Back to Journals » Research and Reports in Urology » Volume 12
Effect of Intracorporeal Human Adipose–Derived Stem Cells (hADSCs) on Corpora Cavernosa Transforming Growth Factor β1 (TGFβ1) and Collagen Type I Concentration in Wistar Rat Priapism Model
Authors Siregar S, Adriansjah R, Sibarani J , Mustafa A
Received 24 September 2019
Accepted for publication 30 December 2019
Published 5 February 2020 Volume 2020:12 Pages 21—27
DOI https://doi.org/10.2147/RRU.S232303
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Safendra Siregar, Ricky Adriansjah, Jupiter Sibarani, Akhmad Mustafa
Department of Urology, Faculty of Medicine, Universitas Padjadjaran, General Hospital Hasan Sadikin, Bandung, Indonesia
Correspondence: Safendra Siregar
Department of Urology, Faculty of Medicine, Universitas Padjadjaran, General Hospital Hasan Sadikin Bandung, 16 Jalan Makmur, Bandung, Indonesia
Tel +62 811 227 180
Email [email protected]
Akhmad Mustafa
Department of Urology, Faculty of Medicine, Universitas Padjadjaran, General Hospital Hasan Sadikin Bandung, 28A Jalan Cikutra Baru Raya, Bandung 40124, Indonesia
Tel +62 811 232 827
Email [email protected]
Introduction: The ischemic process in priapism can lead to displacement of normal tissue with fibrotic tissue, due to collagen deposition, and eventually leads to erectile dysfunction. Many studies have identified that the supernatant of adipose tissue–derived stem cells (ADSCs) significantly ameliorates fibrosis of different tissue, but limited attention has been paid to its efficacy on fibrosis of the corpora cavernosa.
Methods: A total of 22 Wistar rats divided into five groups, with two groups each consisting of five male wistar rats with priapism without human ADSC (hADSC) therapy (group I) and two other groups consisting of five rats with priapism, were given 10 6 cells’ intracorporeal hADSC injection after 12 hours of penile clamping (group II) were euthanized after 2 and 4 weeks of observation. The last group consisted of two rats without any treatment or model (group III). Following euthanasia, penises were harvested for TGFβ 1 and collagen type I measurement using ELISA. Statistical analysis using independent-sample t-tests was done with SPSS 21.0.
Results: Penile TGFβ 1 concentration in the treatment group was significantly lower in the second and fourth weeks of observation (p 2=0.004, p 4=0.003), and collagen type I was significantly lower in the second and fourth weeks (p 2=0.003, p 4=0.011).
Conclusion: Intracorporeal hADSC injection limited the fibrosis process in a priapism model. Although the mechanism was unclear, it may be related to the potential of hADSCs to produce various growth factors that could limit TGFβ 1 and collagen production.
Keywords: human adipose–derived stem cell, collagen type I, priapism, TGFβ 1
Introduction
Priapism is an emergency pathological condition where an erection lasts for more than 4 hours without any sexual stimulation.1,2 The ischemic process in priapism results from blood stasis due to vein occlusion which causes severe hypoxia and acidosis in the corpora cavernosa, leading to fibrosis and damage to the function of the corpora cavernous and permanent erectile dysfunction.3
Histologically, in 12 hours corpora specimens show various changes that eventually lead to normal cavernosal tissue replacement with fibrotic tissue.4,5 The mechanism of fibrotic tissue formation is mainly increased hypoxia-induced growth factors, such as TGFβ1. This theory was proven by Costa et al in 2009, where they analyzed a sample of corpora cavernosa tissue from a patient with history of priapism and compared it with the normal penis. Result of the study showed that there was an increase in collagen deposition and reduction in smooth-muscle components in the corpora cavernosa with a history of priapism.4
Various studies have been conducted to overcome fibrosis of the corpora cavernosa, with the aim of ameliorating erectile dysfunction, such as PDE5 inhibitors,pentoxifylline and eventually surgical treatment with a penile implant. Overall, these treatments focused only on restoration of erectile function, rather than prevention of fibrotic tissue formation.6,7 In recent years, many studies have shown the potential antifibrotic effect of adipose tissue–derived stem cells (ADSCs) in many animal models. ADSCs are mesenchymal SCs that exhibit tissue plasticity and fusiform morphology in vitro. ADSCs have been used as therapy for injured-organ regeneration in animal models and in a few clinical trials. Effects of ADSCs are attributed to their extensive secretomes, which include interleukins, various growth factors, and other proteins that are suitable inducers of tissue regeneration.8–11 Li et al showed that ADSC reduced collagen type I, type III, and α–smooth muscle actin (αSMA) in vitro.12 Spiekman found that ADSCs suppressed TGFβ1 expression and reduced dermal scar formation.13 With recent evidence of antifibrotic effects of ADSCs, this preliminary study aimed to evaluate the potential antifibrotic effect of human ADSCs (hADSCs) in an experimental model of priapism.
