Back to Journals » Breast Cancer: Targets and Therapy » Volume 13
Effect of Heparinoid Moisturizer on Quality of Life in Patients with Acute Radiation Skin Damage Following Hypofractionated Radiation Therapy After Breast-Conserving Surgery: A Randomized Controlled Study
Authors Kawamori J , Itazawa T, Fukushima S, Ito R, Yamauchi H, Sekiguchi K
Received 1 November 2021
Accepted for publication 22 December 2021
Published 31 December 2021 Volume 2021:13 Pages 743—753
DOI https://doi.org/10.2147/BCTT.S347136
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pranela Rameshwar
Jiro Kawamori,1 Tomoko Itazawa,1 Shoko Fukushima,2 Ryoko Ito,1 Hideko Yamauchi,3 Kenji Sekiguchi4
1Department of Radiation Oncology, St. Luke’s International Hospital, Tokyo, Japan; 2Kumagai Clinic, Tokyo, Japan; 3Department of Breast Surgery, St Luke’s International Hospital, Tokyo, Japan; 4Sonoda-kai Radiation Oncology Clinic, Tokyo, Japan
Correspondence: Jiro Kawamori
Department of Radiation Oncology, St. Luke’s International Hospital, 9-1 Akashi-cho, Chuo-ku, Tokyo, 104-8560, Japan
Tel +81 3 5550 7006
Fax +81 3 3544 0649
Email [email protected]
Background: With few research reports on the effects of moisturizer use for dry skin associated with radiotherapy after breast-conserving surgery on patient quality of life (QOL), we conducted a randomized controlled trial to investigate this effect.
Methods: Patients with breast cancer were randomly assigned to receive either heparinoid moisturizer (Group M) or no treatment (Group C). Group M was instructed to apply heparinoid moisturizer during 3 weeks of hypofractionated whole-breast irradiation with or without boost until 4 weeks after completion of irradiation. Skin-related QOL was assessed using the Dermatology Life Quality Index (DLQI) for 7 weeks. The primary endpoint was total DLQI score at 4 weeks after the start date.
Results: In total, 35 patients in Group M and 37 patients in Group C were analyzed. The DLQI total score (2.06 ± 2.17: mean ± SD) at 4 weeks in Group M was slightly lower than in Group C (2.16 ± 2.13) but with no significant difference (p = 0.894). The “Symptoms and feelings” subscore indicated significant worsening at 3 weeks and maintained until 7 weeks in Group C. There was no significant change for this subscore during radiotherapy in Group M, and it significantly increased after radiotherapy (4– 5 weeks) and returned to baseline in 7 weeks. The period of subscore worsening was shorter in Group M than in Group C.
Conclusion: Concomitant and extended use of heparinoid moisturizer with radiation therapy may improve the QOL of breast cancer patients impaired by dry skin for patients with breast cancer.
Keywords: breast cancer, radiation dermatitis, asteatosis, heparinoid moisturizer, skin-related quality of life
Introduction
For patients undergoing radiotherapy after breast-conserving surgery (BCS-RT), acute radiation dermatitis (ARD), along with potentially burdensome symptoms such as hair loss, dry skin and itching, is the most common and inevitable side effect.1 This adverse event is typically Grade 1 or 2 in severity, with Grade 3 rare, and generally these symptoms are mild in intensity. Importantly, ARD is known to have a major negative impact on patients’ skin-related quality of life (QOL), although not on global-QOL.2 Currently, there is no standard therapy or consensus on the optimal management of ARD in patients with breast cancer.3
We have previously shown the effectiveness of heparinoid moisturizer (HP) for ARD. HP is mainly used in dermatology to treat dry skin (asteatosis, xerosis) and its associated diseases such as atopic dermatitis. Management with HP in the prophylactic setting or starting 2 weeks after BCS-RT significantly increased water content in the stratum corneum, thereby improving skin dryness and desquamation.4,5 The amount of sebum also increased with the application of HP.6
To date, several studies have investigated the use of prophylaxis and care of ARD, but few topical agents have demonstrated efficacy in randomized controlled trials (RCTs).7 The primary outcome in most of these studies was prevention or reduction of ARD. Moisturizer is not expected to have anti-inflammatory effects, but they may contribute to the improvement of skin-related QOL, such as reduction of dryness, pain and itching. However, very few RCTs have examined the effect of moisturizer on skin-related QOL.
