Back to Journals » Drug Design, Development and Therapy » Volume 15
Effect of Intravenous Lidocaine on Serum Interleukin-17 After Video-Assisted Thoracic Surgery for Non-Small-Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial
Authors Hou YH, Shi WC, Cai S, Liu H , Zheng Z, Qi FW, Li C, Feng XM, Peng K , Ji FH
Received 21 April 2021
Accepted for publication 20 July 2021
Published 3 August 2021 Volume 2021:15 Pages 3379—3390
DOI https://doi.org/10.2147/DDDT.S316804
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Yong-heng Hou,1,* Wen-cheng Shi,2,* Shu Cai,1,* Hong Liu,3 Zhong Zheng,2 Fu-wei Qi,2 Chang Li,4 Xiao-mei Feng,5,6 Ke Peng,1 Fu-hai Ji1
1Department of Anesthesiology, First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, Taicang First People’s Hospital, Taicang Affiliated Hospital of Soochow University, Taicang, Jiangsu, People’s Republic of China; 3Department of Anesthesiology and Pain Medicine, University of California Davis Health, Sacramento, CA, USA; 4Department of Thoracic Surgery, First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China; 5Department of Anesthesiology, University of Utah health, Salt Lake City, UT, USA; 6Transitional Residency Program, Intermountain Medical Center, Salt Lake City, UT, USA
*These authors contributed equally to this work
Correspondence: Ke Peng; Fu-hai Ji
Department of Anesthesiology, First Affiliated Hospital of Soochow University, 899 Pinghai Road, Suzhou, Jiangsu, 215006, People’s Republic of China
Tel +86-159-6215-5989
; +86-512-6797-2352
Email [email protected]; [email protected]
Purpose: Surgical stress promotes tumor metastasis. Interleukin (IL)-17 plays a pivotal role in cancer progression, and high IL-17 expression predicts poor prognosis of non-small-cell lung cancer (NSCLC). Lidocaine may exert tumor-inhibiting effects. We hypothesize that intravenous lidocaine attenuates surgical stress and reduces serum IL-17 levels during video-assisted thoracic surgery (VATS) for NSCLC.
Methods: This randomized, double-blind, placebo-controlled trial included 60 early-stage NSCLC patients undergoing VATS, into a lidocaine group (n = 30; intravenous lidocaine bolus 1.0 mg/kg, and 1.0 mg/kg/h until the end of surgery) or a normal saline control group (n = 30). The primary outcome was serum IL-17 level at 24 hours postoperatively. The secondary outcomes included serum IL-17 level at the time of post-anesthesia care unit (PACU) discharge, serum cortisol level at PACU discharge and postoperative 24 hours, pain scores (0– 10) from PACU discharge to 48 hours postoperatively, incidences of postoperative nausea and vomiting, dizziness, and arrhythmia during 0– 48 hours postoperatively, and 30-day mortality. Long-term outcomes included chemotherapy, cancer recurrence, and mortality.
Results: The lidocaine group had lower serum IL-17 at 24 hours postoperatively compared with the control group (23.0 ± 5.8 pg/mL vs 27.3 ± 8.2 pg/mL, difference [95% CI] = − 4.3 [− 8.4 to − 0.2] pg/mL; P = 0.038). The lidocaine group also had reduced serum IL-17 (difference [95% CI] = − 4.6 [− 8.7 to − 0.5] pg/mL), serum cortisol (difference [95% CI] = − 37 [− 73 to − 2] ng/mL), and pain scores (difference [95% CI] = − 0.7 [− 1.3 to − 0.1] points) at PACU discharge. During a median follow-up of 10 (IQR, 9– 13) months, 2 patients in the lidocaine group and 6 patients in the control group received chemotherapy, one patient in the control group had cancer recurrence, and no death event occurred.
Conclusion: Intravenous lidocaine was associated with reduced serum IL-17 and cortisol following VATS procedures in early-stage NSCLC patients.
Trial Registration: ChiCTR2000030629.
