Back to Journals » Drug Design, Development and Therapy » Volume 14
Effect of Fimasartan versus Valsartan and Olmesartan on Office and Ambulatory Blood Pressure in Korean Patients with Mild-to-Moderate Essential Hypertension: A Randomized, Double-Blind, Active Control, Three-Parallel Group, Forced Titration, Multicenter, Phase IV Study (Fimasartan Achieving Systolic Blood Pressure Target (FAST) Study)
Authors Chung WB , Ihm SH , Jang SW , Her SH, Park CS, Lee JM, Chang K , Jeon DS, Yoo KD, Seung KB
Received 17 September 2019
Accepted for publication 30 December 2019
Published 23 January 2020 Volume 2020:14 Pages 347—360
DOI https://doi.org/10.2147/DDDT.S231293
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Woo-Baek Chung,1 Sang-Hyun Ihm,2 Sung-Won Jang,3 Sung-Ho Her,4 Chul Soo Park,5 Jong-Min Lee,6 Kiyuk Chang,1 Doo-Soo Jeon,7 Ki-Dong Yoo,8 Ki-Bae Seung1
1Division of Cardiology, Department of Internal Medicine, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea; 2Division of Cardiology, Department of Internal Medicine, Bucheon St. Mary’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea; 3Division of Cardiology, Department of Internal Medicine, St. Paul’s Hospital; 4Daejeon St. Mary’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea; 5Division of Cardiology, Department of Internal Medicine, Yeouido St. Mary’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea; 6Division of Cardiology, Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea; 7Division of Cardiology, Department of Internal Medicine, In-Cheon St. Mary’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea; 8Division of Cardiology, Department of Internal Medicine, St. Vincent’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea
Correspondence: Sang-Hyun Ihm
Division of Cardiology, Department of Internal Medicine, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 14647, 327, Sosa-ro, Bucheon-si, Gyeonggi-do, Republic of Korea
Tel +82-32-340-7027
Fax +82-32-340-2669
Email [email protected]
Ki-Bae Seung
Division of Cardiology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 06591, 222, Banpo-daero, Seocho-gu, Seoul, Republic of Korea
Tel +82-2-2258-1134
Fax +82-2-591-1506
Email [email protected]
Purpose: Head-to-head comparison of the blood pressure (BP) lowering effect of fimasartan versus valsartan, with olmesartan as a reference, on office blood pressure and ambulatory BP.
Patients and Methods: Of the 369 randomly assigned patients in this study, 365 hypertensive patients were referred as the full analysis set and divided into 3 groups with a 3:3:1 ratio (fimasartan group: 155, valsartan group: 157, olmesartan group: 53). After the 2-week single-blind placebo run-in period, initial standard doses of 60-mg fimasartan, 80-mg valsartan, and 10-mg olmesartan were administered for 2 weeks, then forcibly up-titrated higher doses (fimasartan 120 mg, valsartan 160 mg, olmesartan 20 mg) were given for 4 weeks. ABP was measured before and after the 6-week treatment. Primary endpoint was reduction of sitting office systolic BP (SiSBP) of fimasartan compared to valsartan after 6 weeks. Secondary endpoints were reduction of sitting office diastolic BP (SiDBP) and 24 hrs, day-time, and night-time mean systolic and diastolic ABP (ASBP, ADBP) after 6 weeks.
Results: Patients’ mean age was 58.34± 7.68 years, and 289 patients were male (79.18%). After the 6-week treatment, SiSBP reduction of fimasartan and valsartan were − 16.26± 15.07 and − 12.81± 13.87 (p=0.0298) and SiDBP were − 7.63± 9.67 and − 5.14± 8.52 (p=0.0211). Reductions in 24 hrs mean ASBP were − 15.22± 13.33 and − 9.45± 12.37 (p=0.0009), and ADBPs were − 8.74± 7.55 and − 5.98± 7.85 (p=0.0140). Reductions of night-time ASBPs were − 16.80± 15.81 and − 10.32± 14.88 (p=0.0012), and those of night-time ADBPs were − 8.89± 9.93 and − 5.55± 9.70 (p=0.0152). Reduction of BP in olmesartan group did not demonstrate significant difference with fimasartan group in all end-points.
