Back to Journals » Drug Design, Development and Therapy » Volume 14
Effect of Intravenous Injection of Vitamin C on Postoperative Pulmonary Complications in Patients Undergoing Cardiac Surgery: A Double-Blind, Randomized Trial
Authors Wang D , Wang M , Zhang H, Zhu H , Zhang N, Liu J
Received 16 March 2020
Accepted for publication 25 July 2020
Published 11 August 2020 Volume 2020:14 Pages 3263—3270
DOI https://doi.org/10.2147/DDDT.S254150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Dongyue Wang,1,* Min Wang,1,* Hui Zhang,1 He Zhu,1 Na Zhang,1 Jindong Liu2
1Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jindong Liu
Department of Anesthesiology, The Affiliated Hospital of XuZhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
Email [email protected]
Purpose: In this study, the effect of intravenous vitamin C during surgery on the incidence of postoperative pulmonary complications (PPCs) in patients undergoing cardiopulmonary bypass and cardiac surgery was observed, and its protective effect on the lungs was evaluated to provide a reference for clinical medication.
Patients and Methods: Patients undergoing cardiac surgery under cardiopulmonary bypass (CPB) were selected. The patients were divided into group A and group C by random sequence. Patients in group A received intravenous vitamin C 1 g 10 minutes after induction of anesthesia, 10 minutes before cardiac reanimation and at the moment of sternal closure. Patients in group C were intravenously injected with the same volume of saline at the same time. The primary outcome was the postoperative pulmonary complication severity score. Other outcomes were the incidence of PPCs, awakening time, extubation time, length of ICU stay, length of hospital stay, adverse events, oxygenation index (PaO2/FiO2), alveolar arterial oxygen partial pressure difference (A-aDO2), dynamic lung compliance (Cd) and static lung compliance (Cs).
Results: Seventy patients completed the study. Compared to group C, the postoperative pulmonary complication score [2(2– 3) vs 2(1– 2); P=0.009] and the incidence of postoperative pulmonary complications (32.43% vs 12.12%; P =0.043) were lower in group A. There were no significant differences in awakening time, extubation time, length of ICU stay, length of hospital stay, adverse events, PaO2/FiO2, A-aDO2, Cs, and Cd between the two groups (P> 0.05).
Conclusion: In summary, this small randomized trial including low-risk cardiac surgery patients shows that intravenous vitamin C may safely be administered and may be helpful to prevent PPCs after cardiac surgery.
Keywords: cardiac surgery, cardiopulmonary bypass, postoperative pulmonary complications, vitamin C
Introduction
Some scholars define postoperative pulmonary complications (PPCs) as the comprehensive result of respiratory-related complications that occur in the hospital after surgery.1,2 PPCs not only increase the postoperative mortality of patients but also prolong the length of hospital stay and medical costs for patients.3 For decades, the development of cardiopulmonary bypass and surgical technologies for open-heart surgery has continued to benefit patients with cardiovascular disease, but surgical procedures, extracorporeal circulation, protamine application during surgery, mechanical ventilation, lungs undergoing ischemia-reperfusion, and systemic inflammatory reactions lead to varying degrees of pulmonary complications in patients after cardiac surgery. Faced with pulmonary complications after cardiac surgery, the current prevention and treatment methods have limited effects.
