Back to Journals » Clinical Interventions in Aging » Volume 15
Effect of HFE Gene Mutation on Changes in Iron Metabolism Induced by Nordic Walking in Elderly Women
Authors Kortas J , Ziemann E , Antosiewicz J
Received 5 March 2020
Accepted for publication 16 April 2020
Published 13 May 2020 Volume 2020:15 Pages 663—671
DOI https://doi.org/10.2147/CIA.S252661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Walker
Jakub Kortas,1 Ewa Ziemann,2 Jedrzej Antosiewicz3
1Department of Sport, Gdansk University of Physical Education and Sport, Gdansk, Poland; 2Department of Athletics, Strength and Conditioning, Poznan University of Physical Education, Poznan, Poland; 3Department of Bioenergetics and Physiology of Exercise, Medical University of Gdansk, Gdansk, Poland
Correspondence: Jakub Kortas
Department of Sport, Gdansk University of Physical Education and Sport, Kazimierza Górskiego 1, Gdansk 80-336, Poland
Email [email protected]
Jedrzej Antosiewicz
Department of Bioenergetics and Physiology of Exercise, Medical University of Gdansk, Marii Skłodowskiej-Curie 3a, Gdansk 80-210, Poland
Email [email protected]
Background: Excess iron accumulation in human tissue is associated with the diet, lack of exercise, or genetic factors. Iron accumulation increases the risk of acute myocardial infarction, diabetes, and cancer. On the other hand, exercise reduces the risk of several morbidities and influences iron metabolism. Here, we evaluated changes in iron metabolism induced by exercise in elderly women bearing the H63A HFE mutation.
Purpose: To identify a factor that modulates the effect of exercise on iron metabolism. We investigated whether regular exercise induces similar changes in iron metabolism, mainly manifested by lowered body iron stores, in individuals bearing the wild-type (WT) and mutated HFE gene.
Subjects and Methods: Seventy-six women (average age 69.2± 5.6 years old) were enrolled in the study. Thirty-nine women participated in 12 weeks of Nordic walking (NW) training; the remaining participants were assigned to the control group. Based on the H63A HFE mutation status, the NW group was divided into women bearing the mutation (HET, n=12) and women with the WT gene (WT, n=27).
Results: The training resulted in a statistically significant reduction in the serum iron (p=0.03) and ferritin levels (p=0.001); hepcidin levels remained unchanged. No differences in these parameters were noted between the HET and WT groups.
Conclusion: These observations suggest that a reduction in body iron stores might constitute an important aspect of the health-promoting effect of exercise, regardless of the genetic background.
Keywords: physical activity, ferritin, inflammation, glucose
Introduction
Regular exercise induces several adaptive changes in the body, including improvements in the skeletal muscle energy metabolism and an increase in VO2 max.1,2 In addition, exercise reduces low-grade systemic inflammation and brings about many other positive changes, leading to a healthier and longer life.3
One of the major changes induced by exercise is related to iron metabolism. According to several reports, exercise up-regulates hepcidin biosynthesis and increases hepcidin blood levels in athletes.4 Hepcidin inhibits intestinal iron absorption and reduces its liberation from body stores, eg, the liver and macrophages.5 However, the biological significance of increased blood hepcidin levels after exercise is not fully understood. Strenuous exercise can increase the levels of proinflammatory cytokines in the blood and these can up-regulate hepcidin biosynthesis.6,7 Increased blood hepcidin levels lead to a decrease in serum iron levels, as hepcidin blocks iron release from cells, such as enterocytes, hepatocytes, and macrophages, to the blood.8 Hence, reduction of blood iron levels can help to limit the inflammation induced by exercise.9 In addition, interleukin 6 (IL-6) up-regulates hepcidin and anti-inflammatory cytokine levels, eg, IL-10 and IL-1 receptor antagonist (IL-1RA) levels.6,10
By contrast, data on the effects of regular exercise on iron metabolism in the elderly are scarce. Some available data indicate that regular exercise reduces body iron stores, as evaluated on the basis of changes in blood ferritin levels.11 High blood ferritin levels and body iron stores are positively correlated with a risk of morbidities, such as cancer, heart disease, and diabetes.12 For example, high serum ferritin has been associated with elevated glucose levels and insulin resistance.13 In addition, studies in cell culture models demonstrate that high ferritin H levels inhibit insulin signaling. Of note, aging is associated with insulin resistance and iron accumulation. Hence, understanding the effect of exercise on iron metabolism is crucial for recognizing its heath-promoting effects.
