391, was estimated for TB incidence using single population proportion formula (considering HIV infected patients as a single population group as compared to the non-HIV infected population) based on the study in Arba Minch using the following assumption: level of significance (α) = 5%, confidence level = 95%, a margin of error = 4%, incidence proportion of TB = 18%, and 10% attrition rate. Sample size calculation for factors was estimated using Epi info version 7 by taking three factors, INH status, sex, and educational status, with the effect size of 0.313, 0.299, and 0.425 respectively with power = 80%. The sample sizes using the three factors taken from the Arbaminch study22 were (INH = 147, sex = 151, and education = 289). The largest sample size obtained using the single population proportion formula (391) was taken as the sample size of this study. Among the 8 health centers in the town, the two health centers were selected since they were the only health centers that started HIV/AIDS care and treatment services between September 11, 2008, and March 8, 2015. In the beginning, they were checked for the presence of an adequate sample. Then, patients were stratified into two categories based on their INH status; INH completed and never on INH then a sample of patients were selected using simple random sampling (lottery method) from newly and previously enrolled patients from each stratum resulting in 193 INH completed and 198 never on INH patients.
After identifying patients who completed INH starting from September 11, 2008, to March 8, 2015, each individual was followed till either active TB diagnosis (bacteriological or clinical decision) or is censored.
Never on INH individuals were either newly (diagnosed between September 11, 2008 and March 8, 2015) or previously diagnosed (diagnosed before September 11, 2008) HIV infected patients for whom the date of entry was the date of entry into HIV care then followed till either TB diagnosis or censored.
Operational Definitions
Tuberculosis: Is diagnosed using bacteriological confirmation (smear microscopy) or clinical decision.
Clinical TB diagnosis: Is diagnosed when it did not fulfill bacteriological diagnosis (positive smear microscopy) ie diagnosed by clinicians based on either chest x-ray abnormality, histology suggestion (FNAC, biopsy) and failed to improve from repeated broad spectrum antibiotics with clinical suggestive of TB (not using anti-TB).
TB incidence: Occurrence of new TB cases during follow up time period
Separated: Couples who are officially married and living at different places.
INH prophylaxis completed: either Pre-ART or ART individuals taking INH prophylaxis for 6 months to sterilize latent TB infection.
Adherence: Good: ≥95% or ≤3 doses missed per month.
Fair: (85–94)% or 4–8 doses missed per month.
Poor: <85% or ≥9 doses missed per month.8
Functional status: Working: Able to perform usual work in and out of the house.
Ambulatory: Able to perform activities for daily living.
Bedridden: Unable to perform activities for daily living.8
Data Collection Instruments and Procedures
A well structured English version checklist was prepared containing Sociodemographic, clinical, immunological, therapeutic and behavioral factors and it was used to collect data in the charts from June 18 to June 30, 2018 in both ART clinics. The checklist was developed by reviewing related literatures. Charts were extracted by data clerks and the data were collected by the principal investigator.
Data Quality Measures
Data qualities were ensured using a well structured checklist. After selecting chart extractors (data clerks) short training was given on how to select charts randomly for the two groups.
A preliminary review (5%) was done in University of Gondar Referral Hospital to check whether the checklist included adequate information or not.
Data Management and Analysis
After the data were collected, the investigators checked their completeness manually. Then they were entered into Epi info version 7 and exported to SPSS version 22 for analysis. Before the actual data analysis, data were checked for errors, outlying observations, inconsistencies, and missing observations.
Variables with continuous data were explored for their normality via histogram and Shapiro–Wilk statistics and none of the variables were normally distributed. Descriptive statistics were used to report the frequency, prevalence, median and interquartile range of the variables.
The life table was used to estimate the risk of TB occurrence among INH completed and never on INH individuals at different time intervals, and the Kaplan Meier estimate was used to compare the survival experience of the two groups. The Cox regression model was used to identify the independent variables that were associated with the time to TB incidence. Bivariable Cox regression analysis was done first for each variable then variables with P-value ≤0.2 in the bivariable were taken into the multivariable analysis to control the effect of confounders. Variables in the multivariable analysis were considered as significant at P<0.05 and adjusted hazard ratios with 95% confidence level were reported to show the strength of the association.