Vitamin C is the first-line antioxidant in the human body. It is widely used clinically because of its low price, wide range of uses, and high safety. Basic experiments have shown that vitamin C can reduce structural damage in the pulmonary blood vessels and alveoli of mice.4 Based on previous experiments, researchers have also tried to clinically verify the protective effect of vitamin C on the lungs. At present, clinical trials have confirmed that vitamin C can reduce ischemia-reperfusion injury (IRI) in isolated lung and stabilize the function of isolated lung.5 When the body undergoes ischemia-reperfusion, xanthine oxidase formation increases, neutrophil breathing bursts, mitochondrial function is impaired, and catecholamine auto-oxidation results in increased generation of oxygen radicals. Oxygen free radicals cause damage to cell membrane phospholipids, proteins, nucleic acids, and extracellular matrix. Among them, oxygen free radicals produced by neutrophils and xanthine oxidase are the main mediators of pulmonary IRI. Vitamin C can remove oxygen free radicals in the human body and can remove the reactive oxygen species (ROS) in the body through rapid electron transfer to achieve antioxidant functions. Therefore, vitamin C is expected to reduce pulmonary complications in patients undergoing cardiac surgery. The relationship between vitamin C and PPCs after cardiac surgery has never been the focus of research.
Patients and Methods
This was a prospective, double-blind, randomized controlled trial conducted between December 2018 and September 2019 in accordance with the principles of the Declaration of Helsinki. The study was registered in the American Clinical Trial Registry (NCT03756727) and was approved by the Ethics Committee of The Affiliated Hospital of Xuzhou Medical University (Jiangsu, China; XYFY (XYFY2019-KL003-01)). Written informed consent was obtained from the patients before surgery.
Participants
We included patients aged 18–65 years with American Society of Anesthesiologists (ASA) physical status II or III who were scheduled for cardiac surgery under CPB at Affiliated Hospital of Xuzhou Medical University. Patients were excluded for the following reasons: were scheduled for emergency surgery; had a history of cardiac surgery; were allergic to ascorbic acid; had gout, high oxalate urine, uric acid kidney stone, glucose-6-phosphate dehydrogenase deficiency, hemochromatosis, sickle cell anemia, iron myeloblastic anemia, or thalassemia; had a preoperative chest radiograph showing active lung disease; or had a pulmonary arterial systolic blood pressure >60 mmHg. Patients with operation time exceeding 6 hours, withdrawing informed consent, died of non-respiratory diseases during hospitalization are eliminated.
Randomization and Masking
Patients were assigned to receive vitamin C (group A) or saline (group C) in a 1:1 ratio. A random sequence was generated using a computer-generated randomization program and kept within sealed opaque envelopes by an assistant not involved in data collection. On the day of the surgery, the assistant opened the envelope, and prepared the drugs in identical syringes, which covered by opaque paper, drugs injection by the assistant. According to the group, patients in group A would receive vitamin C injection. Patients in group C received the same volume of saline at the same time. Patients, anesthesiologists, and personnel involved in drug formulation, data collector, and blood sample collector were not aware of the groupings. The groupings were announced after the test.
Anesthesia and Intervention
The patients fasted for 8 hours. After each patient entered the room, the venous access of the left upper limb was opened, the mask emitted oxygen, and the oxygen flow was 8 L/min. Electrocardiogram, pulse oxygen saturation, bispectral index, invasive blood pressure, and central venous pressure were monitored. Anesthesia induction involved intravenous injection of midazolam 0.05 mg/kg, etomidate 0.2–0.3 mg/kg, Sufentanil 7 μg/kg, rocuronium bromide 1 mg/kg. Mechanical ventilation was performed after tracheal intubation. The mechanical ventilation mode was volume control ventilation, tidal volume (Vt) 6–8 mL/kg, fraction of inspiration O2 (FiO2) 80–100%, inspiration and expiration ratio (I: E) 1:1.5, respiratory rate (RR) 10–12 times/minute, and end-tidal carbon dioxide partial pressure (PETCO2) was maintained at 35 mmHg ~ 45 mmHg. Anesthesia maintenance involved intravenous infusion of propofol 4–8 mg · kg−1 · h−1 and cis-atracurium 0.08–0.12 mg · kg−1 · h−1, maintained with a bispectral index between 40 and 60. During cardiopulmonary bypass, the mean arterial pressure was maintained at 50–60 mmHg, hematocrit 25% −30%, and blood glucose <200 mg/dl.