Based on our previous studies and on our clinical experience, we hypothesized that moisturizer treatment administered along with radiotherapy may alleviate skin dryness and thereby help to improve patients’ skin-related QOL. We therefore conducted an RCT to evaluate the effect of HP use on skin-related QOL in patients with ARD after BCS-RT.
Dermatology Life Quality Index (DLQI; range 0–30, higher scores indicate worsening quality of daily life) was used as an assessment tool for skin-related QOL.8 This questionnaire consists of 10 questions on QOL associated with skin diseases and has been validated and used in clinical trials, including for the assessment of QOL related to ARD.9,10 As cultural and racial differences in QOL assessed with DLQI may exist, the feasibility of QOL measurement using DLQI for Japanese patients with radiation dermatitis was explored in a pilot study consisting of 20 patients. Based on this, we concluded that QOL measurement using DLQI was feasible in Japanese patients receiving BCS-RT and aimed to investigate this further in a larger patient cohort.11
Patients and Methods
Study Design
This was a prospective RCT to evaluate the effect of HP on the skin-related QOL in patients with ARD associated with BCS-RT. We compared moisturizer treatment (Group M: moisturizer group) with no treatment (Group C: control group).
Patients
Eligible patients were women aged between 40 and 80 years who were scheduled to receive BCS-RT and agreed to skin-related QOL assessment using a questionnaire. Exclusion criteria included patients with wide-spreading or history of chronic skin diseases, sensitivity to heparinoid substances, scheduled to receive systemic chemotherapy excluding trastuzumab or hormone therapy during BCS-RT, with collagen diseases or systemic immune diseases or hemorrhagic blood diseases, and those who were considered otherwise inappropriate.
Procedures
After random assignment to either Group M or Group C, patients were started the assigned treatment following BCS-RT. Radiation treatment was whole-breast irradiation (42.56 Gy in 16 fractions) with or without added tumor bed boost (10–15 Gy in 4–6 fractions; hypofractionated irradiation) according to our hospital protocol. Patients in Group M were instructed to apply approximately 0.5 g of HP (proper amount in accordance with finger-tip unit, HirudoidⓇ Soft Ointment 0.3% or HirudoidⓇ Lotion 0.3%. Maruho Co., Ltd., Osaka, Japan) on their irradiated skin twice daily for 7 weeks after the radiation start date.12 Patients in Group C received no treatment on the skin. All patients were also instructed in general skin care including gently washing with soap and lukewarm water. Topical steroid was used for Grade 2 or above radiation dermatitis or when considered necessary in both groups.
Measurements
Skin-related QOL was assessed using DLQI at the radiation start date and at 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks and 7 weeks after the start date. Physician assessment of radiation dermatitis was performed using the NCI-CTCAE weekly until 3 weeks after the start of irradiation, and then at 5 and 7 weeks thereafter.13 Patients also kept a daily diary to record their application and medication status until the end of the study (Figure 1).
Study Outcomes
The primary endpoint was total DLQI score assessed at 4 weeks after the start of irradiation. Secondary endpoints were total DLQI score at the other evaluations points, DLQI subscores (Symptoms and feelings; Daily activities; Leisure activities; Work or school; Personal relationships; and Treatment score), and time-course in DLQI total score and subscores.
Safety
Adverse events that occurred during the study period were recorded.
