Back to Journals » Clinical and Experimental Gastroenterology » Volume 8
Effect of genistein on basal jejunal chloride secretion in R117H CF mice is sex and route specific
Authors Rayyan E, Polito S, Leung L, Bhakta A, Kang J, Willey J, Mansour W, Drumm M, Al-Nakkash L
Received 1 August 2014
Accepted for publication 15 September 2014
Published 30 January 2015 Volume 2015:8 Pages 77—87
DOI https://doi.org/10.2147/CEG.S72111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Andreas M. Kaiser
Esa Rayyan,1 Sarah Polito,1 Lana Leung,1 Ashesh Bhakta,1 Jonathan Kang,1 Justin Willey,1 Wasim Mansour,1 Mitchell L Drumm,2 Layla Al-Nakkash1
1Department of Physiology, Arizona College of Osteopathic Medicine, Midwestern University, Glendale, AZ, USA; 2Pediatric Pulmonology Division, Case Western Reserve University, Cleveland, OH, USA
Abstract: Cystic fibrosis (CF) results from the loss or reduction in function of the CFTR (cystic fibrosis transmembrane conductance regulatory protein) chloride channel. The third most common CFTR mutation seen clinically is R117H. Genistein, a naturally occurring phytoestrogen, is known to stimulate CFTR function in vitro. We aimed to determine whether route of administration of genistein could mediate differential effects in R117H male and female CF mice. Mice were fed (4 weeks) or injected subcutaneously (1 week) with the following: genistein 600 mg/kg diet (600Gd); genistein-free diet (0Gd); genistein injection 600 mg/kg body weight (600Gi); dimethyl sulfoxide control (0Gi). In male R117H mice fed 600Gd, basal short circuit current (Isc) was unchanged. In 600Gd-fed female mice, there was a subgroup that demonstrated a significant increase in basal Isc (53.14±7.92 µA/cm2, n=6, P<0.05) and a subgroup of nonresponders (12.05±6.59 µA/cm2, n=4), compared to 0Gd controls (29.3±6.5 µA/cm2, n=7). In R117H mice injected with 600Gi, basal Isc was unchanged in both male and female mice compared to 0Gi controls. Isc was measured in response to the following: the adenylate cyclase activator forskolin (10 µM, bilateral), bumetanide (100 µM, basolateral) to indicate the Cl- secretory component, and acetazolamide (100 µM, bilateral) to indicate the HCO3- secretory component; however, there was no effect of genistein (diet or injection) on any of these parameters. Jejunal morphology (ie, villi length, number of goblet cells per villus, crypt depth, and number of goblet cells per crypt) in R117H mice suggested no genistein-mediated difference among the groups. Serum levels of genistein were significantly elevated, compared to respective controls, by either 600Gd (equally elevated in males and females) or 600Gi (elevated more in females versus males). These data suggest a sex-dependent increase in basal Isc of R117H mice and that the increase is also specific for route of administration.
Keywords: genistein, intestine, secretion, CFTR, R117H
Introduction
Genistein is a naturally occurring isoflavonic phytoestrogen, found in high concentrations in soy products.1 We and others have demonstrated genistein’s ability to stimulate the cystic fibrosis transmembrane conductance regulator (CFTR) chloride (Cl−) channel in isolated cells,2–5 and intact isolated tissues.6–10 Both wild-type (Wt) CFTR5 and the most common cystic fibrosis (CF) disease-associated mutation (ΔF508-CFTR) are known to be stimulated by genistein.11,12 Genistein increases the open probability of ΔF508-CFTR to levels analogous to those seen with Wt CFTR,11,12 thus indicating a potential therapeutic benefit of genistein designed for CF treatment.