Keywords: lidocaine, interleukin-17, non-small-cell lung cancer, video-assisted thoracic surgery, surgical stress
Introduction
Non-small-cell lung cancer (NSCLC) is the most diagnosed cancer worldwide and the leading cause of cancer death.1 Over the past decade, video-assisted thoracic surgery (VATS) has been widely performed in patients with NSCLC. A recent national analysis showed that a VATS lobectomy was associated with improved postoperative outcomes compared with an open approach in early-stage NSCLC patients.2 Despite the associated advantages of VATS, postoperative nodal upstaging rate was about 11%, and 5-year survival rate was 66% for early-stage NSCLC patients.2
Surgical stress including the activation of sympathetic nervous system, ischemia-reperfusion injury, hypercoagulation, inflammation, and immunosuppression, promotes tumor metastasis.3,4 Cortisol is a major hormone in response to surgical stress.5 Interleukin-17 (IL-17) is an important proinflammatory cytokine that contributes to lung cancer progression and metastasis.6 A study reported that the expression of IL-17 in NSCLC tissues is an independent prognostic factor for both disease-free and overall survival after surgery.7 In addition, measuring serum levels of IL-17 showed a good diagnostic performance for NSCLC.8 A recent meta-analysis also suggested that the increased IL-17 expression in different samples (including serums, cancer tissues, and pleural effusions) was an indicator of poor prognosis for NSCLC patients.9
Lidocaine is a local anesthetic agent that can be administered intravenously. The intravenous use of lidocaine is of increasing interest to many clinicians. Studies showed that intravenous lidocaine treatment alleviated acute postoperative pain and refractory neuropathic pain, attenuated inflammatory responses, and reduced postoperative complications such as ileus, sore throat, and coughing.10–13 For cancer patients, intravenous lidocaine administration may enhance anti-tumor immunity and inhibit tumor recurrence after surgery.14,15 A recent retrospective cohort study suggested that intravenous lidocaine infusion was associated with an improved postoperative long-term survival after pancreatectomy for pancreatic cancer.16 To date, however, whether intravenous lidocaine treatment would be beneficial for patients undergoing lung cancer surgery remains unknown.
Therefore, this study aims to investigate the effects of intraoperative intravenous administration of lidocaine on serum levels of IL-17 and surgical stress following VATS procedures in early-stage NSCLC patients. We hypothesized that intravenous lidocaine treatment would reduce the serum IL-17 levels and attenuate surgery-induced stress response in these patients.
Methods
Study Design
This investigator-initiated, randomized, double-blind, placebo-controlled trial was approved by the Institutional Review Board of Taicang First People’s Hospital (no. KY-2019-214) on December 25, 2019. The study protocol was registered prior to patient enrollment at the Chinese Clinical Trial Registry (http://www.chictr.org.cn, identifier: ChiCTR2000030629, date of registration: March 8, 2020). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The implementation and reporting of this study followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Study Participants
Patients aged ≥ 18 years with ASA physical status I–III scheduled for elective VATS procedures for early-stage NSCLC were recruited. Preoperative diagnosis of early-stage NSCLC was based on computed tomography results. The exclusion criteria were (1) severe cardiac, pulmonary, renal, or liver dysfunction, (2) second- or third-degree atrioventricular block, (3) neuropsychiatric disorders, (4) immune system disease, (5) allergy to any local anesthetic agent, or (6) history of chemotherapy. Written informed consent was obtained from each participant.
Randomization and Blinding
Randomization was performed by using an online random number generator (http://www.randomization.com), with an allocation ratio of 1:1. Identical opaque sealed envelopes were used to ensure allocation concealment. An independent research assistant prepared the randomization list and sequentially numbered the envelopes. According to the random codes, an independent anesthesia nurse prepared the study solutions in identical syringes containing either lidocaine (Qilu Pharmaceutical Co., Jinan, Shandong, China) or normal saline. Both lidocaine and normal saline are colorless and clear fluids with similar appearance, so there is no way to distinguish them. The participants, care providers, and postoperative observers were all blinded to the group allocation until the completion of final analysis.
Anesthesia and Perioperative Care
Patients were instructed on the use of visual analog scale (VAS, 0–10; 0 indicates no pain, and 10 indicates the most severe pain imaginable) the day before surgery. Baseline serum samples were collected on the morning of surgery. Hook wire localization of lung nodules under computed tomography (CT) was performed approximately 1 hour preoperatively if the nodules were presumably too deep and/or too small to be detected during the surgery. To ensure the efficiency and consistency, all surgical procedures, anesthesia management, and perioperative care were performed by the same medical team.
Patients received no sedative or analgesic premedication. In the operating room, a standard monitoring included electrocardiography, noninvasive blood pressure, and pulse oximetry. In addition, patients received surgical pleth index (SPI, S/5™ Anesthesia Monitor, GE Healthcare, Helsinki, Finland) and bispectral index (BIS, Aspect Medical Systems, Newton, MA, USA) monitoring. General anesthesia was induced with sufentanil 0.4 μg/kg and propofol 2 mg/kg. Endotracheal intubation with a double-lumen endotracheal tube was facilitated with cisatracurium 0.2 mg/kg. The tube location was confirmed by visualization using fiberoptic bronchoscopy. After anesthesia induction, patients received a catheter insertion in the radial artery for continuous arterial pressure monitoring. After placement of a lateral position, single-lung ventilation was performed on the non-surgical side with a tidal volume of 6–8 mL/kg and positive end-expiratory pressure of 5–10 cm H2O. The respiratory frequency and inspired oxygen fraction were adjusted to maintain end-tidal carbon dioxide within 35–45 mmHg and peripheral oxygen saturation ≥ 95%. Lung recruitment maneuvers were performed when clinically indicated.