Statistical Methods
The sample size of this study was calculated to be 72 patients for each group based on the results from our pilot study and previous report (δ, 1.5; 1-β, 0.8; 1-α, 95%; σ, 2.84; drop-out rate, 20%).10,11 The Mann–Whitney U-test was used for the comparison of DLQI total score and DLQI subscores at each time point between the two groups. Wilcoxon signed-rank test with Bonferroni correction was used for the comparison of scores between time points within each group. All tests were two-sided and a p value <0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS Statistics Version 24 (IBM Corp, NY, USA).
Ethical Considerations
The study was approved by the institutional review board at St. Luke’s international Hospital on May 25, 2017 (approval No. 17-R003) and conducted in compliance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects and the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all patients, and signed copies of the consent form were provided to each of these patients. The study was registered at the University Hospital Medical Information Network Clinical Trial Registry (registration No. UMIN000026987).
Results
A total of 306 patients who received BCS-RT between October 2017 and September 2018 were assessed for eligibility to the study and, of these, 75 patients were randomly allocated to Group M and Group C (Figure 2). Three patients subsequently dropped out due to consent withdrawal and 72 patients were analyzed (Group M, 35 cases; Group C, 37 cases). Patient and treatment characteristics were well balanced between the two groups (Table 1). In terms of radiation method, the field-in-field technique was planned for 20 patients, and tangential breast intensity-modulated radiotherapy (IMRT) was planned for 52 patients. V105 and Dmax are indicators of dose distribution uniformity; V105 is breast volume receiving more than 105% of the prescribed dose, and Dmax is maximum dose of irradiated breast. Mean of V105(cc) was 11.65 in Group M, 7.74 in Group C, mean of Dmax (cGy) was 4502.9 in Group M, 4500.0 in Group C.
 |
Table 1 Patients’ Characteristics |
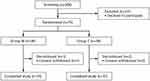 |
Figure 2 Patients flow. Abbreviations: Group M, moisturizer group; Group C, control group. |
Compliance with HP during the treatment period, as assessed by patient diaries, was considered to be good in 99% of applications. All patients developed radiation dermatitis, which were mostly Grade 1 but was Grade 2 in two patients in each group. In both groups, Grade 2 dermatitis developed at 4 weeks after the start of irradiation. Steroid ointment was used for one patient in Group M and three patients in Group C. These four patients were excluded from the analysis after the start of topical steroid use (Tables 2 and 3).
 |
Table 2 Maximum CTCAE Radiation Dermatitis Grade During the Observation Interval |
 |
Table 3 Time Course of CTCAE Radiation Dermatitis Grade During the Observation Interval |
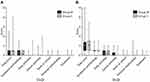
The DLQI total score at the start date was 0.91 ± 1.96 (mean ± standard deviation) in group C and 0.77 ± 1.26 in group M, with no statistical difference between the two groups (p = 0.619). DLQI total score at 4 weeks, the primary endpoint, was 2.16 ± 2.13 in group C and 2.06 ± 2.17 in group M. Although the score was slightly lower in group M, there was no significant difference between the two groups (p=0.894). There was also no difference between the two groups at any other study measurement point (Figure 3A and B; Table 4A).
 |
Table 4 DLQI Total Score (DLQI: Dermatology Life Quality Index) |
The “Symptoms and feelings” subscore, which consists of the majority of DLQI total score, was 1.06 ± 0.74 and 1.06 ± 0.70 in groups C and M, respectively, at 4 weeks (p = 0.988). There were also no differences in the other subscores between the two groups at 4 weeks (Figure 3A and B; Table 5A).
 |
Table 5 DLQI (Dermatology Life Quality Index) Symptoms/Feelings Subscore |
Regarding time-course, the DLQI total score of group C was 0.97 ± 2.01 at the start date and this significantly increased to 2.71 ± 3.21 (p = 0.003), 2.23 ± 2.16 (p = 0.007), and 2.55 ± 2.50 (p = 0.011) at 3, 4, and 5 weeks, respectively. However, by 7 weeks, it was 1.68 ± 1.93 (p=0.158), which was no longer different from baseline. This trend was also observed in group M. There was no significant change up to the second week, but the values were 1.55 ± 1.90 (p = 0.035), 2.06 ± 2.17 (p < 0.001), and 1.97 ± 2.31 (p = 0.006), at 3, 4, and 5 weeks, respectively, representing significant increases compared to start date. However, at 7 weeks, it returned to the baseline level of 1.09 ± 1.00 (p = 1.000) (Table 4B; Figure 4A).