Conclusion: Fimasartan 120-mg treatment demonstrated superior efficacy in reduction of SiSBP, SiDBP, and 24 hrs ASBP and ADBP compared to valsartan 160 mg. Reduction of night-time ASBP from baseline was largest in fimasartan group, suggesting that fimasartan may be effective for recovering dipping pattern.
NCT number: NCT02495324 (Fimasartan Achieving SBP Target (FAST) study).
Keywords: 24 hr ambulatory blood pressure monitoring, angiotensin receptor blocker, essential hypertension, antihypertensive
Introduction
New guidelines from both American1 and European2 society of hypertension emphasized strict blood pressure (BP) control, wherein the American guideline even lowered diagnostic and target office BP level to 130/80 mmHg, and the importance of out-of-office BP measurement in the management of hypertensive patients. Since the first angiotensin II receptor blocker (ARB) losartan was approved to be used for the treatment of hypertension nearly two decades ago,3,4 ARBs have been the major medical treatment of choice for hypertension in the latest guidelines.1,2 In 2019, nine ARBs are available for the treatment of hypertension in Korea, which puts physicians into a dilemma of selecting the best ARB for the patients. The major selection criterion for the antihypertensive drug is its efficacy in lowering BP, and there are several reports that compared the efficacy among ARBs in treating essential hypertension,5–7 but fimasartan was not included in most of the analyses.
Fimasartan was launched in 2011 in Korea; its safety and efficacy in lowering BP have been well established in Korean8–12 and Mexican populations.13 Given that fimasartan is the ninth ARB that is available in the market, comparative studies with other ARBs, which were developed prior to fimasartan, are not sufficient. Previously, the efficacy of fimasartan was compared with losartan,8 valsartan,10 and candesartan.11 In comparison with losartan 50/100 mg, fimasartan 60/120 mg demonstrated a non-inferior BP-lowering effect, especially in sitting office diastolic BP (SiDBP), after 12 weeks of treatment,8 and fimasartan 60 mg demonstrated superior sitting office systolic BP (SiSBP) lowering effect compared to candesartan 8 mg after 12 weeks of treatment.11 In a previous study comparing fimasartan 30 mg with valsartan 80 mg, fimasartan also demonstrated superior BP-lowering effect than valsartan, and changes in SiSBP and SiDBP were significant after 4 weeks of treatment, with the difference maintained even after 8 weeks.10 There are some ambulatory BP (ABP) data on the efficacy of fimasartan;9,12 one parallel-group study compared fimasartan 60 mg, fimasartan 120 mg, and valsartan 80 mg in 1:1:1 manner and another study compared fimasartan 30 mg and valsartan 80 mg. Both studies could not demonstrate the superiority of fimasartan over valsartan in lowering BP because of the relatively small number of patients and the fact that the standard dose (80 mg) of valsartan was being compared with the reduced dose (30 mg) of fimasartan,12 showing the absence of head-to-head comparison.9 Therefore, studies evaluating the BP-lowering effect of fimasartan compared to other ARBs are needed.
In this study, we aimed to evaluate the lowering effect of fimasartan by comparing the sitting office BP- and ABP-lowering effect between fimasartan 120 mg and valsartan 160 mg among patients with mild-to-moderate essential hypertension through a head-to-head comparison. Olmesartan 20 mg was used as reference.
Methods
BP Measurements
Sitting office BPs were measured after resting for 5 mins on a backrest chair, and measurements were taken three times with a 1–2 mins interval; the mean SiSBP and SiDBP were calculated. At the screening visit, brachial artery BP of both arms were measured. The higher BP measurement obtained was used during randomization and following visits using a semi-automated sphygmomanometer [HEM-7080IT, Omron Corporation, Kyoto, Japan]. Every center used the same ABP monitoring (ABPM) device (TM-2403, A&D Company Ltd, Tokyo, Japan) and protocol. Twenty-four hours ABPM was recorded every 15 mins from 7:00 AM to 10:59 PM (day-time) and every 30 mins from 11:00 PM to 6:59 AM the next day (night-time). BP recordings were accepted only if they spanned the full 24 hrs or at least 20 hrs.