General anesthesia was maintained with 1–3% sevoflurane inhalation, and the depth of anesthesia was titrated to a BIS value within 40–60. The intraoperative analgesia strategy aimed to achieve a SPI value of 20–50, which has been validated in previous studies.17–19 An additional sufentanil bolus 0.1 μg/kg was administered when a patient showed insufficient analgesia, such as an increase in mean arterial pressure (MAP) > 20% of the baseline value, heart rate (HR) > 100 beats/min, or SPI value > 50 for more than 20 seconds.17 Patients did not receive regional analgesia (including intercostal or epidural blocks). Additional cisatracurium was administered for muscle relaxation. The nasopharyngeal temperature was maintained at 36–37°C with the use of a warming blanket and an infusion heating device. Frozen section of lung lesion was checked intraoperatively. The patient was excluded from analysis if the pathologic result did not show malignancy.
The baseline hemodynamic values were obtained when patients were comfortably seated in a preoperative area. Hypotension, defined as a reduction in MAP > 20% of the baseline value, was treated with intravenous phenylephrine bolus. Bradycardia, defined as HR < 50 beats/min was treated with intravenous atropine. Hypertension is defined as an increase in MAP > 20% of the baseline value, and tachycardia is defined as HR > 100 beats/min. If hypertension or tachycardia persisted despite sufficient analgesia and depth of anesthesia, intravenous bolus of urapidil or esmolol was given. Lactated Ringer’s solution was infused for intraoperative fluid management. For preventing postoperative nausea and vomiting (PONV), patients received intravenous dexamethasone 5 mg after anesthesia induction and ondansetron 4 mg at the end of surgery. We did not use nonsteroidal anti-inflammatory drugs in this study. After tracheal extubation, patients were transferred to a post-anesthesia care unit (PACU). Postoperative care for patients on the surgical wards were at the discretion of attending physicians according to the institutional clinical practice. For postoperative pain relief, tramadol 50 mg was administered intravenously on request or if a VAS pain score ≥ 4.
Study Interventions
According to the random codes, patients were randomly assigned to the lidocaine group or the control group. As a bolus dose of lidocaine is likely to interfere with study blinding, all patients received the study medications immediately after anesthesia induction. Patients in the lidocaine group received lidocaine bolus of 1.0 mg/kg over 10 min, followed by a continuous infusion at a rate of 1.0 mg/kg/h throughout the surgery. Patients in the control group received the same volume of normal saline instead of lidocaine. The infusion of study medication was stopped at the end of surgery. This infusion regimen of lidocaine was based on the literature and our preliminary investigation. The perioperative intravenous lidocaine infusion (bolus of 1–3 mg/kg followed by 1–3 mg/kg/h) has been shown to reduce postoperative pain and opioid requirement.20 Recent studies suggested that both low dose (1.0–1.5 mg/kg) and high dose (1.5–2.0 mg/kg) of intravenous lidocaine reduced postoperative airway complications.13 Regarding the safety, previous studies showed that an intravenous lidocaine infusion (1.5 mg/kg bolus then 2 mg/kg/h intraoperatively) did not result in toxic plasma concentrations.21,22 Therefore, we tested a low dose of lidocaine (1.0 mg/kg bolus followed by 1.0 mg/kg/h) in our preliminary investigation, and we found that this lidocaine treatment produced analgesic benefits for VATS patients, without any adverse events.
Patient Demographics and Surgical Characteristics
Patient demographic data included age, sex, ASA physical status, comorbidities, and preoperative pulmonary function test (both the absolute values and predicted percentages of forced vital capacity [FVC], forced expiratory volume in 1 second [FEV1], and maximal voluntary ventilation [MVV], FEV1/FVC ratio, and predicted percentages of peak expiratory flow [PEF] and maximum mid-expiratory flow [MMEF] 75/25). Surgical characteristics included preoperative CT-guided localization, tumor category, histologic type, surgical procedure, lymph node dissection, and lymphovascular invasion.