The time-course of the “Symptoms and feelings” subscore of the control group significantly increased from baseline (0.32 ± 0.48) during 3 weeks (1.00 ± 0.84, p=0.004) and was maintained through 7 weeks (0.88 ± 0.64, p=0.007). In contrast, in Group M, the subscore did not significantly change from baseline (0.49 ± 0.74) during radiotherapy (from week 1 through 3 weeks) but significantly increased over 4 weeks (1.06 ± 0.70, p=0.006) through 5 weeks (1.09 ± 0.77, p=0.016) and returned to baseline level at 7 weeks (0.76 ± 0.56, p=0.447) (Figure 4B; Table 5B). The other subscores of the DLQI, “Daily activities”, “Leisure activities”, “Work or school”, “Personal relationships” and “Treatment” were very low throughout the study period for both groups (data not shown).
Safety
All patients experienced Grade 1 or 2 radiation dermatitis, however, there were no adverse reactions that could be attributed to HP use.
Discussion
We evaluated the contribution of HP to the improvement of skin-related QOL of patients with skin damage caused by hypofractionated BCS-RT. The primary endpoint of DLQI total score at 4 weeks was 2.16 ± 2.13 in group C and 2.06 ± 2.17 in group M. Although there was no significant difference between the two groups, there was a slight decrease in the total score by HP. The “Symptoms and Feelings” subscore, which accounted for most of the DLQI total score, also showed no significant difference at 4 weeks. One of the most important reasons for the lack of difference was that the DLQI total score at 4 weeks was unexpectedly not high at around 2.1. The same low values (ie, 1.59 to 2.48) have been also reported previously.14,15 One of these studies noted that DLQI was a relatively insensitive measure in patients undergoing radiotherapy.15 However, in the pilot study, which included 5 patients receiving post-mastectomy radiation therapy (PMRT), the mean value was as high as 4.6 ± 2.8.11 Fourteen patients were treated by hypofractionated radiotherapy, none by IMRT. High values of 3.88 and approximately 3.4 (read by the figure) were also reported by Beamer et al and Hindley et al, respectively, and it is notable that few patients received hypofractionated radiotherapy or IMRT in these studies.2,10
As dose homogeneity in the radiation field improves, ARD becomes less severe.16 Keenan et al reported that a V105 of greater than 30 cc is significantly associated with acute skin toxicity.17 ARD is also affected by Dmax.18 As our study employed the field-in-field technique and tangential breast IMRT, V105 was small and the mean of Dmax was approximately 106% of the prescribed dose, which suggested that dose homogeneity was satisfactory and that dermatitis was mild. Recent studies have shown that hypofractionated radiotherapy reduces ARD compared to conventionally fractionated radiotherapy and provides a better QOL.19–21 Therefore, the effect size (δ) of 1.5 might have been too large when calculating the sample size. However, the smaller value would have increased the number of patients needed and further underpower the results of this study.