Methods
Human ADSC Isolation and Characterization
Human adipose tissue was obtained from adult male/female undergoing surgery who had previously signed an informed consent regarding donation of his/her fat for research. The protocol was approved by the Research and Ethical Committee of Universitas Airlangga Surabaya, which also approved the fat-procurement procedure. Cells were harvested and seeded until passage 3 to achieve greater expansion. Cell characterization and transplantation were conducted in this unique batch of cells obtained from one single fat donor in isolation. We used ADSCs from human adipose tissue for various reasons: the abundance of cells from liposuction aspirate (if we had used rat ADSCs, several rats would have had to be killed to get enough adipose tissue for ADSC isolation; and we aim to use human ADSCs in human patients in the future, so we used them in rats first, since human ADSCd will not express MHC class II and so not create a xenogenic immunologic reaction.
In isolation and culture procedures, cell vitality was 83.4%. Isolated cells were characterized by flow-cytometry assays using the markers CD44+, CD45–, CD73+, CD90+, and CD105+. A total of 22.5×106 cells were obtained and then added to αMEM in 1 mL syringes containing 106 cells for use. All tissue harvesting, cell isolation, culture, and characterization were done at the Tissue and Cell Bank of Dr Soetomo General Hospital Surabaya with sample number 33.E/MSC/Penelitian/AdiposeSwt_01/090519 and declared suitable for application.
Wistar Rat Priapism Model
Eighteen male Wistar rats (n=4/group and two rats for negative-control group) weighing 250–300 g were separated into five groups. All operations were performed under sterile conditions. The animals were anesthetized with a one-shot ketamine injection (75 mg/kg intraperitoneally). Priapism was accomplished as per Sanli et al.14 The tip of a 50 mL syringe was cut to accommodate the device at the base of the flaccid penis, so a vacuum erection device was created. Before application of the vacuum to the penis, a constriction band cut from 16 Fr Foley catheter in 1 mm slices was loaded around the tip of the vacuum erection device. Then, the tip of the syringe was placed on the base of the penis and withdrawn gently to induce erection. After induction of erection, the constriction band was placed on the base of the penis by slipping off the constrictor band from the syringe (Figure 1).
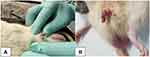 |
Figure 1 Normal rat penis before erection (A), erection induced by vacuum device and constrictor band (B). |
Two groups, each consisting of four male Wistar rats with priapism without hADSC therapy injected with 1 mL saline intracorporeally immediately after 12 hours of penile clamping, were euthanized after 2 and 4 weeks of observation (group I), while two other groups, each consisting of four rats with priapism, were given 106 cells intracorporeal hADSC injections after 12 hours of penile clamping, and immediately after the clamps were released were euthanized after 2 and 4 weeks of observation (group II). The last group consisted of two rats without any treatment or model (group III). After euthanasia, each rat underwent penectomy for TGFβ1 and collagen type I analysis. The tissue was processed and 1 g tissue extracted for TGFβ1 and collagen type I measurement. ELISA measurements were done (FineTest Rat Col1 ELISA kit range 0.156–10 ng/mL and FineTest TGFβ1 ELISA kit range 31.25–2,000 pg/mL). All animals used in this study were housed in solid plastic cages with four rats in each cage. The rats were allowed to eat standard rodent chow and water ad libitum. This study was conducted in the Biochemical and Bioscience Laboratory, Brawijaya University Malang, and was given ethical clearance by the ethical committee. All study subjects received treatment according to animal welfare guidelines as per Russel and Burch.15 For statistical analysis, values are expressed as means ± SD (ng/mL). Groups were compared with independent-sample t-tests. Values of p<0.05 were considered statistically significant. Statistical analysis was conducted using SPSS version 21.0.