The drug was slowly injected by the assistant through a peripheral vein. Patients in group A were injected intravenously with vitamin C 1 g (diluted to 10 mL) with a total of 3 g 10 minutes after induction of anesthesia, 10 minutes before cardiac reanimation and at the moment of sternal closure. Patients in group C were injected intravenously with 10 mL of saline at the same time.
All patients were transferred to the ICU after surgery. Patients continued to receive respiratory and circulatory system support after entering the ICU. The mechanical ventilation mode was volume control ventilation with FiO2 of 40%. The other parameters were set the same as in the operation. After the patient’s breathing and circulation function were stabilized and the patient was conscious, could obey simple instructions (such as blinking, nodding), the muscle strength was restored, the breathing rate was >10 times/minute, the tracheal tube was removed if SpO2≥95% after 5 minutes of inhalation of air.
Observed Parameters
An independent trained anesthesiologist who was blinded to the assigned group checked all measurements. The observed parameters included postoperative pulmonary complication score (patient scoring once a day during the hospital stay, the highest score is the final PPC score); incidence of PPCs (we defined PPCs as PPC score ≥3); and types of disease; oxygenation index (PaO2/FiO2), alveolar arterial oxygen pressure difference (A-aDO2) at 10 minutes after intubation (T0), at the end of the surgery (T1), the first postoperative day (T2) and the third postoperative day (T3); pulmonary dynamic compliance (Cd), and pulmonary static compliance (Cs) at T0 and T1 (Most patients removed the tracheal tube on the night after surgery, so data of Cs Cd at T2 and T3 were not available). The patient’s time from operation to waking up, extubation time, length of ICU stay, length of hospital stay, and postoperative adverse events (nausea, vomiting, abdominal distention, constipation, headache, nightmares, insomnia, heart failure, infection, blood transfusion, atrial fibrillation, acute kidney injury, etc.) were recorded. (Diagnostic criteria for postoperative adverse events see Supplement 1)
The scoring scale (Supplement 2) has been used and continuously optimized since 1992.6–9 Postoperative pulmonary complications were scored on a scale of 0 to 5, with 0 representing no respiratory symptoms and signs, 1 to 4 representing a gradual increase in the severity of the complications, and 5 representing death before discharge.
Statistical Analysis
According to previous studies,6 the mean scores of postoperative pulmonary complications in the experimental and control groups were 1.8 and 2.1; that is, when the average score difference between the two groups was 0.3, the difference in scores between the two groups was statistically significant. It was determined that α = 0.05 and 1-β = 0.80. The sample size was calculated using PASS15.0 software for 68 cases, allowing for a 10% dropout rate. Ultimately, we estimated that 76 patients were needed.
Normally distributed variables were reported as the means ± standard deviations (means ± SDs) and were analyzed with independent sample t-tests and repeated measurements analysis of variance at different time points in each group. Nonnormally distributed data were reported as medians (interquartile ranges) and were analyzed with the Mann–Whitney U-test. The Friedman test was used to compare the different time points in the group. Categorical data were reported as frequencies (%) and were analyzed using the chi-squared test or Fisher’s exact test.
SPSS software (version 25.0; SPSS Inc., IBM, Chicago, IL, USA) was used to conduct all statistical analyses. All tests were two-sided, and P< 0.05 was considered to be statistically significant.
Results
A total of 76 patients were initially randomized. In group C, 1 patient was eliminated from the trial due to an operation time greater than 6 hours. Ultimately, 37 patients participated in the analysis of experimental data. In group A, 5 patients were eliminated, 2 patients withdrew their informed consent, 1 patient had surgery for more than 6 hours, 2 patients died of low cardiac output syndrome before leaving the hospital, 33 patients eventually participated in the analysis of experimental data (Figure 1). The demographic and perioperative characteristics of the two groups were similar, as shown in Table 1 (P> 0.05).
 |
Table 1 Demographic and Perioperative Characteristics by Group |
 |
Figure 1 CONSORT flowchart showing the number of patients at each phase of the study. |
The postoperative pulmonary complications data of the two groups of patients are shown in Figure 2 and Table 2. Compared to group C, the postoperative pulmonary complications score of group A was lower [2(2–3) vs 2(1–2); P= 0.009]. The incidence of postoperative pulmonary complications was also lower in group A (32.43% vs 12.12%; P= 0.043). The scores of patients in group C were mostly in the range of 2 to 3 (81.08%), while the scores of patients in group A were concentrated in the range of 1 to 2 (87.87%).