Study Populations
Patients aged between 19 and 70 years with mild-to-moderate essential hypertension from 8 university hospitals in Korea were enrolled. All enrolled patients provided written informed consent prior to study participation. Patients with SiSBP ≥ 140 mmHg were included in the study. The exclusion criteria for the study followed that of the previous fimasartan ABPM study.12 Patients were excluded if they met any of the following exclusion criteria: (1) SiSBP ≥ 180 mmHg or SiDBP ≥ 110 mmHg at the screening, baseline, and randomization visits; (2) a difference in SiSBP ≥ 20 mmHg and a difference in SiDBP ≥ 10 mmHg between measurements in the same arm at the screening visit; (3) history of and physical or laboratory findings suggestive of secondary hypertension; (4) symptomatic orthostatic hypotension; (5) uncontrolled or severe insulin-dependent diabetes mellitus patients (HbA1c > 9%); (6) ischemic heart disease or peripheral arterial disease patients who underwent percutaneous transcatheter intervention, symptomatic (New York Heart Association symptom class III or IV) congestive heart failure, or valvular heart disease diagnosed within 6 months of screening visit; and (7) creatinine clearance (Cockroft-Gault) <30 mL/min, aspartate aminotransferase or alanine aminotransferase level more than 3 times the upper normal limit, potassium level <3.5 mmol/L or >5.5 mmol/L and other clinically significant abnormal laboratory test results.
Study Design
This study was a randomized, double blind, active control, 3-parallel group, multicenter clinical trial designed to evaluate the superiority of high-dose fimasartan (120 mg) compared to valsartan (160 mg) monotherapy in terms of efficacy and safety.
Medical history taking, blood examinations, blood chemistry, 12-lead electrocardiography (ECG), and chest radiography were performed at the screening visit to evaluate the suitability of the patients (Visit 1). The patients who met the inclusion criteria went through a 2-week placebo run-in period. In those who were on medical therapy for hypertension, previous medications were discontinued, and the placebo was administered. At the baseline visit (Visit 2) after the placebo run-in period, the patients who met the exclusion criteria were not included in the analysis, and 24 hrs ABP of the finally included patients were measured. After the ABP measurement (Visit 3), patients were randomly assigned to three groups in a 3:3:1 ratio (3:3:1 = patients administered fimasartan as the treatment group: those administered valsartan as the control group: those administered olmesartan as the reference group). Each group was administered an initial standard-dose of fimasartan 60 mg, valsartan 80 mg, and olmesartan 10 mg, respectively (from Visit 3), for 2 weeks, and then up-titrated to higher doses of fimasartan 120 mg, valsartan 160 mg, and olmesartan 20 mg, respectively (from Visit 4) for 4 weeks. Final ABPs were measured after a total of 6 weeks of treatment period (Visit 5), and final sitting office BP were measured (Visit 6) after completing the final ABP measurement (Figure 1).
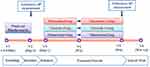 |
Figure 1 Study design. Abbreviations: BP, blood pressure; V, Visit, W, week. |
We used the stratified block randomization method, which stratified the patients into investigational sites in a double-blind study. An independent statistician generated the random assignment numbers using SAS® Version 9.4 (SAS institute, Cary, NC, USA). The placebo run-in period was single blinded, whereas the randomized treatment period was double-blinded.
Efficacy and Safety Evaluation
The primary efficacy was evaluated by the changes in SiSBP from baseline to end of the 6-week-treatment period. The changes in SiSBP and SiDBP after 2 weeks of lower-dose (initial standard dose) treatment and 4 weeks of up-titrated higher-dose treatment were analyzed for the secondary efficacy evaluation. From the ABP parameters, the changes in mean 24 hrs, day-time, and night-time ambulatory systolic BP (ASBP) and diastolic BP (ADBP) were evaluated as secondary efficacy endpoints.
For the safety evaluation, treatment-exposure adverse events (TEAEs), including overall summary and display of adverse events (AEs), fatal and serious AEs, as well as other significant AEs, and clinical laboratory evaluations (i.e. hematology, serum biochemistry, and urinary) were summarized in the safety group. For the other safety analysis, vital signs taking, 12-lead ECG, and physical examinations were performed.