The Schonfeld and Cox-Snell residual tests were used to check proportional hazard assumptions and goodness of fit of the model; the assumptions were satisfied.
Results
Baseline Sociodemographic Characteristics
About 450 charts were extracted from both health centers (Figure 1). Of the 391 HIV infected individuals included in the study, 193 completed INH and 198 never took INH.
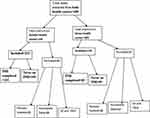 |
Figure 1 Sample size and sampling procedure (included and excluded participants of both health centers) in northwest Ethiopia from September 11, 2008 to March, 2015. |
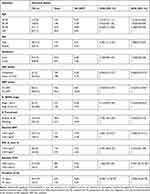
The median age of the study participants was 32 [Inter quartile range (IQR): 27–40] years. The majority, 276 (70.6%) of study participants were females; and 337 (86.1%) were urban dwellers. Ninety-seven (24.8%) study participants were housewives, 89 (22.8%) were daily laborers and 39.9% were illiterates (Table 1).
 |
Table 1 Baseline Socio Demographic Characteristics with INH Status of Study Participants in Northwest Ethiopia from September 11, 2008 to March, 2015, N = 391 |
Baseline Clinical Characteristics of Study Participants
The vast majority, 336 (85.9%) of the study participants were on ART and 158 (40.4%) were stage III patients. The median follow-up time of study participants was 3.001 years (IQR: 2.998–3.001) with minimum and maximum value of 0.01 and 3.001 years respectively while the median follow up time for INH completed individuals and those who did not take is similar (3.001). The median time of study participants since HIV diagnosis was 3.6 (IQR: 3.001–6.489) years. Of all study participants, 312 (79.8%) had working functional status. The median baseline CD4 count was 212 (IQR: 117–359) cells/μL. Among INH completed individuals 89.1% were on ART and 86.5% were on cotrimoxazole preventive therapy (CPT) (Table 2).
 |
Table 2 Baseline Clinical and Immunological Characteristics with INH Status of Study Participants in Northwest Ethiopia from September 11, 2008 to March, 2015, N = 391 |
Incidence of TB Among HIV Infected Individuals
The overall incidence rate of TB among 391 study participants was 38 (3.5/100 PY) [95% CI: 2.55, 4.82] whereas, it was 0.35/100 PY (INH completed) and 7.1/100 PY (never on INH) individuals. Most TB incidences occurred within the first year of follow-up time, in particular within the first 6 months.
According to results from Kaplan Meier and log rank tests, there was a significant difference in acquiring TB during the follow-up period between INH completed and never on INH groups (P-value <0.001). The estimated average TB free time among INH completed individuals was 2.99 years and 2.56 years among never on INH individuals (Figure 2).
 |
Figure 2 Survival function curve both for INH completed and never on INH groups in northwest Ethiopia from September 11, 2008 to March, 2015. |
TB Incidence and Factors Among HIV Infected Individuals
Sociodemographic and Behavioral Factors
TB incidence rate increased as the age of the study participants increased. TB was 29 (7.4%) among urban dwellers while it was 9 (2.3%) among rural dwellers. However, the incidence in rural areas among individuals who never took INH was 9 (25.7%) as opposed to 27 (16.6%) urban dwellers (Table 3). TB incidence rate among farmers was 8.47/100 PY [95% CI: 4.24, 16.94] and smokers had a nearly similar TB incidence rate (3.56/100 PY) as compared to nonsmokers (3.50/100 PY). Alcohol drinkers had a TB incident rate of 2.49/100 PY while non-drinkers had 3.97/100 PY.
Clinical and Immunological (Laboratory) Factors for Active TB Incidence
Out of the total study participants, 15 (19%) individuals with ambulatory and bedridden functional status had TB. However, it was 23 (7.4%) in those with working functional status. Twenty eight (82.4%) of Pre-ART participants who did not take INH developed TB (Table 4).