DLQI was used in this study because radiation-induced skin toxicity has a major impact on skin-related-QOL but not global-QOL.2 The DLQI or Skindex is one of the most frequently used tools in dermatology, while the DLQI is reported to be the most sensitive to skin changes in psoriasis.22 With the minimal clinically important difference of the DLQI reported to be 3.3, it might be difficult to find a difference in DLQI for mainly Grade 1 dermatitis.23
The use of topical corticosteroids is reported to decrease the incidence of moist desquamation and improves skin-related QOL.10,24,25 Five RCTs using steroids and DLQI/Skindex 16 were identified in patients with breast cancer receiving post-operative radiotherapy.10,14,25–27 Amongst these studies, only one showed a significant difference in skin-related QOL.10 In a comparison of topical corticosteroids and moisturizers in breast cancer radiotherapy, a reduction in radiation dermatitis was seen with steroids, but not with moisturizers.28 Moisturizer does not have anti-inflammatory effects and are not expected to reduce ARD.7,15 Variable results have been observed. One study evaluating the effect of Ultra Emu Oil found no significant difference in Skindex-16, while another using emulsion of olive oil and calcium hydroxide showed a significant difference in Skindex-16.29 PMRT might play a role in this difference, although hypofractionated radiotherapy was applied.30
In the current study, HP had no effect on the time-course of change in DLQI total score. Comparing to the start date, there was no significant difference in each group until 2 weeks, and the total score significantly increased from 3 weeks to 5 weeks, and returned to baseline level at 7 weeks. However, the subscore “Symptoms and feelings,” which accounted for the majority of DLQI total scores, increased in Group C from 3 weeks and continued until 7 weeks, while in Group M the significant increase in scores from baseline was limited from 4 weeks to 5 weeks and recovered by 7 weeks. The period of negative effect on “Symptoms and feelings” was therefore shorter for HP. The “Symptoms and feelings” subscore reflects subjective symptoms such as itching or stinging and the objective signs such as erythema or pigmentation. HP might have some effect on shortening the period of subscore worsening over time. However, in the analysis of secondary endpoints, the problem of multiplicity remained even after applying the Bonferroni correction.
Sebum, natural moisturizing factor (NMF), and intercellular lipids such as ceramide play important roles in normal skin moisturization and can be compared to the “bricks and mortar” model of skin structure.31 The barrier made of these factors retains moisture in the stratum corneum and prevents evaporation. However, once irradiation starts, basal cells of the skin begin to be destroyed following an initial dose. Additionally, an inflammatory response with the secretion of histamine and serotonin occurs, as well as a vascular response with capillary dilation and extracapillary cell injury. As a result, erythema and edema occur. The melanin migrates to the more superficial layers of the epidermis. Proximal sweat and sebaceous glands are severely destroyed.1 These processes trigger the breakdown of the barrier function of skin, and patients suffer from skin irritation and skin color change.32
For patients with breast cancer receiving post-operative radiotherapy, hyperpigmentation is the most common and uncomfortable symptom, followed by erythema. However, dry skin is the most uncomfortable experience that patients need management for during radiotherapy.32 HirudoidⓇ is one of several available moisturizers that contain heparinoid, along with other ingredients such as white petrolatum, which are known to supplement stratum corneum water content and sebum.4,6 Pain reduction following HP application has also been reported, although the relief was temporary.4,5 Such effects of HP might contribute to shorten the period of “Symptoms and feelings” subscore worsening. In the present study, as in other reports, no adverse reactions were observed with concurrent use of HP.4 Therefore, using HP during and after radiotherapy is a reasonable approach to help keep skin structure as close to normal as possible.
While there are some guidelines in Europe and North America recommending the application of moisturizer during radiation therapy, the 2015 Japanese Society for Radiation Oncology guidelines indicate to avoid using topical medications in the irradiated area because they may develop more severe ARD.33–37 A traditional concern of topical application has been the potential for increased severity of dermatitis due to the bolus effect of the application layer and secondary radiation produced from the presence of metallic elements.34 However, recent studies have shown that even the presence of metallic elements is only an issue if the topical product is applied too thickly to the skin.38,39
The strength of this study is that it is one of the few RCTs to evaluate the impact of moisturizer on skin-related QOL using the DLQI in a random sample of breast cancer patients.40 A limitation is the lack of case accumulation. The difference between the groups remained statistically non-significant partly because the study lacked statistical power owing to insufficient case enrollment. Although we started the trial expecting to include 72 patients in each group, most candidates preferred to receive moisturizer treatment owing to the efficacy of moisturizer for radiation dermatitis having been widely communicated among patient advocacy groups in Japan. As a result, we prematurely terminated recruitment.