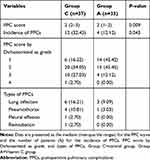 |
Table 2 PPCs of Patients |
 |
Figure 2 Severity of Postoperative Pulmonary Complications. Notes: Group C=control group, Group A=Vitamin C group. |
There were no significant differences in awakening time and extubation time, length of ICU stay and length of hospital stay between the two groups (Table 3). There was no significant difference in PaO2/FiO2, A-aDO2, Cs, and Cd between the two groups (P> 0.05). PaO2/FiO2 and A-aDO2 in the two groups decreased significantly from T1 to T3 compared to T0 (P <0.05). At T1, Cs and Cd in the two groups decreased significantly (P <0.05) (Figure 3).
 |
Table 3 Postoperative Outcomes and Complications of Patients |
There was no significant difference in the incidence of postoperative adverse events between the two groups. The highest incidence of postoperative adverse events was atrial fibrillation, with an incidence of 28.57%, followed by postoperative blood transfusion, and the incidence of adverse events in the digestive system was relatively higher (See Table 3). After follow-up for 30 days after discharge, neither group of patients died or were readmission.
Discussion
The results of this trial support the beneficial effects of intravenous injection of vitamin C 3 g during surgery, which can reduce the severity of PPCs and the incidence of PPCs and have no effect on adverse surgical events. Hence, Vitamin C may be beneficial for PPC in low-risk patients undergoing cardiac surgery. In clinical use, the actual effect of vitamin C is closely related to the route, timing and dose. Vitamin C is absorbed through the intestine after oral administration and can maintain an unbound state in the plasma. In general, a person taking 100 mg of vitamin C a day can maintain a plasma concentration of 60 μmol/L, and the vitamin C concentration in the tissue can reach saturation. When the intake dose is 1 g/d, the plasma concentration can reach saturation. The plasma concentration reached the highest level 2 h after oral ingestion.10 Therefore, the single dose was set to 1 g. Compared with oral administration, intravenous vitamin C reached its peak plasma concentration after injection, and an exponential decrease in plasma concentration was observed, with a drug half-life of approximately 1 h.11 Therefore, this experiment chose intravenous administration. The timing of drug administration should be settled before the patient undergoes ischemia-reperfusion, and at the same time, the times of drug administration and reperfusion should be close. Considering the effectiveness of the intervention method, to avoid the interaction of the drug in the infusion line as much as possible, the timing of drug administration in this study was 10 minutes after anesthesia induction, 10 minutes before the cardiac reanimation, and after the sternum closure. This not only ensured that the plasma vitamin C concentration was maintained at a high level before the occurrence of ischemia-reperfusion injury but also avoided the sequential use of multiple drugs during the induction of anesthesia and the cardiac rebound before interaction.