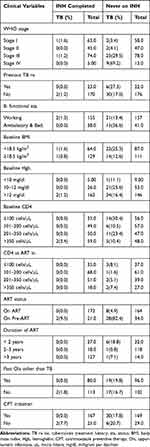 |
Table 4 Clinical and Immunological Factors with INH Status and Cumulative TB Incidence in HIV Infected Individuals in Northwest Ethiopia, September 11, 2008 to March, 2015, N = 391 |
TB incidence rate among individuals who never took INH was higher 7.1/100 PY [95% CI: 5.12, 9.84] as compared to the INH completed, 0.35/100 PY. Individuals who started INH before ART had higher TB incidence rate 1.03/100 PY [95% CI: 0.26, 4.14] than those who started after ART with IR of 0/100 PY. TB incidence rate among individuals who were treated for TB previously was 4.94/100 PY and those individuals who were not treated for TB was 3.32/100 PY. As duration of HIV diagnosis increased, TB incidence decreased. TB incidence rate was higher 0.86/100 PY among individuals with good ART adherence and TB case was zero with fair and poor ART adherence. Interestingly, the incidence rate among individuals with no INH or ART was 86.96/100 PY (Table 5 and Figure 3).
 |
Figure 3 TB Incidence rate with corresponding INH and or ART intervention status in northwest Ethiopia from September 11, 2008 to March, 2015. |
Cox Regression Survival Analysis
In bivariable analysis, sex, INH status, ART status, baseline WHO stage, baseline BMI, baseline functional status and duration of HIV diagnosis were significantly associated with TB incidence.
In multivariable analysis, INH, ART status, WHO stage and baseline CD4 were significantly associated with TB incidence. Individuals who completed INH were protected from TB as compared to individuals who were never on INH [AHR = 0.08, 95% CI: 0.02, 0.37]. On ART individuals were at lower risk of developing TB as compared to pre-ART individuals [AHR = 0.02, 95% CI: 0.01, 0.04]. Stage I and II individuals were at lower risk of developing TB than stage III and IV individuals [AHR = 0.22, 95% CI: 0.08, 0.62]. The hazard of developing TB among patients with baseline CD4 count ≤ 350 was significantly higher than those with CD4 > 350 [AHR = 3.76, 95% CI: 1.39, 10.18] (Table 5).
Discussion
In this study, we determined the incidence of tuberculosis and associated factors among HIV patients who completed INH therapy and those who never took INH. The overall incidence was 3.5/100 PY; factors independently associated with it were INH, ART status, WHO stage, and baseline CD4.
Overall TB incidence was relatively lower as compared to findings in other studies.
The TB incidence rate increased as the age of the study participants increased which was in line with the studies done in SNNPR, Cambodia and Iran in which the incidence rate of TB increased as age increased.23,28,29 This would be due to the decline in immune status as age increased, age related comorbidities and poor standard of living conditions.
Individuals who lived in rural areas (6.6 PY) had two times the TB incidence rate of urban dwellers (3.03 PY). This finding was in line with a study done in South Africa and in contrast to a study done in the Amhara region in which TB burden was higher among urban dwellers.24,25 This discrepancy might be due to the immune status of study participants, living environment, and standard of living conditions.
In our study, smokers had nearly similar TB incidence rate (3.56/100 PY) as compared to non smokers (3.50/100 PY). This finding was against to studies done in Amhara region, Jimma and Mettu Karl hospital, Addis Ababa and Cambodia in which smokers were at higher risk of TB.19,23,24,30 This might be due to differences in the amount of cigarettes smoked/individual/day, duration of smoking, ART status, living standards and immune status.
About 78% of study participants with baseline WHO stage I and II were protected from developing TB as compared stage III and IV. This was comparable with studies done in Addis Ababa and JUSH that showed stage III and IV individuals were at a higher risk of developing active TB.19,20 This might be due to the presence of the better immunity among stage I and II individuals that prevent mycobacterium TB infection and reactivation of latent TB infection into active TB.