The human hemochromatosis protein (HFE) transmits signals from transferrin receptor 2 to the nucleus to regulate the synthesis of hepcidin. H63D HFE and C282Y HFE are the most common HFE mutations. In northern Europe, the H63D HFE mutation occurs in 10–29% of the population, most of whom are heterozygotes.14 Subjects heterozygous for H63D HFE generally exhibit elevated serum iron indices, but the risk of developing iron overload is low.15,16 In general, defective hepcidin synthesis in the liver is postulated to be the central reason for excess iron accumulation.17
Based on the above, we asked whether regular exercise would induce similar changes in iron metabolism, mainly manifested as reduced body iron stores, in individuals bearing the wild-type (WT) and mutated HFE gene. We hypothesized that the decrease in serum ferritin levels in subjects bearing HFE mutations in response to training would be smaller than that in subjects with WT HFE.
Subjects and Methods
Ethics Statement
The study was designed as a randomized control trial. Participants who completed the baseline visit were randomly assigned (1:1) to two groups: the non-training (control) group or the training (Nordic walking, NW) group. Laboratory analyses were conducted at the Medical University of Gdansk (Gdansk, Poland). Physical assessments and training were conducted at the Gdansk University of Physical Education (Gdansk, Poland). The study was officially approved by the Bioethical Committee of the Regional Medical Society in Gdansk; performed in agreement with the Declaration of Helsinki (KB-34/18); and registered as a Clinical Trial NCT03417700. The experiment was verbally described to the subjects before the onset of training and testing. Written informed consent was obtained from all participants prior to the study.
Subjects
After announcements in the local media, press, and in public places in the tri-city area of Gdansk, Gdynia, and Sopot (Poland), 109 elderly women applied to participate in the study (Figure 1). Qualifying tests for the study included standard medical examination of the post-menopausal status. Women with uncontrolled hypertension (diastolic blood pressure over 100 mmHg), a history of cardiac arrhythmia, cardiorespiratory disorders, and orthopedic problems were excluded from the study. Nineteen women did not meet the inclusion criteria. Individuals who passed the initial screening were assigned a number corresponding to the project application number. The participants were then randomly divided into two groups: NW group (n=45) or the control group (n=45). The individuals in the control group were advised to observe their typical diet and physical activity. These individuals declared that they do not exercise regularly. The randomization for experimental group assignment was performed using an online randomization tool (GraphPad QuickCalcs software, https://www.graphpad.com/quickcalcs/randomize1/), using the base of the application number. This allowed for the same number of participants to be assigned to the two groups.9 During the 12 weeks of the study, 14 women were lost to follow-up. The final participant set included 76 women with an average age of 69.2±5.6-years-old.
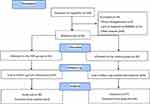 |
Figure 1 Flow diagram of the study.18 |
Experimental Design
Thirty-nine women took part in the training program, 12 of which were heterozygous for H63D HFE. All participants completed at least 80% training units (minimum 29). The 12-week NW training program was conducted based on the authors’ previous training experience with the elderly,19,20 with slight modifications. Specifically, compared with previous studies, the educational aspect of the training was expanded.