Perioperative Data
The perioperative data included (1) HR and MAP values at different time points from baseline to PACU discharge, (2) intraoperative hemodynamic events including hypotension, hypertension, bradycardia, and tachycardia, (3) intraoperative sufentanil consumption, blood loss, fluid infusion, and urine output, and (4) duration of surgery, duration of anesthesia, time to extubation, length of PACU stay, and length of postoperative hospital stay.
Study Outcomes
The primary outcome was the serum level of IL-17 at 24 hours postoperatively. The secondary outcomes included serum IL-17 at the time of PACU discharge, serum cortisol (at PACU discharge and 24 hours postoperatively), VAS pain scores (at PACU discharge and 24 and 48 hours postoperatively), the incidences of PONV, dizziness, and arrhythmia during 0–48 h postoperatively, and 30-day mortality. The long-term outcomes included chemotherapy, local or metastatic cancer recurrence, and mortality. For the long-term follow-up, the electronic medical records were reviewed, and patients or their relatives received a telephone call if necessary.
Enzyme‐linked Immunosorbent Assay
Serum levels of IL-17 and cortisol were measured using specific enzyme-linked immunosorbent assay kits (Bioswamp, Shanghai, China), according to the manufacturer’s instructions. The absorbance value of each sample at 450 nm was obtained on a plate reader (SpectraMax190, Molecular Devices, San Jose, CA, USA). Three replicates were tested for each blood sample. The concentration of each biomarker was determined by using a standard curve approach.
Sample Size Calculation
Sample size calculation was performed using the PASS software (version 11.0.7, NCSS, LCC, Kaysville, UT, USA). A previous study reported that the serum level of IL-17 was 21.1 ± 9.3 (mean ± standard deviation [SD]) pg/mL in early-stage NSCLC patients before any therapy was started.8 The serum level of IL-17 at postoperative 24 hours and the effect of intravenous lidocaine treatment on serum IL-17 in these patients are unknown. Our preliminary results (unpublished data, n = 16) showed that, in early-stage NSCLC patients undergoing VATS procedures without receiving intravenous lidocaine, serum IL-17 level was 19.8 ± 5.2 pg/mL at baseline and 28.1 ± 7.9 pg/mL at postoperative 24 hours. We hypothesized that the lidocaine treatment would reduce serum IL-17 to 22.5 ± 6.3 pg/mL (i.e., a 20% relative reduction) at 24 hours postoperatively. To detect such a between-group difference with a power of 80% at a two-sided alpha error of 0.05, the estimated required sample size was 27 patients per group. Considering a possible dropout rate of 15%, we decided to allocate a total of 64 patients, with 32 in each group.
Statistical Analysis
Continuous data were tested for normal distribution using the Shapiro–Wilk test. Normally distributed data are presented as mean ± SD and analyzed using unpaired t-test or repeated measures analysis of variance followed by Tukey or Sidak test, as appropriate. Skewed data are expressed as median (interquartile range, IQR) and analyzed using Mann–Whitney U-test. Categorical data are shown as numbers (%) of patients and analyzed using χ2 test or Fisher’s exact test. Data of patients’ demographics and surgical characteristics are described using descriptive statistics only. For the primary and secondary outcomes, between-group differences were analyzed using the effect size (mean difference or relative risk) with 95% confidence interval (CI). In addition, subgroup analysis was conducted to investigate the effect of intravenous lidocaine on serum IL-17 levels at postoperative 24 hours, according to age (≤ 50 y vs > 50 y), sex (female vs male), and cancer stage (T1-2 vs Tis).
Statistical analysis was performed and graphs were plotted, with the use of the GraphPad Prism software (version 7.00, GraphPad Software Inc., San Diego, CA, USA). A two-sided P < 0.05 was considered statistically significant.
Results
Study Flow
A total of 95 patients were initially screened from Mar 12, 2020 to Nov 18, 2020. Among those, 31 patients were excluded and 64 were randomized. Four patients were excluded after randomization due to withdrawal of consent before surgery (n = 2), surgery cancellation (n = 1), and no malignancy shown by intraoperative frozen section check (n = 1). Finally, 30 patients in each group completed this study (Figure 1). Until June 30, 2021, two patients in the control group were lost to follow-up. Thus, 30 patients in the lidocaine group and 28 patients in the control group were included in the long-term outcome analysis.