Results
TGFβ1 Concentration
TGFβ1 examination using ELISA showed that there was a concentration difference between group I (control) and group II (hADSC treatment). TGFβ1 concentration was higher in the control group, than the treatment group in the second and fourth weeks of observation. Independent-sample t-tests were used to compare group I and group II at the time of observation, while group III was excluded from comparison and acted only as as tissue-concentration baseline. There were significant differences in TGFβ1 concentration between group I and group II in the second (p=0.004) and fourth weeks of observation (p=0.003). Though there was also a tendency toward TGFβ1-concentration increase in group II,progression was higher in group I (Table 1, Figures 2 and 3).
 |
Figure 2 TGFβ1 standard-error chart: second and fourth weeks of observation. |
 |
Figure 3 TGFβ1-concentration pattern. |
 |
Table 1 Comparison of TGFβ1 Concentrations |
Collagen Type I Concentration
Collagen type I examination using ELISA showed that the collagen type I concentrations were higher in group I than group II in the second and fourth weeks of observation. On independent-sample t-tests, there were significant differences in collagen type I concentration between group I and group II in the second (p=0.003) and fourth weeks of observation (p=0.011; Table 2 and Figures 4 and 5).
 |
Figure 4 Collagen type I standard-error chart: second and fourth weeks of observation. |
 |
Figure 5 Collagen type I–concentration pattern. |
 |
Table 2 Comparison of Collagen Type I Concentration |
Based on Table 2 and Figures 4 and 5,it can be seen that the largest data variation was at 2 weeks for the control, because it had the highest average compared to 2 weeks' therapy.
Discussion
Fibrosis is the end result of various chronic diseases, such as chronic kidney disease, heart failure, hepatic cirrhosis, and various chronic processes in the urogenital organs. In fibrotic tissue, fibroblasts will differentiate into myofibroblasts and produce large amounts of extracellular matrix, characterized by increased collagen type I and type III concentrations. Mesenchymal SCs have successfully prevented the growth of fibrotic tissue in experimental and preclinical studies.16 Mesenchymal SC transplantation is also able to inhibit the formation of fibrotic tissue and to restore the functions of previously disturbed organs, such as the heart, liver, and lungs.12,13,17
This research was conducted in vivo on experimental animals — Wistar rats. The Wistar rat was chosen in this study due to the similarities of its penis structure to humans.18 An increase in TGFβ1 concentration was observed over time in the priapism control group. In the hADSC-therapy group, TGFβ1 concentrations also tended to increase, especially at the second and fourth weeks of therapy. However, the increase in TGFβ1 concentrations in hADSCs therapy group was lower than the priapism control group. This result is in accordance with research conducted by Spiekman, where in vitro hADSC reduced TGFβ1 concentrations and extracellular matrix deposition in petri dishes.13 This research also showed that hADSCs were able to restore fibroblast proliferation to its original level, even after stimulation by TGFβ1. Rivera-Valdes et al in 2017 showed that human ADSC therapy in a renal failure model in Wistar rats significantly decreased renal fibrosis, improved creatinine urea levels, and reduced collagen type I and TGFβ1 levels in the kidney.19,20 This was most likely caused by various secretomes produced by ADSCs, such as VEGF, HGF, GM-CSF, bFGF, BDNF, and IGF1, and various interleukins, such as IL6, IL7, IL8, IL11, and IL10, which contribute to improvement of the tissue microenvironment, but the exact mechanism of how hADSCs inhibit TGFβ1 is still unclear.21,22