The participants met three times a week, 1 h after having a light breakfast, and performed the main session of NW training (10-min warm-up, 45–55-min NW, and 10-min cool-down) at 60–70% intensity of their maximum heart rate. Once a week, each participant received a sport-tester device used for current cardiovascular control (Polar Team2 equipment). The 12 weeks of exercise (36 training units) were divided into three microcycles. During the first microcycle (6 training units), the basic functional efficiency increased, especially chest mobility, and flexibility of the arms and shoulders. Proper walking technique with poles was demonstrated and practiced during that period. The second microcycle (24 training units) was an essential component of the program. Its aim was to improve endurance. It was implemented by a gradual increase in volume (expressed in km walked), which was inherent to increasing the training intensity. In addition, strength and postural muscle-strengthening exercises were performed. The closing microcycle (6 training units) was an attempt to raise the endurance level by intensifying the activity and walking at the fastest possible pace. Further strength exercises, mainly of the back muscles, were performed.
The control group (37 women) was tested twice: at the beginning of the experiment and after 12 weeks of training. Women from this group did not to participate in health training.
The participants were briefed 2 weeks before the experiment, to introduce the experimental procedure and draw attention to their dietary habits, focusing on the source of iron in daily diet. It was recommended that the participants do not change their diet during the experiment. A survey was performed to verify that there were no vegetarians or vegans among the participants, which would have excluded them from the study.
Anthropometric Measurements
Body mass and body composition were determined using a multi-frequency impedance plethysmograph body composition analyzer (In Body 720, Biospace, Korea). Body mass was determined after an overnight fasting, 12 h after any meal and drink. Impedance of segments of the body parts (the trunk, arms, and legs) was measured at six different frequencies (1, 5, 50, 250, 500, and 1000 kHz) using eight-polar tactile-electrode.13
Physical Fitness Measurements
Senior Fitness Test developed by Rikli and Jones,21 which is dedicated to examining the elderly, was used to determine the functional fitness of participants. The test was conducted twice: after the recruitment and after 12 weeks of training. It consists of six items: (1) 30-s chair stand; (2) arm curl; (3) chair sit-and-reach; (4) back scratch; (5) 8-foot up-and-go; and (6) 2-min step. The tests were performed in this order, with 1-min rest between them. Before each test, the evaluator demonstrated the exercise and the participant had an attempt at familiarization, except for the 2-min step test, which the subjects performed only once.
Blood Analysis
At baseline and 1 d immediately after the 12-week training program, blood samples (5 mL) were obtained from the participants, between 07:00 and 08:00 h, following an overnight fast. The serum was obtained by sample centrifugation at 10,009×g for 15 min and stored at –80 °C pending analysis. Red Blood Cells values were corrected by hematocrit values.
Hepcidin levels were determined by enzyme immunoassays using commercial kits (R&D Systems; catalogue nos. DCRP00, Cloud-Clone SEB979Hu, and SEB995Hu). The average intra-assay CV was <10% for all assessments. Glucose levels were determined by using Cobos 6000 analyzer. Serum ferritin levels were determined by using SYSMEX XE 2100, Architect ci 8200, and Test 1 SDL.
Genetic testing for the presence of H63D, S65C, and C282Y HFE was performed using real-time polymerase chain reaction.
Statistical Analysis
Statistical analysis was performed using Statistica 13.1 software. All values are expressed as the mean±standard deviation (SD). Shapiro–Wilk test was used to assess the homogeneity of dispersion from normal distribution. Brown–Forsythe test was used to evaluate the homogeneity of variance. First, baseline differences between groups were evaluated. For a homogenous sample, a paired t-test analysis was performed to identify significantly different results. For a heterogeneous sample, Wilcoxon signed-rank test was used. Further, the effect of 12-week NW training compared with the control group was assessed. For a homogenous sample, the analysis of variance (ANOVA) for repeated measures and a post hoc Tukey’s test for unequal sample sizes were performed to identify significantly different results. For a heterogeneous sample, ANOVA Friedman test and Dunn–Bonferroni post hoc test were used. In addition, analysis of covariance (ANCOVA) was performed to adjust for the between-group effects for potential differences in RBC levels.22 Thereby, RBC levels were included as covariates to adjust for the potential baseline differences in the iron, ferritin, and hepcidin levels. The effect size (partial eta squared, 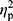 ) was also calculated, with
) was also calculated, with 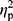 ≥0.01 indicating a small effect; ≥ 0.059 indicating a medium effect; and ≥0.138 indicating a large effect.23 The significance level was set at p<0.05. The relationships between variables were evaluated using the Spearman correlation coefficient.