The effectiveness of genistein in improving the function of ΔF508-CFTR in in vitro cell systems, with half-maximal effective concentration (EC50) of 5 μM, is within the physiological range achievable via diet.2,13 Indeed, micromolar concentrations of genistein can be measured in serum.14 Mice consuming 750 mg/L genistein have been shown to generate plasma genistein concentrations of ~2 μM,15 and moreover, we have previously shown that a diet containing genistein 600 mg/kg food (600Gd) for 4 weeks yields serum concentrations of ~4–8 μM in Wt female and male mice,8 yielding levels that are comparable to a soy milk diet in humans.16
Use of genistein as a pharmacological tool to manipulate tissue function has been demonstrated in several systems: 1) Noël et al17 have demonstrated that subcutaneous (sc) injection of 50 μM genistein (or MPB-07), in the presence of isoprenaline (10 μM), induced salivary secretion in Cftr+/+ mice; 2) acute bilateral application of genistein increases short circuit current (Isc), ie, increases anion secretion, in Wt murine distal colon;18 3) acute application of genistein has been shown to increase current and conductance in depolarized colonic mucosa of normal and CF mice.19 We have previously shown that exposure to dietary genistein (600Gd, for 4 weeks) stimulated basal Cl− secretion across freshly isolated segments of jejunum from Wt female mice, but not in male mice.8 More recently, we have demonstrated that daily sc injections of genistein (600 mg/kg body weight, 600Gi) for periods of 1–2 weeks, elicit stimulation of basal Cl− secretion across freshly isolated segments of jejunum from Wt female and male mice.20,21
The clinically relevant CFTR missense mutation, R117H (replacement of arginine by histidine at residue 117), has a wide range of phenotypic variability and is included in many mutation panels for newborn screening. This membrane-spanning mutation reaches the plasma membrane – ie, the protein is correctly processed – but does not function appropriately, ie, the protein exhibits defective conduction (class IV) and gating (class III) abnormalities.22,23 These R117H mice have been shown to have beneficial responses to a synthetic triterpenoid, CDDO (2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid), as indicated by significant improvements in markers for airway disease.24 Interestingly, relatively recent evidence suggest that the R117H mutation has little impact on Cl− secretion from freshly isolated human rectal biopsies nor on nasal Cl− secretion.25
Additional evidence suggests that beneficial effects of genistein on CFTR-mediated Cl− secretion may be a result of 1) either potentiation of the CFTR Cl− channel by binding to CFTR and the stabilization of the CFTR channel into its open state13 or 2) the promotion of CFTR retention in the plasma membrane.26 To date, there have been no studies to assess the effect of dietary genistein or daily sc genistein injections on intestinal function in R117H CF mice. In this study, we have compared the effects of 4 week consumption of a genistein-enriched diet (600Gd) or a genistein-free diet (0Gd) with the effects of 1 week treatment consisting of daily sc injections of genistein (600Gi) or vehicle control (0 mg/kg body weight/day, 0Gi) on small intestinal (jejunal) epithelial anion secretion (transepithelial short circuit current, Isc, using freshly excised intestinal segments) in R117H female and male mice. In addition, we examine the effect of genistein on intestinal morphology. Based on our previous work describing genistein’s action on Wt jejunal Cl− secretion, we predict that genistein would increase intestinal Isc in R117H male and female mice to likely intermediate levels to those that we have previously noted in Wt female mice fed the same genistein-containing diet8 or the levels in Wt-male and Wt female mice injected with the same genistein dose.20
Materials and methods
Mice
Male and female mice carrying the R117H CFTR mutation were generously provided by the CF Mouse Models Core at Case Western Reserve University (Cleveland, OH, USA) and were housed in an animal care facility with 12:12-hour light–dark cycle, two mice per cage, until used. Mice consumed food and water ad libitum. Body weight was measured weekly during the studies and general health was monitored daily. Mice were randomly assigned to either genistein-containing diet (600 mg genistein/kg food, 600Gd) or genistein-free (0 mg genistein/kg food, 0Gd) diet and were fed either of these two diets for 4 weeks. Mice of a separate subgroup were randomly assigned to either genistein-injected (600 mg genistein/kg body weight, 600Gi) or dimethyl sulfoxide (DMSO) control-injected group (0Gi) for a period of 1 week. Mice were asphyxiated in an atmosphere of 100% CO2, followed by surgical thoracotomy to induce pneumothorax. Animal care and treatments were conducted in accordance with established guidelines, and all protocols were approved by the Midwestern University Institutional Animal Care and Use Committee.