In the present study, skin damage was mild (Grade 1), and the difference between the two groups could not be adequately detected in the DLQI total score. However, in the “Symptom/Feelings” subscore, the duration of deterioration in skin-related QOL was shorter in the moisturizer group. This suggests that it may be preferable to evaluate skin-related QOL using VAS scale by symptoms such as pain and soreness instead of DLQI.4,41
The impact of HP on skin-related QOL was small in the present study, in which BCS-RT was frequently performed using hypofractionation and IMRT. However, ARD is expected to be more severe with conventionally fractionated radiotherapy, 3D-conformal radiotherapy, or PMRT that includes the skin as a target, and moisturizer may further improve skin-related QOL. Future clinical trials are awaited, but the control cohort with no application are expected to be difficult to collect. New evaluation tools to evaluate skin-related QOL under mild ARD are also awaited.
Conclusions
The administration of HP to ARD caused by BCT-RT did not affect the DLQI total score at 4 weeks after the start of irradiation. The DLQI total score significantly worsened from 3 to 5 weeks and recovered at 7 weeks, with or without HP application. Regarding the “Symptoms and feelings” subscore, which represented a major part of the change in DLQI, the worsening period was shorter and the patient recovered earlier with HP. Our study indicated that extended use of HP concomitant with radiation therapy may improve skin-related QOL of patients with breast cancer experiencing radiation-induced dry skin.
Acknowledgments
The authors would like to express our gratitude to all patients registered in this trial. We are also grateful to Chiori Haga for data collection, and to Martin Guppy PhD for editorial support. This research was a collaborative research and funded by Maruho Co., Ltd. Osaka, Japan. The abstract of this paper was presented at the American Society for Radiation Oncology 61st Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in Int J Radiat Oncol Biol Phys. 2019; 105(1) Supplement, 1 September 2019, Page E56: [https://doi.org/10.1016/j.ijrobp.2019.06.2390].
Disclosure
Jiro Kawamori, Ryoko Ito and Kenji Sekiguchi received lecture fees from Maruho Co., Ltd. Osaka, Japan. Hideko Yamauchi reports grants from Eiken Kagaku, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. McQuestion M. Evidence-based skin care management in radiation therapy: clinical update. Semin Oncol Nurs. 2011;27(2):e1–17. doi:10.1016/j.soncn.2011.02.009
2. Beamer LC, Grant M. Longitudinal trends in skin-related and global quality of life among women with breast radiodermatitis: a pilot study. Eur J Oncol Nurs. 2018;33:22–27. doi:10.1016/j.ejon.2018.01.008
3. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933–2948. doi:10.1007/s00520-013-1896-2
4. Sekiguchi K, Akahane K, Ogita M, et al. Efficacy of heparinoid moisturizer as a prophylactic agent for radiation dermatitis following radiotherapy after breast-conserving surgery: a randomized controlled trial. Jpn J Clin Oncol. 2018;48(5):450–457. doi:10.1093/jjco/hyy045
5. Sekiguchi K, Ogita M, Akahane K, et al. Randomized, prospective assessment of moisturizer efficacy for the treatment of radiation dermatitis following radiotherapy after breast-conserving surgery. Jpn J Clin Oncol. 2015;45(12):1146–1153. doi:10.1093/jjco/hyv155
6. Ogita M, Sekiguchi K, Akahane K, et al. Damage to sebaceous gland and the efficacy of moisturizer after whole breast radiotherapy: a randomized controlled trial. BMC Cancer. 2019;19(1):125. doi:10.1186/s12885-019-5334-9
7. Yee C, Wang K, Asthana R, et al. Radiation-induced skin toxicity in breast cancer patients: a systematic review of randomized trials. Clin Breast Cancer. 2018;18(5):e825–e840. doi:10.1016/j.clbc.2018.06.015
8. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi:10.1111/j.1365-2230.1994.tb01167.x
9. Beamer LC, Grant M. Using the dermatology life quality index to assess how breast radiodermatitis affects patients’ quality of life. Breast Cancer (Auckl). 2019;13:1178223419835547. doi:10.1177/1178223419835547
10. Hindley A, Zain Z, Wood L, et al. Mometasone furoate cream reduces acute radiation dermatitis in patients receiving breast radiation therapy: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2014;90(4):748–755. doi:10.1016/j.ijrobp.2014.06.033
11. Fukushima S, Kawamori J, Haga C, Yamauchi H, Sekiguchi K Effect of radiation dermatitis on quality of life in patients following radiation therapy after breast-conserving surgery: prospective observational study.