The incidence of pulmonary complications after cardiac surgery is 10–25%.10 Patients undergoing cardiovascular surgery can suffer from IRI during the operation. The final organ damage of patients is mostly related to IRI.12 During CPB, the lungs undergo IRI after ischemia and hypoxia-reperfusion, which also makes the incidence of pulmonary complications after cardiac surgery higher than in other types of surgery.2
The results of this study showed that patients who received vitamin C intraoperatively had lower PPCs scores and a lower incidence of PPCs than patients who did not. These findings indicate that vitamin C can prevent and reduce postoperative pulmonary complications in patients undergoing cardiac surgery, suggesting that vitamin C may play a protective role when the lungs undergo ischemia-reperfusion injury. The exact protective mechanism is not clear. Studies have shown that vitamin C is very active in transport between plasma and cells. Higher intracellular vitamin C concentrations can inhibit type 2A protein phosphatase and protect the endothelial cell barrier,13 which helps restore blood vessel responsiveness to vasoactive drugs,14 thereby improving microcirculation blood flow.15
There were no significant differences in awakening time, extubation time, length of ICU stay, and length of hospital stay between the two groups of patients. First, the patient did not continue to use vitamin C injection from the day of surgery to the time of discharge. Studies have shown that in patients undergoing stress reactions after surgery, the content of vitamin C in the body was decreased; thus, the demand for vitamin C after surgery increased accordingly, and the post-surgical requirement for patients with simple surgery is 500 mg/d. The demand for patients with complex surgery is correspondingly higher.16 Second, patients will continue to receive treatment in the ward after surgery; it is impossible to achieve a unified treatment of patients, which may also affect the outcome. In addition, the sample size is calculated based on the PPC score, which may not make the difference between these indicators statistically significant.
From the comparison of postoperative adverse events of patients, there was no statistically significant difference between the two groups in the observed adverse events. It can be considered that intravenous vitamin C will not increase the incidence of postoperative adverse events in this study. However, studies17,18 have shown that vitamin C can cause adverse reactions, which are rare. Among all the adverse events observed, the incidence of AF was the highest. Existing evidence indicating whether perioperative use of vitamin C can reduce the incidence of postoperative AF is still inconsistent.19–21 This difference in results may be due to different regimens of vitamin C.
In consideration of the safety of the intervention method, this study did not establish a larger dose test group, but used the intermediate dose of the instruction of 2 to 4 g/day, that is, 3 g/day.
Due to ethical constraints, this study did not use a larger experimental group and was not able to perform vitamin C supplementation over a longer period of time. Second, the vitamin C concentrations in the patients’ plasma were not monitored during the perioperative period; therefore, it could not be determined whether the patients had vitamin C deficiencies for a longer period after the operation. More research is needed to determine the optimal dose and timing of vitamin C during the perioperative period. Third, our conclusions are based on a relatively small sample size, so the number of patients recruited may not be sufficient to detect the effect of vitamin C on lung function and length of hospital stay. Fourth, this was a single-center study. In the populations in other regions, whether a single-day use of vitamin C can improve postoperative pulmonary complications remains to be determined. Therefore, more high-quality, multicenter, simple randomized trials are still needed to confirm these findings.
Conclusion
In summary, this small randomized trial including low-risk cardiac surgery patients shows that intravenous vitamin C may safely be administered and may be helpful prevent PPCs after cardiac surgery.
Data Sharing Statement
We will share all of the individual de-identified participant data that underlie the results reported in this article. Other study-related documents, including study protocol and statistical analysis plan, will be made available. Data are available indefinitely at https://doi.org/10.6084/m9.figshare.11986980.v1. Anyone who wishes to access the data can acquire them immediately following publication and no end date. For further data, proposals should be directed to [email protected] to Dr. Wang.