Sample Size Determination
To demonstrate the superiority of fimasartan over the control drug valsartan in terms of the SiSBP-lowering effect in patients with essential hypertension, this study used the following statistical hypothesis.
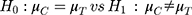
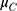 : Change in SiSBP of valsartan from the baseline at week 6
: Change in SiSBP of valsartan from the baseline at week 6
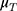 : Change in SiSBP of fimasartan from the baseline at week 6
: Change in SiSBP of fimasartan from the baseline at week 6
Change from the baseline at week 6 = week 6 – baseline
To calculate the number of subjects in this study, we referred to the clinical SiSBP drop results (−17.84 ± 12.52 mmHg) in the previous study.8 For the subject numbers of valsartan, the weighted mean (−13.60 mmHg) and pooled SD (12.54 mmHg) from the references8,14 that presented clinical SiSBP-lowering effects were used. Based on the abovementioned data, the differences in the clinical SiSBP-lowering effect between fimasartan and valsartan were set at 4.24 mmHg, the standard deviation of the difference among groups was assumed to be 12.54 mmHg, and the number of test subjects was calculated using the following formula:
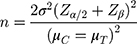
The number of subjects at the significance level of 5% (two sided) and a statistical power of 80% was calculated to be 138 per group, and 154 patients were required per group when considering a 10% dropout rate. In this study, olmesartan was considered as a reference group in addition to the treatment group (fimasartan) and control group (valsartan); thus, 360 people were needed by setting the allocation ratio to 3:3:1 (154 patients for fimasartan, 154 patients for valsartan, and 52 patients for olmesartan).
Statistical Analysis
For the demographic data, descriptive statistics (number of subjects, mean, standard deviation) are presented for continuous variables, and categorical variables are presented as the frequency (N) and percentage (%). Analysis of variance (ANOVA) or Kruskal–Wallis test was used to identify differences in the continuous variables among the groups. Chi-squared test or Fisher’s exact test was used to analyze the categorical variables.
This study followed the intention-to-treat principle, and the data obtained from the subjects were largely divided into the safety set, the full analysis set (FAS), and the per-protocol set (PPS). The primary analysis set for efficacy evaluation was the FAS, but the analysis was also performed for the PPS. The result of safety evaluation was presented for the safety set. For the efficacy variables, descriptive statistics are presented for each visit. Changes from baseline were assessed using the paired t-test or Wilcoxon signed-rank test. For the primary efficacy analysis, the descriptive statistics are presented for the SiSBP results of each visit (baseline, 6 weeks) and changes from the baseline within group, and the paired t-test or Wilcoxon signed-rank test were performed to analyze the changes within each group. An analysis of covariance (ANCOVA) with baseline values as covariates and treatment groups as factors was used to test whether difference among treatment groups was significant. For the differences among the groups, covariance analysis was performed, with the treatment group as a factor and baseline SiSBP as a covariate. Then, least squares mean and standard error are presented for each treatment group. Least squares (LS) mean for difference, the p-value, and 95% confidence interval for the control and test groups are presented.
For the secondary efficacy analysis, the changes in SiSBP after treatment with investigational drugs at 2 weeks from baseline, the changes in SiDBP after treatment with investigational drugs at 2 and 6 weeks from baseline, the controlled SBP rate (SiSBP<140 mmHg) after 2 weeks or 6 weeks of treatment, and target BP rate (SiSBP<140 mmHg and SiDBP<90 mmHg), the changes of mean ASBP and ADBP for 24 hrs, day-time, and night-time at 6 weeks from baseline, and the changes in night-time dipping pattern assessed by ABP monitoring after treatment were presented. For safety analysis, all TEAEs were standardized by MedDRA 20.0 and analyzed by System Organ Class (SOC) and Preferred Term (PT). For subjects who experienced TEAEs, ADRs, serious TEAEs/ADRs, AEs/ADRs leading to IP discontinuation, or fatal damages from AEs/ADRs during the study, the incidence number of subjects, number of events, and the 95% confidence interval of the incidence were presented.