Diets
Casein-based diets were purchased from Dyets Inc. (Bethlehem, PA, USA) and contained either 600Gd or 0Gd.8 Importantly, diets contained equivalent amounts of protein (20.3 g), carbohydrate (66 g), and fat (5 g), with an estimated energy content of 16.28 kJ/g. Diets were formulated as a powder and mice were allowed to free feed. All mice assigned to the injection studies were fed genistein-free (0Gd) diet. Diet composition is described previously in the study by Al-Nakkash et al.8
Bioelectric measurement of intestinal secretion
Via an abdominal incision, ~5 cm of middle portion of jejunum was removed and placed in ice-cold oxygenated Krebs bicarbonate ringer (KBR) buffer. Each mouse yielded three to four jejunum pieces, isolated as described previously.18,27–29 Jejunum sections mounted in the Ussing chambers had 0.3 cm2 exposed surface area. Transepithelial short circuit current (Isc, reported as μA/cm2) was measured via an automatic voltage clamp (VCC-600; Physiologic Instruments, San Diego, CA, USA) and the experimental conditions and methods were as previously described.8,20,21,30 Intestinal tissue pieces were constantly maintained in 1 μM indomethacin (minimizing tissue exposure to endogenously generated prostanoids due to manipulation and mounting of the tissue).31 Glucose (10 mM) was added to the serosal KBR bath and mannitol (10 mM) was substituted for glucose in the mucosal KBR bath to avoid an inward current due to Na+-coupled glucose transport.30 Once mounted, the serosal side was exposed to tetrodotoxin (0.1 μM), minimizing variations in intrinsic intestine neural tone.32 Intrinsic neural tone limits the absorptive capacity of the murine mucosa and neural block is denoted by a decrease in Isc.
Experimental protocols
Tissues were exposed to KBR buffer for 20 minutes and steady-state basal Isc was measured at that time. Cyclic adenosine monophosphate-dependent anion secretion was assessed by bilateral application of 10 μM forskolin (at 20 minutes) and steady-state forskolin response was measured at 50 minutes. Addition of bumetanide (100 μM, serosal), a Na+/K+/2Cl− cotransporter, evaluates the Cl− secretory component. Addition of acetazolamide (100 μM bilateral) assesses the secretory component represented by HCO3−. At the end of each experiment, glucose (10 mM, mucosal) was added to stimulate Na+-coupled glucose transport to assess tissue viability (denoted by >10% increase in Isc). In a subgroup of mice, basal Isc was determined, and at time 20 minutes, 100 μM of phosphodiesterase inhibitor was added (bilateral), and at time 40 minutes, 200 μM was added. Tissues that failed to respond to glucose within this parameter were discarded. Experiments were performed in the presence of KBR buffer (Cl−/HCO3−present), containing the following (in mM):115 NaCl, 25 NaHCO3, 5 KCl, 1.2 MgCl2, and 1.2 CaCl2, pH 7.4.
Histology and morphology
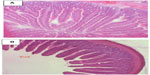
Freshly isolated pieces of jejunum were embedded and flash frozen in Optimal Cutting Temperature compound (Tissue-Tek OCT compound; Sakura Finetek USA, Torrance, CA, USA). Frozen sliced sections (8–10 μm) of murine jejunum were stained with a standard hematoxylin and eosin protocol, prior to performing the morphometric analyses to evaluate basic histological measurements. In brief, sections were exposed to the following wash protocol: hematoxylin 30 seconds, water rinse 10 seconds, Scott’s solution 5 seconds, water rinse 10 seconds, 95% ethanol 5 seconds, eosin 15 seconds, rinses with 95% ethanol 10 seconds, 100% ethanol rinse 10 seconds, followed by treatment with Histo-Clear for 15 seconds. Crypt depth, villi length, and numbers of goblet cells per crypt and villus were measured using AxioVision LE (Carl Zeiss, freeware), from images of hematoxylin-and-eosin-stained jejunum sections. All images were taken at ×10 magnification. Averages of measurements were taken from six separate slices per frozen section of jejunum (ie, per mouse), with data being presented as the average of seven mice per group.
Serum genistein
At the time the mice were euthanized, blood samples were obtained by cardiac puncture, and the serum was separated by centrifugation and then stored at −80°C. Serum samples were analyzed for genistein level by high-performance liquid chromatography using a modification of the methodology of Franke et al.33 Values represent the average of duplicate serum samples.
Chemicals
Forskolin was purchased from Calbiochem (La Jolla, CA, USA) and stored as a 10 mM stock in DMSO at -20°C. Genistein was purchased from LC Laboratories (Woburn, MA, USA) and acetazolamide was purchased from MP Biomedicals (Solon, OH, USA). All other chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA).