This study also found an increase in type I collagen concentration over time in the priapism control group. In the hADSC-therapy group, the concentration of type I collagen also tended to increase at the second and fourth weeks of therapy, but when compared with the control group, the concentration of type I collagen in the human ADSC-therapy group was lower than the control group. This is in accordance with by Li et al, who showed inhibition of collagen I, collagen type III, and αSMA expression in hypertrophic wound tissue in vitro. In addition, ADSC scan also suppress collagen deposition in the matrix of the tissue, thus correcting the fibrosis of the cicatrices ex vivo. The study also showed the antifibrotic effect of ADSC by regulating the p38–MAPK pathway, thereby decreasing type I and type III collagen expression and correcting fibrotic tissue.13
Another finding of this study is that an increase in TGFβ1 and type I collagen concentrations over time in the second and fourth weeks of monitoring was observed. This showed that the effect of hADSC administration at the beginning of the treatment did not prevent the progression of the ongoing fibrosis. This finding was not demonstrated by previous studies that examined the antifibrotic effect of hADSCs in other experimental animals, as they only assessed the effect of ADSCs in a single observation.22–25 Increased collagen deposition in both the control and the hADSC-therapy group is thought to have been caused by the increase in TGFβ1 concentrations in the second and fourth weeks of monitoring. With the presence of TGFβ1, molecular signals for fibroblast differentiation and fibroblast collagen production will continue to exist. The main issue of this finding is the progressively increasing concentrations of TGFβ1 in the HADSC-therapy group in the fourth week of monitoring. Several possibilities may have caused this problem, such as penile tissue damage, including blood vessels that supply the tissue of the penis in the process of priapism modeling, due to mechanical pressure caused by the rubber applied at the base of the penis. With reduced blood flow, the potency of tissue repair by ADSCs to the implanted penis tissue might have been decreased due to reduced oxygen supply. Another thing that will have to be considered is the necessity for periodic ADSC therapy with optimal dosages to maintain the microenvironment, in order to optimize prevention of fibrosis. In addition, a limitation of this study was a lack of assessment of collagen type III and αSMA), which might be useful in further characterizing fibrotic tissue composition and inhibition by hADSCs. TGF and collagen tissue assessment using PCR and Western blot would also be superior to ELISA to detect tissue gene expression. Another limitation in this study was that we did not evaluate histopathological changes in the corpora cavernosa or functional changes that might have contributed greatly to the study results.
Although an ability to repair damaged tissue without the formation of scar tissue is thought to be ideal, in most chronic inflammatory diseases the regeneration process cannot be completed by merely parenchymal cell regeneration, even in tissue with fast regeneration capability. The damaged tissue must be repaired by replacing parenchymal cells with scar tissue, eventually causing significant fibrosis. As such, ADSCs are an interesting alternative to prevent the process of fibrosis in various tissue injuries in general, and offer possibilities for the prevention of erectile dysfunction due to cavernous corpora fibrosis in priapism. However, further research is needed.
Conclusion
Intracorporeal hADSC administration decreased TGFβ1 and collagen type I concentration in a low-flow priapism model. This is possibly an interesting novel treatment for fibrotic tissue prevention in low-flow priapism. However, further research should be done to investigate the exact mechanism of fibrosis prevention in cavernosa tissue by hADSCs and suitable routes and timing of administration of hADSCs before we could use them safely in human subjects.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34:631–642. doi:10.1016/j.ucl.2007.08.006