Results
Study Population
The participation status of the study subjects is shown in Figure 2. A total of 668 patients underwent a screening test and 369 patients were randomized to the groups (155 in the fimasartan group, 161 in the valsartan group, and 53 in the olmesartan group) who were all administered the investigational drugs. Among the randomized patients, 48 patients did not continue (fimasartan group: 23, valsartan group: 19, olmesartan group: 6), whereas 321 patients completed the study. Of the 369 randomly assigned patients in the trial, 365 subjects were defined as FAS (155 in the fimasartan group, 157 in the valsartan group, and 53 in the olmesartan group), whereas the other 4 did not meet the primary efficacy evaluation criteria after baseline screening. Of the 365 subjects in the FAS, 311 subjects were included in the PPS (127 in the fimasartan group, 137 in the valsartan group, and 47 in the olmesartan group), whereas the other 54 patients dropped out and displayed significant protocol violations.
 |
Figure 2 Study disposition. **Reason for drop out: (1) withdrawal of subject’s consent, (2) protocol violation, (3) lack of efficacy, (4) adverse events, (5) lost to follow-up, and (6) other reasons. |
Demographic and baseline characteristics of the study subjects in FAS are shown in Table 1. The mean age of patients was 58.34±7.68 years, and the study patients consisted of 289 (79.18%) men and 76 (20.82%) women. The mean body mass index was 25.75±2.98 kg/m2. There were 76 smokers (20.82%), 182 non-smokers (49.86%), and 107 former smokers (29.32%). There were 250 drinkers (68.49%) and 115 non-drinkers (31.51%). The demographic and baseline characteristics were not significantly different among the treatment groups.
 |
Table 1 Baseline Clinical Characteristics (Full Analysis Set) |
Efficacy
The results of the primary efficacy endpoint, the changes in SiSBP after 2 weeks of initial standard dose treatment and followed by 4 weeks of higher-dose treatment of fimasartan, valsartan, and olmesartan are shown in Figure 3. Reduction of SiSBP in the FAS after 6 weeks of treatment from the baseline were −16.26±15.07 mmHg in the fimasartan group, −12.81±13.87 mmHg in the valsartan group, and −14.78±16.12 mmHg in the olmesartan group, showing significant difference from baseline (p<0.0001, p <0.0001, and p <0.0001, respectively). Comparing the adjusted mean changes (LS mean±SE) between the fimasartan and valsartan groups (−16.27±1.13 mmHg and −12.80±1.12 mmHg), the difference of LS mean was −3.47±1.59 mmHg (p=0.0298), indicating that fimasartan showed superiority in terms of SiSBP-lowering effect. Compared with the olmesartan group as a reference, the difference of LS mean± SE of the fimasartan group vs olmesartan group was −1.62±2.39 mmHg, indicating that fimasartan was more effective lowering SiSBP than olmesartan, but the difference was not statistically significant (p = 0.4991) (Figure 4).
 |
Figure 4 Changes in sitting office systolic blood pressure (SiSBP) from baseline. (*p=0.0298, valsartan vs fimasartan, **p=0.4991 olmesartan vs fimasartan). |
In the results of the secondary efficacy endpoint, there was a significant decrease in the change of SiSBP in all treatment groups after 2 weeks of initial standard dose treatment compared with baseline (p<0.0001 in all three groups). The fimasartan group showed a significantly greater decrease by −4.31±1.50 mmHg than the valsartan group (p=0.0044). The fimasartan group showed a significantly greater reduction of SiDBP by a difference of −2.35±0.91 mmHg (p=0.0106) at 2 weeks and −2.23±0.96 mmHg (p=0.0211) at 6 weeks from baseline than the valsartan group. Twenty-four hours ABP profiles are demonstrated in Figure 5, which showed that night-time ASBP difference of fimasartan is greatest between the baseline and at 6 weeks. The calculated reduction of night-time ASBP (−16.80±15.81 mmHg) in the fimasartan group was greatest than that of day-time ASBP (−14.39±14.95 mmHg) of the fimasartan group with clinical significance (p<0.0001) (Figure 6). The change of ABP measurement at 6 weeks compared with baseline is demonstrated in Table 2. The mean ASBP and ADBP data for 24 hrs, day-time, and night-time after 6 weeks of treatment compared with the baseline showed a significant decrease among all groups (p<0.0001 in all three groups). The fimasartan group showed a significantly greater decrease by a difference of −5.68±1.68 mmHg (p=0.0009) for ASBP and −2.46±0.99 mmHg (p=0.0140) for ADBP than the valsartan group. The day-time mean ASBP and ADBP after 6 weeks of treatment significantly decreased among all groups compared with baseline (p<0.0001 in all three groups). Moreover, the fimasartan group showed a significantly greater decrease in day-time ASBP by a difference of −5.22±1.86 mmHg (p=0.0054) than the valsartan group. The fimasartan group showed greater reduction in day-time ADBP by a difference of −2.06±1.06 mmHg (p=0.0535) than the valsartan group, but the difference was not statistically significant. The mean night-time ASBP and ADBP significantly decreased after 6 weeks of treatment compared to baseline in all groups (p<0.0001 for all three groups). In the fimasartan group, the night-time ASBP significantly decreased by a difference of −6.34±1.94 mmHg (p=0.0012) and the night-time ADBP decreased by a difference of −3.07±1.26 mmHg (p=0.0152).