 |
Figure 1 CONSORT flow diagram. Notes: Adapted from: Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3):e1000251. Copyright: © 2010 Schulz et al. Creative Commons Attribution License.37Abbreviation: CONSORT, Consolidated Standards of Reporting Trials. |
Patient Demographics and Surgical Characteristics
The two groups are comparable in terms of demographic and surgical characteristic data (Table 1). The mean age was 51.3 ± 8.6 and 50.9 ± 11.6 years old in the lidocaine and control groups, respectively. Preoperative pulmonary function test results were available in 29 patients in the lidocaine group and in 28 patients in the control group. The mean predicted percentages of MVV were 92.5% (SD, 16.7%) and 92.4% (SD, 21.4%) in the lidocaine and control groups, respectively. The median FEV1/FVC ratio was 0.80 (IQR, 0.75–0.83) in the lidocaine group and 0.80 (IQR, 0.70–0.85) in the control group.
 |
Table 1 Patient Demographics and Surgical Characteristics |
All patients were diagnosed as early-stage NSCLC, based on the 8th edition Lung Cancer Stage Classification system.23 Tis (carcinoma in situ) was diagnosed in 16 patients (53.3%) in the lidocaine group and in 12 patients (40%) in the control group. For both groups, 90% of patients underwent segmentectomy or lobectomy procedures. Lymph node dissection was performed in 13 and 11 patients in the lidocaine and control groups, respectively. Of these, 1 patient in the lidocaine group and 3 patients in the control group had lymphovascular invasion in the pathological examination.
Perioperative Data
The perioperative data are shown in Table 2. The lidocaine treatment did not lead to increased incidences of intraoperative hemodynamic events. Intraoperative hypotension and bradycardia were successfully treated with phenylephrine and atropine, respectively. Hypertension and tachycardia were treated with sufentanil alone or sufentanil plus propofol. The HR values were comparable between the two groups at different time points, except that the lidocaine group had lower values of HR at the time of skin incision and tracheal extubation (Figure 2A). The MAP values were also comparable between groups, except that the lidocaine group showed a lower value of MAP at the time of skin incision (Figure 2B). The median total dose of lidocaine was 168 (IQR, 130–201) mg in the lidocaine group. The two groups had similar length of PACU stay and postoperative hospital stay.
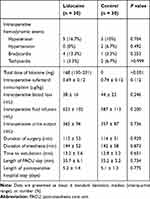 |
Table 2 Perioperative Data |
Primary and Secondary Outcome
The lidocaine group had a significantly lower serum IL-17 at 24 hours postoperatively than that in the control group (23.0 ± 5.8 pg/mL vs 27.3 ± 8.2 pg/mL, mean difference = −4.3 pg/mL, 95% CI, −8.4 to −0.2 pg/mL; P = 0.038) (Table 3 and Figure 3A). The lidocaine treatment led to reduced serum IL-17 (difference = −4.6 pg/mL, 95% CI, −8.7 to −0.5 pg/mL; P = 0.024) (Figure 3A), serum cortisol (difference = −37 ng/mL, 95% CI, −73 to −2 ng/mL; P = 0.036) (Figure 3B), and VAS pain scores (difference = −0.7, 95% CI, −1.3 to −0.1; P = 0.019) at the time of PACU discharge (Table 3). There were no between-group differences in other secondary outcomes. No death event occurred during 30 days after surgery. The subgroup analysis is depicted in Figure 4. The effect of intravenous lidocaine on serum IL-17 at postoperative 24 hours was mainly evident in patients who were older (aged > 50 y) or with a higher T category (T1 or T2).
 |
Table 3 Postoperative Outcomes |
Long-Term Outcomes
During a median follow-up of 10 (IQR, 9–13) months, 2 patients in the lidocaine group and 6 patients in the control group received chemotherapy (Table 3). One patient with lymphovascular invasion in the control group had cancer recurrence postoperatively. No patient died during the follow-up period.
Discussion
In this randomized, double-blind, placebo-controlled trial, intraoperative intravenous administration of lidocaine reduced postoperative serum levels of IL-17 after VATS procedures in early-stage NSCLC patients. In addition, the lidocaine treatment reduced serum cortisol levels and VAS pain scores in the early postoperative period. To the best of our knowledge, this is the first randomized controlled study evaluating the effects of intravenous lidocaine infusion on the prognostic factor of serum IL-17 and surgical stress in NSCLC patients undergoing VATS procedures.