Statistics
Data are expressed as mean ± standard error of mean (SEM). Numbers in parentheses are numbers of tissues used from separate individual mice. One-way analysis of variance with Newman–Keuls multiple comparison test or t-tests was performed using GraphPad (GraphPad Software, Inc., La Jolla, CA, USA), and P<0.05 was considered statistically significant.
Results
Mouse weights and serum genistein
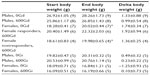
During the 4 week diet and 1 week injection studies, mouse weights were monitored (Table 1). As shown in Table 1, significant increases in weight (from start weight) were observed in females fed 0Gd and in a subgroup of females fed 600Gd. Interestingly, there was no difference in the final body weight of females fed 600Gd that concomitantly exhibited an increase in basal Isc (responders) versus those that did not have increased basal Isc (nonresponders). Mice in the injection study did not gain as much weight as those on the diet studies, likely due to the fact that diet mice were fed and maintained for additional duration (4 weeks on the diet study versus the 1 week injection studies).
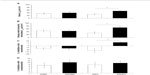
Serum genistein concentrations are shown in Figure 1. Serum genistein levels were significantly elevated by genistein treatment in both male and female mice, regardless of route of administration (diet or injection). Of note, in both male and females alike, diet route of administration yielded significantly greater serum genistein levels compared to the injected counterparts. Interestingly, there was no difference in the serum genistein level of those females fed 600Gd that concomitantly exhibited an increase in basal Isc (responders: 740.3±271.7 ng/mL, n=3) versus those that did not have increased basal Isc (nonresponders: 1094.8±592.1 ng/mL, n=4).
Ussing chamber bioelectric measurements
Diet study
Basal Isc was significantly increased in a subgroup of female mice fed 600Gd for 4 weeks (53.14±7.92 μA/cm2, n=6) compared to those fed 0Gd (29.30±6.47 μA/cm2, n=7, P<0.05; Figures 2 and 3A). Interestingly, another subgroup of female mice fed 600Gd for 4 weeks did not exhibit an increase in basal Isc (12.05±6.59 μA/cm2, n=4; Figure 2). Moreover, we found that basal anion secretion in jejuna removed from male mice fed 600Gd for 4 weeks (39.20±11.72 μA/cm2, n=9) was comparable to those from male mice fed 0Gd (34.89±7.67 μA/cm2, n=9; Figure 3A). The effect of bilateral application of 10 μM forskolin was determined, and steady-state forskolin-stimulated Isc was significantly increased only in the subgroup of females that had exhibited a significantly elevated basal Isc (Figure 3B). Addition of bumetanide (100 μM, serosal), to assess the Cl− secretory component, only resulted in a significant increase in the percentage inhibition with bumetanide in the subgroup of 600Gd females with elevated basal Isc (Figure 3C). There was no effect of acetazolamide (100 μM, bilateral) on the HCO3− contribution toward the Isc, in any of the groups (Figure 3D).
Injection study
Basal Isc was unchanged after 1 week of daily genistein injections (600Gi), compared to 0Gi controls, for both male and female R117H mice (Figure 4A). In R117H mice, in either sex with either 600Gi or 0Gi, we found no change in Isc following the addition of bilateral application of 10 μM forskolin, bumetanide (100 μM, serosal), or acetazolamide (100 μM, bilateral) (Figure 4B–D).
Role of phosphodiesterase enzymes
To determine the role of phosphodiesterase enzymes on basal Isc in R117H male and female mice, we examined the effect of IBMX (3-isobutyl-1-methylxanthine, a nonspecific phosphodiesterase enzyme inhibitor, bilateral). Despite a trend for an increased Isc following addition of IBMX, there was no significant effect of either 100 μM or 200 μM IBMX on basal Isc in male and female jejuna (Figure 5), likely due to the large variability in the responses between mice. There was a trend for a greater increase in Isc with IBMX in females versus males, but again, large variability nullified this potential effect.