 |
Table 2 Comparison of Ambulatory Blood Pressure (Full Analysis Set) |
 |
Figure 5 24-hr systolic blood pressure profiles of fimasartan (A), valsartan (B), and olmesartan (C). |
Safety
Among the 369 enrolled patients who received the investigational drugs after randomization, 61 AEs (TEAEs) occurred in 49 patients (13.28%). As the study was designed for forced up-titration to higher-dose treatment, the overall safety results are summarized in Table 3. Based on the safety evaluation, there was no difference in the incidence rate of AEs and ADRs among the groups, and there were no unusual findings during the trial compared with previously known AEs. Although not presented in the table, the most frequent TEAE was headache in 11 subjects (2.98%) [6 subjects (3.87%) in the fimasartan group, 4 subjects (2.48%) in the valsartan group, 1 subject (1.89%) in the olmesartan group], followed by dizziness in 5 subjects (1.36%) [3 subjects (1.94%) in the fimasartan group, 1 subject (0.62%) in the valsartan group, 1 subject (1.89%) in the olmesartan group].
 |
Table 3 Overall Summary of TEAEs and ADRs (Safety Set) |
Discussion
This study demonstrated the head-to-head comparison of fimasartan and valsartan to evaluate which has superior efficacy in reducing BP among mild-to-moderate Korean hypertensive patients, using olmesartan as reference. Given that fimasartan is a late runner in the class of ARBs, comparison with earlier ARBs to prove its BP-lowering effect is somewhat inevitable. Later ARBs, such as olmesartan15,16 and telmisartan,5,17 also underwent a head-to-head comparison with losartan, valsartan, and irbesartan to evaluate their efficacy in the earlier days. Fimasartan had undergone several comparative studies with losartan,8 valsartan,9,10,12 and candesartan,11 but previous studies focused on the evaluation of the BP-lowering effect of fimasartan not of its comparative efficacy with other ARBs. This study is the first fimasartan study that evaluated its comparative efficacy in lowering BP and proved its superiority over valsartan in terms of SiSBP reduction. Given that olmesartan showed superior BP-lowering effect in most of the previous comparative studies,15,16 olmesartan was used as the reference drug.
The result of primary endpoint clearly demonstrated that fimasartan 120 mg has superior efficacy in SiSBP reduction compared with valsartan 160 mg. In comparison with a previous losartan study,8 the primary endpoint was reduction of SiDBP after 4 weeks of standard-dose treatment and followed by 4 weeks of higher-dose treatment, which was similar to the design of the present study but the dose was up-titrated in patients with SiDBP >90 mmHg after 4 weeks of standard dose treatment, not forced up-titration. The reduction of SiSBP after 8 weeks of fimasartan treatment was −17.84±12.52 mmHg, which seemed to be greater that the reduction obtained from the present study (−16.26±15.07 mmHg). The primary endpoint was also SiDBP in comparison study with candesartan11 and the reduction of SiSBP after 8 weeks of treatment of standard-dose fimasartan was −20.4±11.6 mmHg and higher-dose fimasartan was −22.1±13.4 mmHg. Both previous studies8,11 were conducted on younger patients, a small number of patients with less male ratio, and with longer treatment period than the current study, which may be the reason why the reduction of SiSBP was larger in the previous studies than in the present study.