Activation of the sympathetic nervous system is the most obvious pathophysiological response to surgical stress. Catecholamines released into the bloodstream activate adrenergic receptors, resulting in hemodynamic fluctuations. The activation of the sympathetic nervous system inhibits cell-mediated immunity, suppresses programmed cell death, and stimulates inflammation, macrophage infiltration, angiogenesis, and epithelial–mesenchymal transition.24 The above-mentioned effects combined contribute to tumor invasion and metastasis.24 A recent review summarizes the effects of commonly used anesthetic agents on immunosuppression after surgery.25 Ketamine induces apoptosis of T-lymphocytes, and midazolam has little impact on cytotoxic T-lymphocytes. Ketamine and thiopental inhibit the activity of natural killer (NK) cells, while propofol does not. Volatile anesthetics induce T-lymphocyte apoptosis, reduce the activity of NK cells, and enhance angiogenesis. In addition, the use of opioids is associated with suppressed NK cell activity.26 However, a recent large-scale multicenter trial demonstrated that propofol anesthesia with paravertebral block did not reduce recurrence after primary breast cancer resection surgery compared with sevoflurane anesthesia with opioids.27 Therefore, the relationship among surgical stress, anesthetics, and cancer recurrence has not been fully elucidated.
Experimental studies have shown the tumor-inhibiting effects of lidocaine. In lung cancer A549/DDP cells, lidocaine inhibited cell migration and induced cell apoptosis through downregulating miR-21.28 Zhang et al. found that lidocaine inhibited cancer cell proliferation via suppressing the expression of GOLT1A in cultured human lung adenocarcinoma cells and in patients’ lung cancer samples.29 A recent study showed that lidocaine reduced pulmonary metastasis after breast cancer surgery in mice during sevoflurane anesthesia.30 Recent clinical studies also suggested that intravenous lidocaine treatment may produce favorable anti-tumor effects in cancer patients. Perioperative intravenous administration of lidocaine inhibited the expression of neutrophil extracellular trapping and matrix metalloproteinase 3, an important mechanism related to tumor metastasis, in women undergoing primary breast tumor resection.31 For pancreatic cancer patients undergoing pancreatectomy, lidocaine infusion was associated with an overall improved survival after surgery.16 In our study, intravenous lidocaine treatment reduced the expression of serum levels of IL-17 in NSCLC patients undergoing VATS procedures. A mean difference of −4.3 pg/mL in serum IL-17 at postoperative 24 hours was found between the lidocaine and control groups. Measuring tumor tissue or serum IL-17 levels presents a useful diagnostic and prognostic value for NSCLC patients.7,8 Specifically, the mean difference in serum IL-17 of NSCLC patients was −6.3 pg/mL in early vs advanced lung cancer stages, −4.7 pg/mL in absence vs presence of lymph node metastases, and −4.8 pg/mL in absence vs presence of distant metastases.8 Thus, the mean difference of −4.3 pg/mL in serum IL-17 levels in our study exhibits its clinical significance, suggesting the tumor-inhibiting effects of intravenous lidocaine.
The non-opioid, lidocaine, may contribute to multimodal pain management and enhanced recovery after surgery. A previous study showed that intravenous lidocaine (1.5 mg/kg, followed by 1.5 mg/kg/h) significantly reduced the incidence and severity of persistent postsurgical pain and secondary hyperalgesia after breast cancer surgery.32 Preventing central hyperalgesia is a possible mechanism underlying the analgesic effects of intravenous lidocaine. A recent study showed that intravenous lidocaine (initiated at 1–1.5 mg/kg/h and titrated to serum lidocaine level at 3–5 μg/mL) produced effective analgesia in patients with acute rib fracture pain.10 For patients with refractory neuropathic pain, repeated lidocaine infusions (3 mg/kg, once a week, for 4 consecutive weeks) provided effective short-term pain relief.11 However, several studies did not find significant opioid-sparing or analgesic effects of intravenous lidocaine.33–35 Our results showed that intraoperative lidocaine treatment slightly reduced VAS pain scores (a mean difference of −0.7) at the time of PACU discharge. Although the improvement of 0.7 in the 0–10 VAS pain scores is statistically significant, it is not clinically meaningful.36 This is likely attributable to the minimal invasive nature of VATS procedures.
This study has several limitations. First, as a single-center study with a small sample size, the generalizability of the current results may be limited. Second, postoperative use of opioids or other medications were not measured or controlled for, which could have affected our results. Third, a standardized strategy to titrate intraoperative anesthesia and analgesia was applied in both groups; however, we did not record data on end-tidal concentrations of sevoflurane or SPI values in our patients. Fourth, patients in the lidocaine group received a relatively low dose of intravenous lidocaine when compared with previous studies.21,22 Thus, the optimal dose of lidocaine for NSCLC patients undergoing VATS needs further investigation. Last, although we carried out a median postoperative follow-up of 10 months, this study mainly focused on serum IL-17 and other postoperative outcomes in the early postoperative period. Therefore, further clinical trials with a larger sample size are needed to assess the long-term benefits of intravenous lidocaine infusion for NSCLC patients undergoing VATS procedures.