Jejunum histology
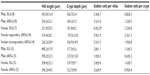
To determine whether there were genistein-mediated or sex-dependent effects on intestinal morphology, histological sections of jejunum were stained using hematoxylin and eosin and analyzed for wall thickness, villi length, crypt depth, and numbers of goblet cells within entire villi and crypts using Axiovision software. Data are shown in Table 2, and representative hematoxylin-and-eosin-stained histological images are shown in Figure 6. Number of goblet cells per villus was significantly greater in the female 0Gd group compared to their male counterparts (P<0.05), which may indicate a greater mucus production in R117H females versus males, that were fed the casein-based genistein-free diet. There were no other effects of sex or genistein (regardless of route of administration) on the parameters measured.
Discussion
We provide here the first evidence that chronic consumption of dietary genistein (600Gd, for 4 weeks) increases basal Isc in freshly isolated jejunum segments removed from a population of female R117H mice, but not in R117H males.
Dietary isoflavones (such as genistein) are found in soy and are digested in an average daily diet. Soy-rich diets can generate micromolar serum genistein concentrations.34 Consumption of genistein-containing diets correlates with elevated serum levels of genistein: 1 μM serum genistein concentrations can be obtained in rats consuming a diet of 750 μg genistein/g/day,35 and serum levels of ~1.5 μM or ~0.5 μM serum genistein in mice have been detected after consumption of 1,000 or 500 mg/kg dietary genistein, respectively, for 4 weeks.36 Moreover, we have previously demonstrated that Wt female and Wt male mice fed 600Gd for 4 weeks have serum genistein levels of ~8 μM and ~4 μM respectively, which correlated with a significant genistein-mediated increase in basal Isc in females (~36 μA/cm2), but not in males.8 Furthermore, we have recently demonstrated that serum genistein levels significantly increased in both 600Gi-treated females (after 1 week to ~9 μM) and 600Gi-treated males (after 2 weeks to ~4 μM), and this translated into significant genistein-mediated increases in basal Isc (of ~85 μA/cm2) in both Wt females and Wt males. Thus, in Wt mice, sc injections produced similar potentiative effects on basal jejunal Isc in both female and male mice, and, moreover, these increases in Isc were considerably greater than the increases seen via the diet route of administration. This was not the case with the R117H mice, and while serum genistein levels were substantially greater in 600Gi-treated males and females, basal Isc was not modified. We demonstrate here that serum genistein levels in R117H males and females injected with 600Gi are significantly greater compared to the levels found after dietary route of administration, 600Gd. Moreover, R117H female mice fed genistein diet have significantly greater serum genistein levels (942±319 ng/mL, n=7) compared to males fed 600Gd (336±71 ng/mL, n=9), which may contribute to the disparity and sex-dependent basal Isc increases. However, in these R117H mice, compared to our previous Wt-mice studies, it is notable and distinct that increases in serum genistein do not correlate consistently with increases in basal Isc. This disparity may be a consequence of the fact that the serum genistein levels attained in R117H mice are not comparable to those in Wt mice. Clearance rates of serum genistein may well be different between R117H and Wt mice, in addition to sex-dependent clearance rates, because evidence suggests that after consuming the same genistein-rich diets, female mice have greater circulating levels than their male counterparts.37,38
In the murine intestine, the major route for Cl− exit across the apical membrane is via the CFTR Cl− channel,39–41 although it is widely accepted that other candidate pathways (ClC-2 and/or ClC-4) exist for this apical membrane Cl− exit.42,43 The presence of ClC-2 and ClC-4 is thought to be responsible for the lack of severe intestinal impact observed in a subset of CF mice.43 In addition, members of the family of Ca2+-activated Cl− channels (CLCA), mCLCA2 and mCLCA3, have been proposed to be involved in intestinal function of both CF and Wt mice.44 Whether or not our observed increase in Isc following the 4-week genistein-feeding regimen in the subgroup of female R117H mice is due to activation of CLC channels remains to be seen.