≥0.01 indicating a small effect; ≥ 0.059 indicating a medium effect; and ≥0.138 indicating a large effect.23 The significance level was set at p<0.05. The relationships between variables were evaluated using the Spearman correlation coefficient.
Results
Effectiveness of the Training on Functional Level
No differences between the study groups in terms of anthropometric (Table 1), hematological (Table 1), and functional fitness variables (Table 2) were apparent. No participants exhibited any signs of inflammation.
 |
Table 1 Anthropometric and Hematological Characteristic of the Study Participants |
 |
Table 2 Effect of 12 Weeks of NW Training on Physical Fitness |
Participation in the NW training did not change the body composition (∆Weight=1%; ∆Fat=2%; ∆SMM=0%; ∆FFM=0%; ∆TBW=1%). These observations were in agreement with earlier studies.24,25 After 12 weeks of training, a significant improvement in the physical fitness of subjects was noted (Table 2). The strength of the leg (p=0.00) and arm muscles (p=0.02) was improved. The endurance (p=0.00) and flexibility (p=0.00) were increased. No differences between the genotype groups were apparent (0.08<p<0.90).
General Effect of Training on Iron Metabolism
The aim of the study was to determine whether the NW training induced changes in iron metabolism and whether the changes were the same in elderly women with different HFE genotype. NW resulted in a statistically significant drop in the serum levels of iron and ferritin, while the levels of hepcidin remained unchanged (Figure 2). A small inter-group effect in favor of NW was observed in terms of changes in the ferritin levels (p=0.04, 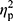 =0.06). Multiple pairwise post hoc t-test analysis revealed significant differences in the individuals pre- and post-testing (0.001<p<0.03). These changes were not associated with the RBC levels, which remained unchanged after the training (4.67±0.38 vs 4.68±0.30, p=0.70). Further, no correlation between changes in RBC and hepcidin levels was noted (r=–0.05, p=0.77).
=0.06). Multiple pairwise post hoc t-test analysis revealed significant differences in the individuals pre- and post-testing (0.001<p<0.03). These changes were not associated with the RBC levels, which remained unchanged after the training (4.67±0.38 vs 4.68±0.30, p=0.70). Further, no correlation between changes in RBC and hepcidin levels was noted (r=–0.05, p=0.77).
Analysis of hepcidin levels accounting for the baseline iron (p=0.28, 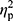 =0.02), ferritin (p=0.36,
=0.02), ferritin (p=0.36, 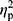 =0.01), and RBC levels (p=0.32,
=0.01), and RBC levels (p=0.32, 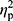 =0.02) did not reveal significant differences between groups. In other words, hepcidin levels were unchanged after adjusting for baseline differences.
=0.02) did not reveal significant differences between groups. In other words, hepcidin levels were unchanged after adjusting for baseline differences.
Next, we evaluated whether changes in the serum iron concentration were associated with inflammation. No significant changes in inflammatory markers were apparent. Changes in the levels of C reactive protein (in the range of 0.2–0.7 pg/mL) and IL-6 (in the range of 0.1–0.6 pg/mL) were similar in both genotype groups.