In conclusion, intraoperative intravenous lidocaine administration was associated with reduced serum IL-17 after VATS procedures for early-stage NSCLC. In addition, intravenous lidocaine treatment may help to attenuate surgical stress response and potentiate analgesia in the early postoperative period. The current findings support future studies investigating the effects of intravenous lidocaine on long-term outcomes of early-stage NSCLC patients after VATS procedures.
Data Sharing Statement
The data supporting the results reported in the manuscript can be assessed with approval from the corresponding author on reasonable request.
Ethics Approval
This study involving human participants was reviewed and approved by the Institutional Review Board of Taicang First People’s Hospital (no. KY-2019-214). The participants provided their written informed consent to participate in this study.
Acknowledgments
We would like to thank the patients, research assistants, anesthesia and surgical nurses, and all other healthcare providers for their support and participation in this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. These authors have contributed equally to this work and share first authorship: Yong-heng Hou, Wen-cheng Shi, and Shu Cai.
Funding
This work was supported by the National Natural Science Foundation of China (82072130 and 81873925), Natural Science Foundation of Jiangsu Province (BK20191171), Science and Technology Development Plan Clinical Trial Project (SLT201909), and Jiangsu Provincial Medical Youth Talent (QNRC2016741). The funders had no role in the study design, data collection, data analysis, interpretation, or writing of the manuscript.
Disclosure
The authors reported no conflicts of interest for this work and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Yang CJ, Kumar A, Klapper JA, et al. A national analysis of long-term survival following thoracoscopic versus open lobectomy for stage I non-small-cell lung cancer. Ann Surg. 2019;269(1):163–171. doi:10.1097/SLA.0000000000002342
3. Chen Z, Zhang P, Xu Y, et al. Surgical stress and cancer progression: the twisted tango. Mol Cancer. 2019;18(1):132. doi:10.1186/s12943-019-1058-3
4. Hogan BV, Peter MB, Shenoy HG, Horgan K, Hughes TA. Surgery induced immunosuppression. Surgeon. 2011;9(1):38–43. doi:10.1016/j.surge.2010.07.011
5. Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr. 2013;37(5 Suppl):21S–29S. doi:10.1177/0148607113496117
6. Wu F, Xu J, Huang Q, et al. The role of interleukin-17 in lung cancer. Mediators Inflamm. 2016;2016:8494079. doi:10.1155/2016/8494079
7. Chen X, Wan J, Liu J, et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69(3):348–354. doi:10.1016/j.lungcan.2009.11.013
8. Xu C, Hao K, Yu L, Zhang X. Serum interleukin-17 as a diagnostic and prognostic marker for non-small cell lung cancer. Biomarkers. 2014;19(4):287–290. doi:10.3109/1354750X.2014.908954
9. Wang XF, Zhu YT, Wang JJ, et al. The prognostic value of interleukin-17 in lung cancer: a systematic review with meta-analysis based on Chinese patients. PLoS One. 2017;12(9):e0185168. doi:10.1371/journal.pone.0185168
10. Lii TR, Aggarwal AK. Comparison of intravenous lidocaine versus epidural anesthesia for traumatic rib fracture pain: a retrospective cohort study. Reg Anesth Pain Med. 2020;45(8):628–633. doi:10.1136/rapm-2019-101120
11. Kim YC, Castaneda AM, Lee CS, Jin HS, Park KS, Moon JY. Efficacy and safety of lidocaine infusion treatment for neuropathic pain: a randomized, double-blind, and placebo-controlled study. Reg Anesth Pain Med. 2018;43(4):415–424. doi:10.1097/AAP.0000000000000741
12. Beaussier M, Delbos A, Maurice-Szamburski A, Ecoffey C, Mercadal L. Perioperative use of intravenous lidocaine. Drugs. 2018;78(12):1229–1246. doi:10.1007/s40265-018-0955-x
13. Yang SS, Wang NN, Postonogova T, et al. Intravenous lidocaine to prevent postoperative airway complications in adults: a systematic review and meta-analysis. Br J Anaesth. 2020;124(3):314–323. doi:10.1016/j.bja.2019.11.033
14. Chamaraux-Tran TN, Piegeler T. The amide local anesthetic lidocaine in cancer surgery-potential antimetastatic effects and preservation of immune cell function? A narrative review. Front Med. 2017;4:235. doi:10.3389/fmed.2017.00235