Individuals with baseline CD4 count ≤ 350 were 3.76 times at higher risk of TB as compared to those with CD4>350. Comparable findings were obtained in studies done in Harrar which was 62% higher in those with CD4 ≤ 350; in the Amhara region, it was 1.71 times at higher risk among those with CD4<200; in South Africa individuals with CD4 count <100 cells/μL had 2.22 times higher risk compared to CD4 >349.24,25,31 Although TB can occur at any CD4 count, individuals with advanced WHO stage and severe immune suppression are at increased risk of conversion of latent TB infection to active TB; this might be the causes for these similarities.
The overall incidence rate of TB for the cohort was found to be 3.5/100 PY whereas the incidence of TB among INH completed individuals was 0.35/100 PY and 7.1 in those who never took INH. These findings were nearly similar with studies done in SNNPR overall TB (2.6/100 PY), among INH (0.7/100 PY) and for those who did not take INH (6.1/100 PY); in another study done in public health facilities of Addis Ababa, overall TB was 6.7/100 PY, INH group was 0.21/100 PY and among never on INH groups 7.18/100PY.11,28
INH completed individuals were 92% at lower risk of developing TB compared to those never on INH. In line with this study, INH completed individuals were 96%, 85% and 68% at lower risk of developing TB according to studies conducted in public health facilities of Addis Ababa, SNNPR and Arba Minch Hospital respectively.11,22,28 This increased INH effectiveness might be due to synergistic effect with ART in the prevention of TB, good INH adherence, being on CPT and better immune status.
On ART patients were 98% at lower risk of developing TB disease compared to the Pre-ART counterparts. In support of this study, a study in Jimma and Mettu Karl hospital, not on ART participants (pre-ART) were 3.1 times more at higher risk compared to being on ART; the risk was also reduced by half in the study conducted in Harrar.30,31
There was no TB incident case among individuals who were on both INH and ART. Similarly, a study in South Africa showed TB incidence was reduced by 89% among these groups of people. Moreover, a study done in SNNPR, TB incidence was reduced only by 57% among these groups of individuals.25,28 This might be due to the synergistic effect of INH and ART, being on CPT, good INH adherence and good clinical care.
As the duration of HIV diagnosis increased, the incidence rate of TB was decreased. In contrast to this finding, in Guangxi, China individuals with a long duration of HIV diagnosis were at higher risk of TB.32 This might be due to the inclusion of nearly half of the participants from INH completed, ART status, and having good medication adherence.
Strength and Limitations of This Study
It will be used as a baseline source of information for researchers who are interested to further investigate TB/HIV co-infection and the effectiveness of INH against active TB incidence among different HIV infected individuals and health institutions.
Being a retrospective study, variables such as viral load, diabetes mellitus, and other chronic diseases were missed from the charts during data collection these variables were significantly associated with TB incidence in related studies. We were also unable to determine the amount of alcohol, cigarettes, and khat being consumed. Since we excluded partially treated individuals we might have resulted in an overestimation of INH efficacy despite their numbers being small, 25.
Conclusions and Recommendations
According to this study, INH status, Baseline CD4 count, baseline WHO stage and ART status were significantly associated with TB incidence. Provision of INH for 6 months was significantly associated with a reduction of TB incidence. Individuals who were never on INH had 20 times higher TB incidence rate (7.1/100 PY) than INH completed individuals (0.35/100 PY). Individuals who started INH before ART had higher TB incidence rate than those started after ART. Individuals who were both INH completed and on ART were 100% protected from new TB incidence. However, ART alone was more protective than INH alone. As individuals lived longer and being on ART for a long time, TB incidence was decreased. Therefore, it is recommended to strengthen the provision of INH prophylaxis to all individuals in all health institutions with HIV/AIDS care and treatment centers after screening active TB and excluding contra indication.
Abbreviations
AIDS, acquired immune deficiency syndrome; ART, anti retro viral therapy; BMI, body mass index; CD4, cluster of différentiation; CPT, cotrimoxazole preventive therapy; HIV, human immune deficiency virus; HURH, Hawassa University Referral Hospital; INH, isoniazid; IPT, isoniazid preventive therapy; IQR, inter quartile range; IR, incidence rate; JUSH, Jimma University Specialized Hospital; OIs, opportunistic infections; PMTCT, prevention of mother to child transmission; PY, person year; TB, tuberculosis; UOGRH, University of Gondar Referral Hospital; WHO, World Health Organization; CI, confidence interval; SNNPR, southern nations nationalities and peoples region.