Genistein’s ability to improve epithelial function, namely, to increase epithelial secretion, has remained both intriguing and questionable. Mall et al6 suggested that genistein activated both Wt- and ΔF508-CFTR in oocytes and non-CF human tissue, yet they did not garner support for genistein’s use as a pharmacological tool in CF. More recently, Yu et al45 demonstrated that combined application of curcumin with genistein did in fact synergistically rescue the gating defect associated with G551D-CFTR. Various other CFTR activators have been tested by several laboratories in the past decade. Such small-molecule correctors have varying effects on CF epithelia and, not surprisingly, appear to be CF mutation-dependent moieties.46 Caputo et al47 suggested that felodipine and PG-01 acted on the CFTR mutations E193K, G970R, and G551D, whereas the sulfonamide SF-01 was not as effective. A therapeutic potential has been hypothesized for the naturally occurring coumarin compound osthole, given its ability to stimulate ΔF508-CFTR-mediated Cl− secretion in colonic mucosa.48 In addition, the compound RP193 (a modified pyrrolo[2,3-b]pyrazine derivative) has been shown to potentiate ΔF508- and G551D-CFTR activity in the presence of low forskolin concentrations in cell culture systems.49 Ivacaftor (VX-770) has been shown in vitro to increase ΔF508 and G551D channel opening in recombinant cells, to increase Cl− secretion in human CF bronchial epithelia,50 to improve lung function indices in CF subjects who have the G551D mutation,51 and more recently, to effectively activate a wider range of CFTR gating mutations in vitro, eg, G1349D, S1255P, S1251N, G1244E, G551S, and G178R.52
Interestingly, the R117H CFTR mutation has received less attention in the field of potential candidate pharmacomodulators. This is likely a consequence of the fact that the R117H missense mutation has a combination of both conductance and gating dysfunction, thus functional rectification may be challenging.22,25 Utilizing a cell culture system, Clancy et al53 have previously shown activation of R117H CFTR by adenosine and its nucleotides. We conclude that given the variety of CFTR activators available, the mechanism(s) involved in the activation of CFTR (and its many mutated forms) are no doubt both numerous, and complex.
While the mechanism(s) underlying the genistein-mediated (600Gd) increase in jejunum anion secretion in the subpopulation of R117H females is(are) currently unclear, our data suggest the following: 1) it is not a result of changes in intestinal morphology, ie, not related to changes in crypt depth (responders: 96.5±13.8 μm, n=4, versus nonresponders: 106.9±14.9 μm, n=4) or number of goblet cells in crypts (responders: 2.3±1.3, n=4, versus nonresponders: 1.9±0.8, n=4); 2) it is not related to change in body weight (ie, growth of those mice fed the 600Gd); however, there was a trend for these responding mice to gain somewhat more weight at the end of the study (responders: 1.92±0.94 g, n=6, versus nonresponders: 1.36±0.25 g, n=4); 3) it is not related to changes in serum genistein levels (responders: 740±272 ng/mL, n=3, versus nonresponders: 1,095±592 ng/mL, n=4); 4) it is related to an increase in Cl− secretion, as evidence by the significantly elevated bumetanide-sensitive Isc in responders versus nonresponders. This study provides the first evidence that consumption of dietary genistein (600Gd), a naturally occurring isoflavone, can mediate an increase in basal intestinal anion secretion in a population of R117H female mice.
Acknowledgments
The authors thank Dr G Rottinghaus (Veterinary Medical Diagnostic Laboratory, University of Missouri-Columbia, MO, USA) for analysis of serum genistein. This work was supported by the Soy Health Research Program (to LA), the National Institutes of Health (grant number R15 DK071625-01A2 to LA), and the Midwestern University intramural funds (to LA). We also gratefully acknowledge the CF Mouse Models Core of the Case Western Reserve University and thank Alma Wilson for maintaining the R117H mouse colony.
Disclosure
The authors report no conflicts of interest in this work.