15. Ramirez MF, Tran P, Cata JP. The effect of clinically therapeutic plasma concentrations of lidocaine on natural killer cell cytotoxicity. Reg Anesth Pain Med. 2015;40(1):43–48. doi:10.1097/AAP.0000000000000191
16. Zhang H, Yang L, Zhu X, et al. Association between intraoperative intravenous lidocaine infusion and survival in patients undergoing pancreatectomy for pancreatic cancer: a retrospective study. Br J Anaesth. 2020;125(2):141–148. doi:10.1016/j.bja.2020.03.034
17. Gruenewald M, Willms S, Broch O, Kott M, Steinfath M, Bein B. Sufentanil administration guided by surgical pleth index vs standard practice during sevoflurane anaesthesia: a randomized controlled pilot study. Br J Anaesth. 2014;112(5):898–905. doi:10.1093/bja/aet485
18. Ryu KH, Kim JA, Ko DC, Lee SH, Choi WJ. Desflurane reduces intraoperative remifentanil requirements more than sevoflurane: comparison using surgical pleth index-guided analgesia. Br J Anaesth. 2018;121(5):1115–1122. doi:10.1016/j.bja.2018.05.064
19. Funcke S, Pinnschmidt HO, Wesseler S, et al. Guiding opioid administration by 3 different analgesia nociception monitoring indices during general anesthesia alters intraoperative sufentanil consumption and stress hormone release: a randomized controlled pilot study. Anesth Analg. 2020;130(5):1264–1273. doi:10.1213/ANE.0000000000004388
20. Vigneault L, Turgeon AF, Cote D, et al. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anaesth. 2011;58(1):22–37. doi:10.1007/s12630-010-9407-0
21. Kaba A, Laurent SR, Detroz BJ, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106(1):
22. Hans GA, Lauwick SM, Kaba A, et al. Intravenous lidocaine infusion reduces bispectral index-guided requirements of propofol only during surgical stimulation. Br J Anaesth. 2010;105(4):471–479. doi:10.1093/bja/aeq189
23. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi:10.1016/j.chest.2016.10.010
24. Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15(9):563–572. doi:10.1038/nrc3978
25. Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 2018;16(1):8. doi:10.1186/s12967-018-1389-7
26. Duan MC, Zhong XN, Liu GN, Wei JR. The Treg/Th17 paradigm in lung cancer. J Immunol Res. 2014;2014:730380. doi:10.1155/2014/730380
27. Akbay EA, Koyama S, Liu Y, et al. Interleukin-17A promotes lung tumor progression through neutrophil attraction to tumor sites and mediating resistance to PD-1 blockade. J Thorac Oncol. 2017;12(8):1268–1279. doi:10.1016/j.jtho.2017.04.017
28. Yang Q, Zhang Z, Xu H, Ma C. Lidocaine alleviates cytotoxicity-resistance in lung cancer A549/DDP cells via down-regulation of miR-21. Mol Cell Biochem. 2019;456(1–2):63–72. doi:10.1007/s11010-018-3490-x
29. Zhang L, Hu R, Cheng Y, et al. Lidocaine inhibits the proliferation of lung cancer by regulating the expression of GOLT1A. Cell Prolif. 2017;50(5):e12364. doi:10.1111/cpr.12364
30. Freeman J, Crowley PD, Foley AG, et al. Effect of perioperative lidocaine, propofol and steroids on pulmonary metastasis in a murine model of breast cancer surgery. Cancers. 2019;11(5):613. doi:10.3390/cancers11050613
31. Galos EV, Tat TF, Popa R, et al. Neutrophil extracellular trapping and angiogenesis biomarkers after intravenous or inhalation anaesthesia with or without intravenous lidocaine for breast cancer surgery: a prospective, randomised trial. Br J Anaesth. 2020;125(5):712–721. doi:10.1016/j.bja.2020.05.003
32. Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clin J Pain. 2012;28(7):567–572. doi:10.1097/AJP.0b013e31823b9cc8
33. Weibel S, Jelting Y, Pace NL, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6:CD009642.
34. Dewinter G, Moens P, Fieuws S, Vanaudenaerde B, Van de Velde M, Rex S. Systemic lidocaine fails to improve postoperative morphine consumption, postoperative recovery and quality of life in patients undergoing posterior spinal arthrodesis. A double-blind, randomized, placebo-controlled trial. Br J Anaesth. 2017;118(4):576–585. doi:10.1093/bja/aex038
35. Terkawi AS, Durieux ME, Gottschalk A, Brenin D, Tiouririne M. Effect of intravenous lidocaine on postoperative recovery of patients undergoing mastectomy: a double-blind, placebo-controlled randomized trial. Reg Anesth Pain Med. 2014;39(6):472–477. doi:10.1097/AAP.0000000000000140
36. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
37. Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3):e1000251.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