Acknowledgments
The authors thank Dr. Liu for his support in conducting this study and the colleagues of the group for their cooperation in data collection. This work was supported by the Department of Anesthesiology, Affiliated Hospital of Xuzhou Medical University. No commercial funding was received.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32(2):88–105. doi:10.1097/EJA.0000000000000118
2. Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–1350. doi:10.1097/ALN.0b013e3181fc6e0a
3. Mathis MR, Duggal NM, Likosky DS, et al. Intraoperative mechanical ventilation and postoperative pulmonary complications after cardiac surgery. Anesthesiology. 2019;131(5):1046–1062. doi:10.1097/ALN.0000000000002909
4. Koike K, Ishigami A, Sato Y, et al. Vitamin C prevents cigarette smoke-induced pulmonary emphysema in mice and provides pulmonary restoration. Am J Respir Cell Mol Biol. 2014;50:347–357. doi:10.1165/rcmb.2013-0121OC
5. Shaghaghi H, Kadlecek S, Siddiqui S, et al. Ascorbic acid prolongs the viability and stability of isolated perfused lungs: A mechanistic study using 31P and hyperpolarized 13C nuclear magnetic resonance. Free Radic Biol Med. 2015;89:62–71. doi:10.1016/j.freeradbiomed.2015.06.042
6. Costa Leme A, Hajjar LA, Volpe MS, et al. Effect of intensive vs moderate alveolar recruitment strategies added to lung-protective ventilation on postoperative pulmonary complications: a randomized clinical trial. JAMA. 2017;317(14):1422–1432. doi:10.1001/jama.2017.2297
7. Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437. doi:10.1056/NEJMoa1301082
8. Hulzebos EH, Helders PJ, Favié NJ, et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. 2006;296:1851–1857. doi:10.1001/jama.296.15.1851
9. Kroenke K, Lawrence VA, Theroux JF. Operative risk in patients with severe obstructive pulmonary disease. Arch Intern Med. 1992;152(5):967–971. doi:10.1001/archinte.1992.00400170057011
10. Hill A, Wendt S, Benstoem C, et al. Vitamin C to improve organ dysfunction in cardiac surgery patients-review and pragmatic approach. Nutrients. 2018;10(8):974. doi:10.3390/nu10080974
11. Levine M, Rumsey SC, Daruwala R, et al. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–1423. doi:10.1001/jama.281.15.1415
12. Desai M, Gurusamy KS, Ghanbari H, et al. Remote ischaemic preconditioning versus no remote ischaemic preconditioning for vascular and endovascular surgical procedures. Cochrane Database Syst Rev. 2011;12:CD008472.
13. Han M, Pendem S, Teh SL, et al. Ascorbate protects endothelial barrier function during septic insult: role of protein phosphatase type 2A. Free Radic Biol Med. 2010;48:128–135. doi:10.1016/j.freeradbiomed.2009.10.034
14. Tyml K. Vitamin C and microvascular dysfunction in systemic inflammation. Antioxidants (Basel). 2017;6(3):49.
15. Oudemans-van Straaten HM, Spoelstra-de Man AM. Vitamin C revisited. Crit Care. 2014;18(4):460. doi:10.1186/s13054-014-0460-x
16. Fukushima R. Vitamin C requirement in surgical patients. Curr Opin Clin Nutr Metab Care. 2010;13(6):669–676. doi:10.1097/MCO.0b013e32833e05bc
17. Spoelstra-de Man AME, Elbers PWG. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit Care. 2018;22:70. doi:10.1186/s13054-018-1996-y
18. Putzu A, Daems AM, Lopez-Delgado JC, et al. The effect of Vitamin C on clinical outcome in critically Ill patients: a systematic review with meta-analysis of randomized controlled trials. Crit Care Med. 2019;47:774–783. doi:10.1097/CCM.0000000000003700
19. Bjordahl PM, Helmer SD, Gosnell DJ, et al. Perioperative supplementation with ascorbic acid does not prevent atrial fibrillation in coronary artery bypass graft patients. Am J Surg. 2012;204(6):
20. Antonic M, Lipovec R, Gregorcic F, et al. Perioperative ascorbic acid supplementation does not reduce the incidence of postoperative atrial fibrillation in on-pump coronary artery bypass graft patients. J Cardiol. 2017;69:98–102. doi:10.1016/j.jjcc.2016.01.010
21. Dehghani MR, Madjidi N, Rahmani A, et al. Effect of oral vitamin C on atrial fibrillation development after isolated coronary artery bypass grafting surgery: a prospective randomized clinical trial. Cardiol J. 2014;21:492–499. doi:10.5603/CJ.a2013.0154
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