Data Sharing Statement
All relevant information is included within the manuscript. The data upon which the result was based can be available upon request.
Ethics Approval and Consent to Participate
This research was conducted after ethical clearance was obtained from the Ethical Review Board of School of Medicine, University of Gondar. The Department of Internal Medicine wrote an official letter to the Ethical Review Board of School of Medicine. After ethical approval, the Ethical Review Board of School of Medicine wrote official letter to the town health bureau then the heath bureau to both health centers. As this study was a retrospective cohort, verbal consent was requested from and approved by Heads of Health Centers to review and use medical records of the study participants. In addition, confidentiality of study participants was maintained by avoiding personal identifiers during data collection and all check lists were kept locked.
The study was conducted in accordance with the Declaration of Helsinki 1964 and its consecutive amendments.
Acknowledgments
We would like to thank University of Gondar, College of Medicine and Health Sciences and all staff members of the Department of Internal Medicine. We would also like to thank the heads, data clerks and all ART clinics staff members of Gondar and Azezo Health Centers who helped us a lot during data collection. In addition, we would also like to thank Mr. Kindie Fentahun Muche for his comments in the analysis part of this paper.
Author Contributions
All authors made a significant contribution to the work reported; in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No external funding was provided to conduct this research. Since it was conducted in a resource limited country (Ethiopia), the research publication charge will be covered by the World Bank
Disclosure
All authors report no competing interests in this work.
References
1. Cain PK, McCarthy DK, Heilig MC, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362(8):707–716. doi:10.1056/NEJMoa0907488
2. Raviglione MC. Tuberculosis - the Essentials.
3. World Health Organization. Global tuberculosis report, end TB 2019.
4. Addis Ababa Report on National TB/HIV sentinel surveillance. 2015.
5. Wondimeneh Y, Muluye D, Belyhun Y. Prevalence of pulmonary tuberculosis and immunological profile of HIV co-infected patients in Northwest Ethiopia. BMC Res Notes. 2012;5:331. doi:10.1186/1756-0500-5-331
6. Nigatu DF, Kiros DM, Mamo MS, et al. Ethiopian consolidated national guidelines for HIV prevention, care and treatment- final version December 17, 2014.
7. Africa DohRoS. National tuberculosis management guidelines. 2014.
8. Keder DF, Getachew MM, Mamo SS, et al. National consolidated guidelines for comprehensive HIV prevention, care and treatment. 2018.
9. Volmink J, Woldehanna S. Treatment of latent tuberculosis infection in HIV infected persons (sytematic review). Cochrane Lib. 2004;3.
10. Fitzgerald DW, Desvarieux M, Severe P, Joseph P, Johnson WD, Pape JW. Effect of post-treatment isoniazid on prevention of recurrent tuberculosis in HIV-1-infected individuals: a randomised trial. The Lancet. 2000;356:1470–1474. doi:10.1016/S0140-6736(00)02870-1
11. Semu M, Fenta TG, Medhin G, Assefa D. Effectiveness of isoniazid preventative therapy in reducing incidence of active tuberculosis among people living with HIV/AIDS in public health facilities of Addis Ababa, Ethiopia: a historical cohort study. BMC Infect Dis. 2017;17:1–8. doi:10.1186/s12879-016-2109-7
12. Gordin MFM, Miller JP, Brown C, et al. A controlled trial of isoniazid in persons with anergy and human immunodeficiency virus infection who are at high risk for tuberculosis. N Engl J Med. 1997;337(5):315–320. doi:10.1056/NEJM199707313370505
13. Word Vision. Technical guideline for tuberculosis (TB) and TB-HIV program implementation. World Vision. 2017;13:87–95.
14. Ahn JV, Bailey H, Malyuta R, Volokha A, Thorne C. Factors associated with non-disclosure of HIV status in a cohort of childbearing HIV-positive women in Ukraine. AIDS Behav. 2016;20(1):174–183. doi:10.1007/s10461-015-1089-8
15. World Health Organization. Global tuberculosis report. 2018.
16. World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource constrained settings. Geneva, Switzerland: World Health Organization; 2011.