Subgroup Analysis of Genotype
Next, we analyzed the subjects representing the two genotype subgroups, WT and HET. No differences were noted between the HET and WT groups (Figure 3). Changes in iron, ferritin, and hepcidin levels were similar in individuals representing both genotypes. Further, the changes in ferritin levels after 12 weeks of training were statistically significant in both genotype groups (WT: 101.47±67.75 vs 82.23±55.77, p=0.002; HET: 103.34±42.70 vs 79.94±30.03, p=0.01).
Subgroup Analysis of Changes in Ferritin Levels
Considering the changes in serum ferritin levels of all participants, a decrease in serum iron levels after 12 weeks (by 11 μg/dL, 12%, p=0.07) was observed only in the subgroup with decreased ferritin levels (n=59). In the subgroup with increased ferritin levels (n=17), serum iron levels did not change. Hepcidin levels were constant in both subgroups (p=0.99).
Subgroup Analysis of Baseline Ferritin Levels
Baseline serum iron concentration in subjects with low ferritin levels (n=47) (cut-off of 100 ng/mL) was significantly lower (p=0.04) than that in subjects with high ferritin levels (baseline concentration exceeding 100 ng/mL) (n=15). A greater decrease in serum iron levels was observed in women with high ferritin baseline levels than in women with low ferritin (13 μg/dL, 14%, p=0.14 vs 5 μg/dL, 6%, p=0.75, respectively). Hepcidin levels were constant in both subgroups (p=0.99).
For a general overview of the changes in various parameters, we evaluated the correlations between the variables studied. As noted previously,19 the glucose and ferritin levels were correlated (r=0.32, p=0.005, CI: 0.10–0.52). However, we observed that these changes were associated with the HFE genotype (Figure 4), but the correlation was statistically significant only in the WT group [WT: r=0.39, p=0.002, CI= 0.1–0.6; HET: r=0.18, p=0.47, CI: (–0.3)–0.6].
 |
Figure 4 Correlation between glucose and ferritin levels at baseline in WT and HET groups. |
Discussion
We have previously shown that NW training leads to some adaptive changes in elderly women, as manifested by increased physical performance, decreased blood cholesterol levels, and reduced body iron stores.19,25,26 In the present study, we confirmed that NW induces a reduction in serum ferritin levels, which is a good marker of body iron stores in the absence of inflammation.27 Detailed analysis of the general significant decrease of serum ferritin levels indicated that some subjects did not respond to the training, albeit the reason for this was not known. Hence, the main aim of the present study was to determine whether NW training induced the same changes in iron metabolism in elderly women heterozygous for the H63D HFE gene and in women homozygous for the WT gene.
In the current study, changes in the iron stores induced by regular exercise were not manifest in all subjects, and a minor proportion of subjects were classified as non-responders. Some individuals heterozygous for the H63D HFE gene are characterized by elevated serum iron levels; however, only a small percentage of them excessively accumulate iron.28 Conversely, a study involving elderly subjects demonstrated that 12.9% of participants over-accumulated stored iron, while only 2.7% were iron-deficient.29 This and many other studies strongly indicate that aging is associated with iron accumulation in the body. Therefore, understanding why in some individuals training does not modulate body iron stores may be important for defining the health-promoting outcomes of exercise. The reason for this phenomenon is not known, but certainly, genetic factors might contribute to excessive iron accumulation in tissues. Recently, using animal model and cell cultures, we demonstrated that impaired insulin signaling leads to iron accumulation in the skeletal muscle tissue.30 It cannot be excluded that regular exercise, which is known to increase insulin sensitivity, may impede aging-associated iron accumulation in tissues. High iron stores or serum ferritin levels are correlated with the risk of heart attack, cancer, and diabetes.12 Hence, reduction of serum ferritin levels induced by NW training should be considered as one possible mechanism of the beneficial effects of exercise.