12. Long CC, Finlay AY. The finger-tip unit–a new practical measure. Clin Exp Dermatol. 1991;16(6):444–447. doi:10.1111/j.1365-2230.1991.tb01232.x
13. National Cancer Institute, editor. Common Terminology Criteria for Adverse Events V4.03 (CTCAE). Bethesda, MD: National Institutes of Health; 2010.
14. Meghrajani CF, Co HS, Arcillas JG, Maano CC, Cupino NA. A randomized, double-blind trial on the use of 1% hydrocortisone cream for the prevention of acute radiation dermatitis. Expert Rev Clin Pharmacol. 2016;9(3):483–491. doi:10.1586/17512433.2016.1126506
15. Wells M, Macmillan M, Raab G, et al. Does aqueous or sucralfate cream affect the severity of erythematous radiation skin reactions? A randomised controlled trial. Radiother Oncol. 2004;73(2):153–162. doi:10.1016/j.radonc.2004.07.032
16. Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–2092. doi:10.1200/JCO.2007.15.2488
17. Keenan LG, Lavan N, Dunne M, McArdle O. Modifiable risk factors for acute skin toxicity in adjuvant breast radiotherapy: dosimetric analysis and review of the literature. Med Dosim. 2019;44(1):51–55. doi:10.1016/j.meddos.2018.01.004
18. Shiba S, Okamoto M, Kiyohara H, et al. Clinical advantage of chest-wall post-mastectomy radiation therapy without Bolus. In Vivo (Brooklyn). 2018;32(4):961–965. doi:10.21873/invivo.11335
19. Arsenault J, Parpia S, Goldberg M, et al. Acute toxicity and quality of life of hypofractionated radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2020;107(5):943–948. doi:10.1016/j.ijrobp.2020.03.049
20. Hickey BE, James ML, Lehman M, et al. Fraction size in radiation therapy for breast conservation in early breast cancer. Cochrane Database Syst Rev. 2016;7:CD003860. doi:10.1002/14651858.CD003860.pub4
21. Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: a randomized clinical trial. JAMA Oncol. 2015;1(7):931–941. doi:10.1001/jamaoncol.2015.2666
22. Bronsard V, Paul C, Prey S, et al. What are the best outcome measures for assessing quality of life in plaque type psoriasis? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24(Suppl 2):17–22. doi:10.1111/j.1468-3083.2009.03563.x
23. Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230(1):27–33. doi:10.1159/000365390
24. Haruna F, Lipsett A, Marignol L. Topical management of acute radiation dermatitis in breast cancer patients: a systematic review and meta-analysis. Anticancer Res. 2017;37(10):5343–5353. doi:10.21873/anticanres.11960
25. Ho AY, Olm-Shipman M, Zhang Z, et al. A randomized trial of mometasone furoate 0.1% to reduce high-grade acute radiation dermatitis in breast cancer patients receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2018;101(2):325–333. doi:10.1016/j.ijrobp.2018.02.006
26. Miller RC, Schwartz DJ, Sloan JA, et al. Mometasone furoate effect on acute skin toxicity in breast cancer patients receiving radiotherapy: a Phase III double-blind, randomized trial from the North Central Cancer Treatment Group N06C4. Int J Radiat Oncol Biol Phys. 2011;79(5):1460–1466. doi:10.1016/j.ijrobp.2010.01.031
27. Ulff E, Maroti M, Serup J, Falkmer U. A potent steroid cream is superior to emollients in reducing acute radiation dermatitis in breast cancer patients treated with adjuvant radiotherapy. A randomised study of betamethasone versus two moisturizing creams. Radiother Oncol. 2013;108(2):287–292. doi:10.1016/j.radonc.2013.05.033