2. Broderick GA. Campbell-Walsh urology. In: Ch 28: Priapism.
3. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116–120. doi:10.1111/j.1743-6109.2004.10117.x
4. Costa WS, Felix B, Cavalcanti AG, Medeiros J
5. Munarriz R, Park K, Huang YH, et al. Reperfusion of ischemic corporal tissue: physiologic and biochemical changes in an animal model of ischemic priapism. Urology. 2003;62:760–764. doi:10.1016/S0090-4295(03)00484-9
6. Gonzalez-Cadavid NF, Rajfer J. Treatment of Peyronie’s disease with PDE5 inhibitors: an antifibrotic strategy. Nat Rev Urol. 2010;7:215–221. doi:10.1038/nrurol.2010.24
7. Zahran AR, Daiem HA, Youssif M. Does pentoxifylline enhance the recovery of erectile function after a T-shunt procedure for prolonged ischaemic priapism? A prospective randomised controlled trial. Arab J Urol. 2012;10:425–428. doi:10.1016/j.aju.2012.05.006
8. Alexeev V, Arita M, Donahue A, Bonaldo P, Chu M-L, Igoucheva O. Human adipose-derived stem cell transplantation as a potential therapy for collagen VI-related congenital muscular dystrophy. Stem Cell Res Ther. 2014;5:21. doi:10.1186/scrt411
9. Harn H-J, Lin S-Z, Hung S-H, et al. Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function. Cell Transplant. 2012;21:2753–2764. doi:10.3727/096368912X652959
10. Kamada Y, Yoshida Y, Saji Y, et al. Transplantation of basic fibroblast growth factor-pretreated adipose tissue-derived stromal cells enhances regression of liver fibrosis in mice. AJP Gastrointest Liver Physiol. 2008;296:G157–G167. doi:10.1152/ajpgi.90463.2008
11. Seki A, Sakai Y, Komura T, et al. Adipose tissue-derived stem cells as a regenerative therapy for a mouse steatohepatitis-induced cirrhosis model: Seki, Sakai, et al. Hepatology. 2013;58:1133–1142. doi:10.1002/hep.26470
12. Li Y, Zhang W, Gao J, Liu J, Wang H, Li J. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther. 2016;7:102. doi:10.1186/s13287-016-0356-6
13. Spiekman M. Human Adipose Tissue-Derived Stromal Cells Suppress TGF-Β Induced Fibroblast Differentiation of Human Dermal Fibroblasts: Implications for Scar Formation. Cardiovascular Regenerative Medicine group Department of Pathology and Medical Biology University Medical Centre Groningen; 2014.
14. Sanli O, Armagan A, Kandirali E, et al. TGF-beta1 neutralizing antibodies decrease the fibrotic effects of ischemic priapism. Int J Impot Res. 2004;16(6):492–497. doi:10.1038/sj.ijir.3901261
15. Russell WMS, Burch RL. The Prin - Ciples of Humane Experimental Technique. London, UK: Methuen; 1959:238.
16. Arno AI, Amini-Nik S, Blit PH, et al. Effect of human Wharton’s jelly mesenchymal stem cell paracrine signaling on keloid fibroblasts. Stem Cells Trans Med. 2014;3:299–307. doi:10.5966/sctm.2013-0120
17. Lam MT, Nauta A, Meyer NP, Wu JC, Longaker MT. Effective delivery of stem cells using an extracellular matrix patch results in increased cell survival and proliferation and reduced scarring in skin wound healing. Tissue Eng Part A. 2013;19:738–747. doi:10.1089/ten.tea.2012.0480
18. Goswami S, Inamdar M, Pandre M, Jamwal R, Dethe S. Erectogenic and aphrodisiac effects of butea frondosa Koenig ex Roxb. In Rats: Involvement of Enzyme Inhibition. Evidence-Based Complementary and Alternative Medicine: eCAM. 2013;(2013:874894. doi:10.1155/2013/874894
19. Rivera-Valde´s JJ, Garcı ´a-bañuelos J, Salazar-Montes A, et al. Human adipose derived stem cells regress fibrosis in a chronic renal fibrotic model induced by adenine. PLoS One. 2017;12(12):e0187907. doi:10.1371/journal.pone.0187907
20. Zuk P. Adipose-derived stem cells in tissue regeneration: a review. Int Scholarly Res Notices. 2013. doi:10.1155/2013/713959
21. Salgado AJBOG, Reis RLG, Sousa NJC, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther.2010;5:103–110. doi:10.2174/157488810791268564
22. Castiglione F, Hedlund P, Van der Aa F, et al. Intratunical injection of human adipose tissue–derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of peyronie’s disease. Eur Urol. 2013;63(3):551–560. doi:10.1016/j.eururo.2012.09.034
23. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells.2007;25:2739–2749. doi:10.1634/stemcells.2007-0197
24. Huang W, Wang T, Zhang D, et al. Mesenchymal stem cells overexpressing CXCR4 attenuate remodeling of postmyocardial infarction by releasing matrix metalloproteinase-9. Stem Cells Dev. 2012;21:778–789. doi:10.1089/scd.2011.0126
25. Harn HJ, Lin SZ, Hung SH, et al. Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function. Cell Transplant. 2012;21:2753–2764. doi:10.3727/096368912X652959
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