17. Ethiopa FMoHo. National comprehensive HIV care and treatment training for health care providers, practical manua. ART guideline. 2014.
18. Sileshi B, Deyessa N, Girma B, Melese M, Suarez P. Predictors of mortality among TB-HIV Co-infected patients being treated for tuberculosis in Northwest Ethiopia: a retrospective cohort study. BMC Infect Dis. 2013;13(297):1471–2334. doi:10.1186/1471-2334-13-297
19. Kibret KT, Yalew AW, Belaineh BG, Asres MM. Determinant factors associated with occurrence of tuberculosis among adult people living with HIV after antiretroviral treatment initiation in Addis Ababa, Ethiopia: a case control study. PLoS One. 2013;8(5):e64488. doi:10.1371/journal.pone.0064488
20. Assebe LF, Reda HL, Wubeneh AD, et al. The effect of isoniazid preventive therapy on incidence of tuberculosis among HIV-infected clients under pre-ART care, Jimma, Ethiopia: a retrospective cohort study. BMC Public Health. 2015;15(1):346. doi:10.1186/s12889-015-1719-0
21. Fekadu S, Teshome W, Alemu G. Prevalence and determinants of Tuberculosis among HIV infected patients in south Ethiopia. J Infect Dev Ctries. 2015;9(8):898–904. doi:10.3855/jidc.5667
22. Abossie A, Yohanes T. Assessment of isoniazid preventive therapy in the reduction of tuberculosis among aRT patients in arba Minch hospital, ethiopia. Ther Clin Risk Manag. 2017;13:361. doi:10.2147/TCRM.S127765
23. Choun TK, Thai S, Pe R, et al. Incidence and risk factors for tuberculosis in HIV-infected patients while on antiretroviral treatment in Cambodia. Trans R Soc Trop Med Hyg. 2013;107(4):235–242. doi:10.1093/trstmh/trt001
24. Mitku AA, Dessie ZG, Muluneh EK, Workie DL. Prevalence and associated factors of TB/HIV co-infection among HIV Infected patients in Amhara region, Ethiopia. Afr Health Sci. 2016;16(2):588–595. doi:10.4314/ahs.v16i2.29
25. Goluba JE, Pronykc P, Mohapie L, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23(5):631–636. doi:10.1097/QAD.0b013e328327964f
26. Ethiopia CSAo. Ethiopian population projection census 2015. 2015.
27. Simbayi LC, Kalichman SC, Strebel A, Cloete A, Henda N, Mqeketo A. Disclosure of HIV status to sex partners and sexual risk behaviours among HIV-positive men and women, Cape Town, South Africa. Sex Transm Infect. 2007;83(1):29–34. doi:10.1136/sti.2006.019893
28. Yirdaw KD, Jerene D, Gashu Z, et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoS One. 2014;9(8):e104557. doi:10.1371/journal.pone.0104557
29. Molaeipoor L, Poorolajal J, Mohraz M. Predictors of tuberculosis and human immunodeficiency virus co-infection: a case-control study. Epidemiol Health. 2014;36. doi:10.4178/epih/e2014024
30. Taha M, Deribew A, Tessema F, Assegid S, Duchateau L, Colebunders R. Risk factors of active tuberculosis in people living with HIV/AIDS in southwest ethiopia: a case control study. Ethiop J Health Sci. 2011;21(2):131–140. doi:10.4314/ejhs.v21i2.69053
31. Geleto A, Abate D, Egata G. Intensified tuberculosis case finding, implementation of isoniazid preventive therapy and associated factors among people living with human immunodeficiency virus at public health facilities of Harari Region, Eastern Ethiopia: a cross-sectional study. Int J Health Sci. 2017;11(1):1.
32. Cui Z, Lin M, Nie S, Lan R. Risk factors associated with Tuberculosis (TB) among people living with HIV/AIDS: a pair-matched case-control study in Guangxi, China. PLoS One. 2017;12(3):e0173976. doi:10.1371/journal.pone.0173976
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.