28. Uysal B, Gamsız H, Dincoglan F, et al. Comparative evaluation of topical corticosteroid and moisturizer in the prevention of radiodermatitis in breast cancer radiotherapy. Indian J Dermatol. 2020;65(4):279–283. doi:10.4103/ijd.IJD_607_18
29. Rollmann DC, Novotny PJ, Petersen IA, et al. Double-blind, placebo-controlled pilot study of processed ultra emu oil versus placebo in the prevention of radiation dermatitis. Int J Radiat Oncol Biol Phys. 2015;92(3):650–658. doi:10.1016/j.ijrobp.2015.02.028
30. Chitapanarux I, Tovanabutra N, Chiewchanvit S, et al. Emulsion of olive oil and calcium hydroxide for the prevention of radiation dermatitis in hypofractionation post-mastectomy radiotherapy: a randomized controlled trial. Breast Care (Basel). 2019;14(6):394–400. doi:10.1159/000496062
31. Rawlings AV. Molecular basis for stratum corneum maturation and moisturization. Br J Dermatol. 2014;171(Suppl 3):19–28. doi:10.1111/bjd.13303
32. Lee J, Park W, Choi DH, et al. Patient-reported symptoms of radiation dermatitis during breast cancer radiotherapy: a pilot study. Qual Life Res. 2017;26(7):1713–1719. doi:10.1007/s11136-017-1526-4
33. BC Cancer Agency. Symptom management guidelines: radiation dermatitis; 2018. Available from: http://www.bccancer.bc.ca/nursing-site/Documents/16.%20Radiation%20Dermatitis.pdf.
34. Bolderston A, Lloyd NS, Wong RK, Holden L, Robb-Blenderman L. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support Care Cancer. 2006;14(8):802–817. doi:10.1007/s00520-006-0063-4
35. D’Haese S, Van Roy M, Bate T, Bijdekerke P, Vinh-Hung V. Management of skin reactions during radiotherapy in Flanders (Belgium): a study of nursing practice before and after the introduction of a skin care protocol. Eur J Oncol Nurs. 2010;14(5):367–372. doi:10.1016/j.ejon.2009.10.006
36. Nystedt KE, Hill JE, Mitchell AM, et al. The standardization of radiation skin care in British Columbia: a collaborative approach. Oncol Nurs Forum. 2005;32(6):1199–1205. doi:10.1188/05.ONF.1199-1205
37. Japanese Society for Radiation Oncology, editor. Radiotherapy Q & A for Patients and Family, 2015 Edition. Tokyo: Kanehara-shuppan; 2015. (in Japanese).
38. Baumann BC, Verginadis II, Zeng C, et al. Assessing the validity of clinician advice that patients avoid use of topical agents before daily radiotherapy treatments. JAMA Oncol. 2018;4(12):1742–1748. doi:10.1001/jamaoncol.2018.4292
39. Iyama A, Matsuyama T, Matsumoto E, et al. Effect of metal-containing topical agents on surface doses received during external irradiation. J Radiat Res. 2018;59(6):794–799. doi:10.1093/jrr/rry078
40. Dalenc F, Ribet V, Rossi AB, et al. Efficacy of a global supportive skin care programme with hydrotherapy after non-metastatic breast cancer treatment: a randomised, controlled study. Eur J Cancer Care (Engl). 2018;27(1):e12735. doi:10.1111/ecc.12735
41. Leonardi MC, Gariboldi S, Ivaldi GB, et al. A double-blind, randomised, vehicle-controlled clinical study to evaluate the efficacy of MAS065D in limiting the effects of radiation on the skin: interim analysis. Eur J Dermatol. 2008;18(3):317–321. doi:10.1684/ejd.2008.0396
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.