The ABP-lowering effect of 30 mg,12 60 mg, and 120 mg9 treatment of fimasartan for 8 weeks has been previously reported. Previous ABP studies compared fimasartan 30 mg and valsartan 80 mg and demonstrated comparable BP lowering in mean 24 hrs, day-time, and night-time ASBP and ADBP, similar to the results of the present study but did not demonstrated statistically significant difference. Only the fimasartan 30 mg ABP study12 showed a similar result to this study wherein the reduction of night-time ASBP was greater than the reduction of day-time ASBP. The 60 mg and 120 mg fimasartan study did not show similar results (day-time reduction was larger than night-time reduction), because the definition of night-time was different (10 PM to 7 AM) in both the reduced dose study and the present study (11 PM to 7 AM); result may not sufficient to compare with the other fimasartan ABP studies.
The concept of “Perfect 24 hrs BP control”, suggested by some hypertension experts,18 indicates that the important treatment targets of BP management include not only the maintenance of the mean 24 hrs BP to <130/80 mmHg but also the maintenance of dipping pattern and reduction of morning BP surge. Maintaining adequate dipping of nocturnal BP is the second step of the perfect 24 hrs BP control, which follows the first step that is to achieve the mean 24 hrs BP level < 130/80 mmHg. Night-time ABP is now a therapeutic target for the reduction of cardiovascular risk,19 and a Korean ABP observational study has reported that high nocturnal BP, not non-dipping pattern, is a better predictor for left ventricular hypertrophy in essential hypertension patients.20 Finally, reducing morning BP surge <45 mmHg, the third step for the perfect BP control, is important for the prevention of cardiovascular events.21 Recently, a study comparing the effect of valsartan and olmesartan in non-dipper hypertensive patients,22 showed that evening administration of valsartan and olmesartan was effective for recovering dipping pattern, but the study was focused on the chronotherapeutic effects of valsartan and olmesartan and their comparative efficacy. There is still little data on comparative efficacy of night-time ABP-lowering effect among ARBs. The ABP data from this study demonstrated that the reduction in night-time ASBP in the fimasartan group was the largest even during morning administration, whereas the olmesartan group showed a larger reduction in mean day-time ASBP compared to mean night-time ASBP in this study. Considering that the reduction of night-time ABP is important for maintaining the circadian rhythm and dipping BP pattern, the result may suggest that fimasartan is effective for maintaining dipping pattern of the BP circadian rhythm. Subgroup analysis involving non-dipper hypertensive patients is need for further understanding.
Given that this study was designed for forced up-titration to higher dose, safety of the patients who participated in the study was of major concern. The AEs and ADRs reported in this study were similar in each treatment group, and the differences in the incidence of AEs and ADRs among the groups were not statistically significant. The AEs in the fimasartan, valsartan, and olmesartan groups were not unusual in comparison with the previously known adverse drug reactions. In addition, no AE or serious AE (serious TEAE) that caused death was reported, and most of the patients who experiences AEs and ADRs that resulted in discontinuation of drug administration recovered without further treatment.
Conclusion
The head-to-head comparison showed that fimasartan 120 mg had superior SiSBP-lowering effect than valsartan 160 mg. Moreover, fimasartan demonstrated a significant efficacy in reducing SiDBP compared to valsartan. In terms of ABP reduction, fimasartan showed greater reduction in mean 24 hr, day-time, and night-time ASBP and ADBP than valsartan. The amount of ABP reduction in night-time ASBP was largest, suggesting that fimasartan is effective for the recovery of night-time dipping pattern. On the other hand, comparisons with olmesartan showed comparable results in both office BP and ABP. In addition, there were no significant differences in the incidence of AEs and ADRs. The AEs experienced by some patients in the fimasartan, valsartan, and olmesartan groups were not unusual in comparison with the previously known ADRs. In addition, no adverse event or serious AE (serious TEAE) that caused death was reported, and most patients with AEs and ADRs that resulted in the discontinuation of drug administration recovered without further treatment.
Ethics Approval and Informed Consent
The Institutional Review Board of the Catholic University of Korea, Seoul, Republic of Korea reviewed and approved the study protocol and monitored this study which was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All enrolled patients provided informed consent prior to study participation.