In addition, improved physical performance after training was observed in the current study, suggesting some positive changes in skeletal muscle metabolism. Many mitochondrial proteins, myoglobin, and others whose synthesis is induced by training contain iron. We did not observe changes in hemoglobin and RBC values after the NW training; however, based on the measurements, we cannot exclude the possibility that the total hemoglobin content in circulation increased. Indeed, several studies demonstrated increased erythropoiesis after training.31 Hence, the NW-induced drop in body iron stores can be a result of adaptive changes in the skeletal muscle and increased erythropoiesis.
Interestingly, in the current study, the correlation between serum ferritin and fasting blood glucose levels was observed before and after 12 weeks of NW training. These data support an earlier observation that higher blood ferritin levels are associated with a higher risk of insulin resistance and elevated blood glucose levels.32,33 However, in the current study, the observed decrease in serum ferritin levels was not associated with decreased blood glucose.
Regular exercise might decrease serum iron levels by increasing hepcidin biosynthesis, which could limit intestinal iron accumulation, and iron release into the blood by the liver and other tissues. A substantial number of studies demonstrate that a single bout of exercise increases serum hepcidin levels,34 but the data on the effects of regular exercise are scarce.20 We here observed that NW training did not induce significant changes in serum hepcidin levels despite a significant reduction in serum iron levels. These observations indicate that factors other than serum iron contribute to the regulation of hepcidin biosynthesis after NW training.35
Further, we observed that serum iron levels dropped significantly after 12 weeks of training, suggesting hepcidin activity. Of note, there was no significant difference in serum iron levels in subjects bearing H63D HFE and WT HFE. These observations contrast with those of previous studies showing that heterozygotes may have higher serum iron levels than the WT. High serum iron levels are associated with an increased risk of cancer and some other diseases.36 This is likely because of an increased iron-dependent formation of reactive oxygen species. Hence, lowering serum iron levels by regular training should be considered as a positive health-promoting measure.
In conclusion, the presented data strongly indicate that regular exercise significantly lowers body iron stores and blood iron levels, which could be considered as a health-promoting effect of exercise. The observed changes in iron metabolism were not related to the genetic background. Studies of professional athletes show that a drop in iron stores may be related to intestinal bleeding upon overtraining and stress.37 Hence, the original hypothesis that individuals bearing mutations in the HFE gene would not respond to NW training by lowering serum iron and ferritin levels was not confirmed. However, a study with a higher number of participants is needed to determine the effect of exercise on serum iron levels.
Data Sharing Statement
All individual deidentified participant data are available for the next 5 years on reasonable request from the first author Jakub Kortas (email: [email protected]).
Acknowledgments
The authors would like to thank Joanna Mackie for language assistance and all individuals who participated in the study.
Funding
This research was supported by the National Science Centre OPUS_15, project no. 2018/29/B/NZ7/02094. The sponsor had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Ziemann E, Grzywacz T, Luszczyk M, Laskowski R, Olek RA, Gibson AL. Aerobic and anaerobic changes with high-intensity interval training in active college-aged men. J Strength Cond Res Nat Strength Cond Assoc. 2011;25(4):1104–1112. doi:10.1519/JSC.0b013e3181d09ec9 PubMed PMID: 20661160.
2. Gan Z, Fu T, Kelly DP, Vega RB. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res. 2018;28:969–980. 10.1038/s41422-018-0078-7. PubMed PMID: 30108290; PubMed Central PMCID: PMCPMC6170448.
3. Ziemann E, Zembron-Lacny A, Kasperska A, et al. Exercise training-induced changes in inflammatory mediators and heat shock proteins in young tennis players. J Sport Sci Med. 2013;12(2):282–289. PubMed PMID: ISI:000319866300010.
4. Tomczyk M, Kortas J, Flis D, Skrobot W, Camilleri R, Antosiewicz J. Simple sugar supplementation abrogates exercise-induced increase in hepcidin in young men. J Int Soc Sports Nutr. 2017;14:10. doi:10.1186/s12970-017-0169-8 PubMed PMID: 28428736; PubMed Central PMCID: PMCPMC5397733.
5. Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi:10.1126/science.1104742 PubMed PMID: 15514116.
6. Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi:10.1172/JCI20945 PubMed PMID: 15124018; PubMed Central PMCID: PMCPMC398432.
7. Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103(3):1093–1098. Epub 2007/ 03/10. doi: 10.1152/japplphysiol.00080.2007. PubMed PMID: 17347387.
8. Ganz T, Nemeth E. Regulation of iron acquisition and iron distribution in mammals. Biochimica et biophysica acta. 2006;1763(7):690–699. doi:10.1016/j.bbamcr.2006.03.014 PubMed PMID: 16790283.
9. Sonnweber T, Theurl I, Seifert M, et al. Impact of iron treatment on immune effector function and cellular iron status of circulating monocytes in dialysis patients. Nephrol Dial Transplant. 2011;26(3):977–987. doi:10.1093/ndt/gfq483 PubMed PMID: 20826742.
10. Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–1162. doi:10.1152/japplphysiol.00164.2004 PubMed PMID: 15772055.
11. Lakka TA, Nyyssonen K, Salonen JT. Higher levels of conditioning leisure time physical activity are associated with reduced levels of stored iron in Finnish men. Am J Epidemiol. 1994;140(2):148–160. doi:10.1093/oxfordjournals.aje.a117225
12. Salonen JT, Nyyssonen K, Salonen R. Body iron stores and the risk of coronary heart disease. N Engd J Med. 1994;331(17):
13. Bozzini C, Girelli D, Olivieri O, et al. Prevalence of body iron excess in the metabolic syndrome. Diabetes Care. 2005;28(8):2061–2063. doi:10.2337/diacare.28.8.2061 PubMed PMID: 16043762.
14. Merryweather-Clarke AT, Pointon JJ, Jouanolle AM, Rochette J, Robson KJ. Geography of HFE C282Y and H63D mutations. Genet Test. 2000;4(2):183–198. doi:10.1089/10906570050114902 PubMed PMID: 10953959.
15. Alexander J, Kowdley KV HFE-associated hereditary hemochromatosis. Genet Med. 2009;11(5):307–313. Epub 2009/ 05/16. doi: 10.1097/GIM.0b013e31819d30f2. PubMed PMID: 19444013.
16. Zaloumis SG, Allen KJ, Bertalli NA, et al. Natural history of HFE simple heterozygosity for C282Y and H63D: a prospective 12-year study. J Gastroenterol Hepatol. 2015;30(4):719–725. doi:10.1111/jgh.12804 PubMed PMID: 25311314; PubMed Central PMCID: PMCPMC4782752.
17. Gehrke SG, Kulaksiz H, Herrmann T, et al. Expression of hepcidin in hereditary hemochromatosis: evidence for a regulation in response to the serum transferrin saturation and to non-transferrin-bound iron. Blood. 2003;102(1):371–376. [PubMed PMID: 12637325]. doi:10.1182/blood-2002-11-3610
18. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357(9263):1191–1194. [PubMed PMID: 11323066]. doi:10.1016/S0140-6736(00)04337-3
19. Kortas J, Kuchta A, Prusik K, et al. Nordic walking training attenuation of oxidative stress in association with a drop in body iron stores in elderly women. Biogerontology. 2017;18(4):517–524. doi:10.1007/s10522-017-9681-0 10.1007/s10522-017-9681-0. PubMed PMID: 28229255; PubMed Central PMCID: PMCPMC5514214.