References
Murphy PA. Phytoestrogen content of processed soybean products. Food Technol. 1982;36:60–64. | |
Al-Nakkash L, Hu S, Li M, Hwang T-C. A common mechanism for cystic fibrosis transmembrane conductance regulator protein activation by genistein and benzimidazolone analogs. J Pharmacol Exp Ther. 2001;296:464–472. | |
Moran O, Zegarra-Moran O. A quantitative description of the activation and inhibition of CFTR by potentiators: genistein. FEBS Lett. 2005;579:3979–3983. | |
Schmidt A, Hughes LK, Cai Z, et al. Prolonged treatment of cells with genistein modulates the expression and function of the cystic fibrosis transmembrane conductance regulator. Br J Pharmacol. 2008;153:1311–1323. | |
Illek B, FIscher H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol. 1995;268:C886–C893. | |
Mall M, Wissner A, Seydewitz HH, et al. Effect of genistein on native epithelial tissue from normal individuals and CF patients and on ion channels expressed in Xenopus oocytes. Br J Pharmacol. 2000;130:1884–1892. | |
Baker MJ, Hamilton KL. Genistein stimulates electrogenic Cl− secretion in the mouse jejunum. Am J Physiol. 2004;287:C1636–C1645. | |
Al-Nakkash L, Clarke LL, Rottinghaus GE, Chen YJ, Cooper K, Rubin LJ. Dietary genistein stimulates anion secretion across female murine intestine. J Nutr. 2006;136:2785–2790. | |
Chao P-C, Hamilton KL. Genistein stimulates electrogenic Cl− secretion via phosphodiesterase modulation in the mouse jejunum. Am J Physiol. 2009;297:C688–C698. | |
Tuo B, Wen G, Seidler U. Differential activation of the HCO3− conductance through the cystic fibrosis transmembrane conductance regulator anion channel by genistein and forskolin in murine duodenum. Br J Pharmacol. 2009;158:1313–1321. | |
Al-Nakkash L, Hwang T-C. Activation of CFTR by pharmacological modulators. Pediatr Pulmonol Suppl. 1997;13:Abstract #68. | |
Hwang T-C, Wang F, Zeltwanger S, Yang I, Reenstra W. Genistein potentiates wild-type and ÐF508-CFTR channel activity. Am J Physiol. 1997;273:C988–C998. | |
Wang F, Zeltwanger S, Yang I, Nairn A, Hwang T-C. Actions of genistein on CFTR channel gating. J Gen Physiol. 1998;111:477–490. | |
Barnes S, Sfakianos J, Coward L, Kirk M. Soy isoflavonoids and cancer prevention. Underlying biochemical and pharmacological issues. Adv Exp Med Biol. 1996;401:87–100. | |
Hsieh CY, Santell R, Haslam S, Helferich W. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–3838. | |
Xu X, Wang H, Murphy P, Cook L, Hendrich S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr. 1994;124:825–832. | |
Noël S, Strale PO, Dannhoffer L, et al. Stimulation of salivary secretion in vivo by CFTR potentiators in Cftr+/+ and Cftr-/- mice. J Cyst Fibros. 2008;7:128–133. | |
Goddard CA, Evans M, Colledge W. Genistein activates CFTR-mediated Cl- secretion in the murine trachea and colon. Am J Physiol. 2000;279:C383–C392. | |
Cuthbert A. Assessment of CFTR chloride channel openers in intact normal and cystic fibrosis murine epithelia. Br J Pharmacol. 2001;132:659–668. | |
Al-Nakkash L, Batia L, Bhakta M, et al. Stimulation of murine intestinal secretion by daily genistein injections: gender-dependent differences. Cell Physiol Biochem. 2011;28:239–250. | |
Al-Nakkash L. Genistein stimulates jejunal chloride secretion via sex-dependent, estrogen receptor or adenylate cyclase mechanisms. Cell Physiol Biochem. 2012;30:137–150. | |
Sheppard DN, Rich DP, Ostedgaard LS, Gregory RJ, Smith AE, Welsh MJ. Mutations in CFTR associated with mild-disease-form Cl-channels with altered pore properties. Nature. 1993;362:160–164. | |
Carson MR, Winter MC, Travis SM, Welsh MJ. Pyrophosphate stimulates wild-type and mutant cystic fibrosis transmembrane conductance regulator Cl− Channels. J Biol Chem. 1995;270:20466–20472. | |
Nichols DP, Ziady AG, Shank SL, Eastman JF, Davis PB. The triterpenoid CDDO limits inflammation in preclinical models of cystic fibrosis lung disease. Am J Physiol Lung Cell Mol Physiol. 2009;297:L828–L836. | |
de Nooijer RA, Nobel JM, Arets HG, et al. Assessment of CFTR function in homozygous R117H-7T subjects. J Cyst Fibros. 2011;10:326–332. | |
Lim CH, Bijvelds MJ, Nigg A, et al. Cholesterol depletion and genistein as tools to promote F508delCFTR retention as the plasma membrane. Cell Physiol Biochem. 2007;20:473–482. | |
Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated HCO3− secretion across murine duodenum. Am J Physiol. 1998;274:G718–G726. | |
Grubb BR, Boucher RC. Enhanced colonic Na+ absorption in cystic fibrosis mice versus normal mice. Am J Physiol. 1997;272:G393–G400. | |
Grubb BR. Ion transport across the jejunum in normal and cystic fibrosis mice. Am J Physiol. 1995;268:G505–G513. | |
Clarke LL, Grubb BR, Gabriel SE, Smithies O, Coller BH, Boucher RC. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science. 1992;257:1125–1128. | |
Clarke LL, Harline MC. CFTR is required for cAMP inhibition of intestinal Na+ absorption in a cystic fibrosis mouse model. Am J Physiol. 1996;270:G259–G267. | |
Sheldon RJ, Malarchik ME, Fox DA, Burks TF, Porreca F. Pharmacological characterization of neural mechanisms regulating mucosal ion transport in mouse jejunum. J Pharmacol Exp Ther. 1988;249:572–582. | |
Franke AA, Custer LJ, Wang W, Shi CY. HPLC analysis of isoflavonoids and other phenolic agents from human fluids. Proc Soc Exp Biol Med. 1998;217:263–273. | |
Hendrich S, Lee KW, Xu X, Wang HJ, Murphy PA. Defining food components as new nutrients. J Nutr. 1994;124:1789S–1792S. | |
Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J Nutr. 1997;127:263–269. | |
Bhandari A, Crawford SE, Huang L, Reenstra WW. Effects of oral genistein in mice. Pediatr Pathol Mol Med. 2003;22:131–141. | |
Doerge DR, Twaddle NC, Banks EP, Jefferson WN, Newbold RR. Pharmacokinetic analysis in serum of genistein administered subcutaneously to neonatal mice. Cancer Lett. 2002;184:21–27. | |
Morris SM, Akerman GS, Warbritton AR, et al. Effect of dietary genistein on cell replication indices in C57BL6 mice. Cancer Lett. 2003;195:139–145. | |
Grubb BR. Ion transport across the normal and CF neonatal murine intestine. Am J Physiol. 1999;277:G167–G174. | |
Anderson MP, Welsh MJ. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991;88:6003–6007. | |
Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A, Boucher RC. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(-/-) mice. Proc Natl Acad Sci U S A. 1994;91:479–483. | |
Rozmahel R, Wilschanski M, Matin A, et al. Modulation of disease severity in cystic fibrosis transmembrane conductance regulator deficient mice by a secondary genetic factor. Nat Genet. 1996;12:280–287. | |
Gyomorey K, Garami E, Galley K, Rommens JM, Bear CE. Non-CFTR chloride channels likely contribute to secretion in the murine small intestine. Pflugers Arch. 2001;443:S103–S106. | |
Leverkoehne I, Holle H, Anton F, Gruber AD. Differential expression of calcium-activated chloride channels (CLCA) gene family members in the small intestine of cystic fibrosis mouse models. Histochem Cell Biol. 2006;126:239–250. | |
Yu YC, Miki H, Nakamura Y, et al. Curcumin and genistein additively potentiate G551D-CFTR. J Cyst Fibros. 2011;10:243–252. | |
Pedemonte N, Lukacs GL, Du K, et al. Small-molecule correctors of defective deltaF508 cellular processing identified by high throughput screening. J Clin Invest. 2005;115:2564–2571. | |
Caputo A, Hinzpeter A, Caci E, et al. Mutation-specific potency and efficacy of cystic fibrosis transmembrane conductance regulator chloride channel potentiators. J Pharmacol Exp Ther. 2009;330:783–791. | |
Yang H, Xu LN, Sui YJ, et al. Stimulation of airway and intestinal mucosal secretion by natural coumarin CFTR activators. Front Pharmacol. 2011;2:1–5. | |
Dannhoffer L, Billet A, Jollivet M, Melin-Heschel P, Faveau C, Becq F. Stimulation of wild-type, F508del- and G551D-CFTR chloride channels by non-toxic modified pyrrolo[2,3-b]pyrazine derivatives. Front Pharmacol. 2011;2:1–10. | |
Van Goor F, Hadida S, Grootenhuis PDJ, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106:18825–18830. | |
Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. | |
Yu H, Burton B, Huang CJ, et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros. 2012;11:237–245. | |
Clancy JP, Ruiz FE, Sorscher EJ. Adenosine and its nucleotides activate wild-type and R117H CFTR through an A2B receptor-coupled pathway. Am J Physiol. 1999;276:C361–C369. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.