20. Kortas J, Prusik K, Flis D, et al. Effect of Nordic Walking training on iron metabolism in elderly women. Clin Interv Aging. 2015;10:1889–1896. PubMed PMID: 26664101; PubMed Central PMCID: PMCPMC4669095. doi:10.2147/CIA.S90413
21. Rikli RE, Jones CJ. Senior Fitness Test Manual: Human Kinetics. 2013.
22. Nash R, Bunce C, Freemantle N, Dore CJ, Rogers CA; Ophthalmic Statistics G. Ophthalmic Statistics Note 4: analysing data from randomised controlled trials with baseline and follow-up measurements. Br J Ophthalmol. 2014;98(11):1467–1469. PubMed PMID: 25107901; PubMed Central PMCID: PMCPMC4215292. doi:10.1136/bjophthalmol-2014-305614
23. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
24. Gmiat A, Jaworska J, Micielska K, et al. Improvement of cognitive functions in response to a regular Nordic walking training in elderly women - a change dependent on the training experience. Exp Gerontol. 2018;104:105–112. PubMed PMID: 29432893. doi:10.1016/j.exger.2018.02.006
25. Prusik K, Kortas J, Prusik K, et al. Nordic walking training causes a decrease in blood cholesterol in elderly women supplemented with Vitamin D. Front Endocrinol (Lausanne). 2018;9:42. doi:10.3389/fendo.2018.00042 PubMed PMID: 29515518; PubMed Central PMCID: PMCPMC5826219.
26. Gmiat A, Mieszkowski J, Prusik K, et al. Changes in pro-inflammatory markers and leucine concentrations in response to Nordic Walking training combined with vitamin D supplementation in elderly women. Biogerontology. 2017;18(4):535–548. doi:10.1007/s10522-017-9694-8 10.1007/s10522-017-9694-8. PubMed PMID: 28316011
27. Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014. doi:10.1039/c3mt00347g. PubMed PMID: 24549403.
28. Chambers V, Sutherland L, Palmer K, et al. Haemochromatosis-associated HFE genotypes in English blood donors: age-related frequency and biochemical expression. J Hepatol. 2003;39(6):925–931. doi:10.1016/S0168-8278(03)00471-9 PubMed PMID: 14642607
29. Fleming DJ, Jacques PF, Tucker KL, et al. Iron status of the free-living, elderly Framingham Heart Study cohort: an iron-replete population with a high prevalence of elevated iron stores. Am J Clin Nutr. 2001;73(3):638–646. doi:10.1093/ajcn/73.3.638 PubMed PMID: 11237943.
30. Halon-Golabek M, Borkowska A, Kaczor JJ, et al. hmSOD1 gene mutation-induced disturbance in iron metabolism is mediated by impairment of Akt signalling pathway. J Cachexia Sarcopenia Musc. 2018;9(3):557–569. doi:10.1002/jcsm.12283 PubMed PMID: 29380557.
31. Mairbaurl H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front Physiol. 2013;4:332. doi:10.3389/fphys.2013.00332 PubMed PMID: 24273518; PubMed Central PMCID: PMCPMC3824146.
32. Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care. 2004;27(10):2422–2428. doi:10.2337/diacare.27.10.2422 PubMed PMID: 15451911
33. Lee BK, Kim Y, Kim YI. Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean National Health and Nutrition Examination Survey 2008. Metabolism. 2011;60(10):1416–1424. doi:10.1016/j.metabol.2011.02.008 PubMed PMID: 21489582.
34. Antosiewicz J, Kaczor JJ, Kasprowicz K, et al. Repeated “all out” interval exercise causes an increase in serum hepcidin concentration in both trained and untrained men. Cell Immunol. 2013; 283 (1–2): 12–17. Epub 2013/ 07/16. doi: 10.1016/j.cellimm.2013.06.006. PubMed PMID: 23850671.
35. Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochimica et biophysica acta. 2012;1823(9):1434–1443. doi:10.1016/j.bbamcr.2012.01.014 PubMed PMID: 22306005; PubMed Central PMCID: PMC4048856.
36. Mainous AG
37. de Oliveira EP, Burini RC. The impact of physical exercise on the gastrointestinal tract. Curr Opin Clin Nutr Metab Care. 2009;12(5):533–538. doi:10.1097/MCO.0b013e32832e6776 PubMed PMID: 19535976.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.