Data Sharing Statement
Raw data of this study are available from the Boryung Pharmaceutical Co. Ltd.
Disclosure
The study drugs were supplied by Boryung Pharmaceutical Co. Ltd.
The authors have indicated that they have no other conflicts of interest with regard to the content of this article.
References
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. doi:10.1161/HYP.0000000000000066
2. Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2018;36(12):2284–2309. doi:10.1097/HJH.0000000000001961
3. Carr AA, Prisant LM. Losartan: first of a new class of angiotensin antagonists for the management of hypertension. J Clin Pharmacol. 1996;36(1):3–12. doi:10.1002/j.1552-4604.1996.tb04146.x
4. Messerli FH, Weber MA, Brunner HR. Angiotensin II receptor inhibition. A new therapeutic principle. Arch Intern Med. 1996;156(17):1957–1965. doi:10.1001/archinte.1996.00440160069010
5. Abraham HM, White CM, White WB. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. 2015;38(1):33–54. doi:10.1007/s40264-014-0239-7
6. Tsoi B, Akioyamen LE, Bonner A, et al. Comparative efficacy of angiotensin II antagonists in essential hypertension: systematic review and network meta-analysis of randomised controlled trials. Heart Lung Circ. 2018;27(6):666–682. doi:10.1016/j.hlc.2017.06.721
7. Dimou C, Antza C, Akrivos E, et al. A systematic review and network meta-analysis of the comparative efficacy of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in hypertension. J Hum Hypertens. 2019;33(3):188–201. doi:10.1038/s41371-018-0138-y
8. Lee SE, Kim YJ, Lee HY, et al. Efficacy and tolerability of fimasartan, a new angiotensin receptor blocker, compared with losartan (50/100 mg): a 12-week, Phase III, multicenter, prospective, randomized, double-blind, parallel-group, dose escalation clinical trial with an optional 12-week extension phase in adult Korean patients with mild-to-moderate hypertension. Clin Ther. 2012;34(3):
9. Lee H, Kim KS, Chae SC, Jeong MH, Kim DS, Oh BH. Ambulatory blood pressure response to once-daily fimasartan: an 8-week, multicenter, randomized, double-blind, active-comparator, parallel-group study in Korean patients with mild to moderate essential hypertension. Clin Ther. 2013;35(9):1337–1349. doi:10.1016/j.clinthera.2013.06.021
10. Youn JC, Ihm SH, Bae JH, et al. Efficacy and safety of 30-mg fimasartan for the treatment of patients with mild to moderate hypertension: an 8-week, multicenter, randomized, double-blind, Phase III clinical study. Clin Ther. 2014;36(10):1412–1421. doi:10.1016/j.clinthera.2014.07.004
11. Lee JH, Yang DH, Hwang JY, et al. A randomized, double-blind, candesartan-controlled, parallel group comparison clinical trial to evaluate the antihypertensive efficacy and safety of fimasartan in patients with mild to moderate essential hypertension. Clin Ther. 2016;38(6):1485–1497. doi:10.1016/j.clinthera.2016.04.005
12. Lee HY, Kim CH, Song JK, et al. 24-hour blood pressure response to lower dose (30 mg) fimasartan in Korean patients with mild to moderate essential hypertension. Korean J Intern Med. 2017;32(6):1025–1036. doi:10.3904/kjim.2016.094
13. Cardona-Munoz EG, Lopez-Alvarado A, Conde-Carmona I, et al. Safety and efficacy of fimasartan in Mexican patients with grade 1–2 essential hypertension. Arch Cardiol Mex. 2017;87(4):316–325. doi:10.1016/j.acmx.2017.01.001
14. Hedner T, Oparil S, Rasmussen K, et al. A comparison of the angiotensin II antagonists valsartan and losartan in the treatment of essential hypertension. Am J Hypertens. 1999;12(4):414–417. doi:10.1016/S0895-7061(99)00082-5
15. Stumpe KO. Olmesartan compared with other angiotensin II receptor antagonists: head-to-head trials. Clin Ther. 2004;26(Suppl A):A33–A37. doi:10.1016/S0149-2918(04)90144-0
16. Zannad F, Fay R. Blood pressure-lowering efficacy of olmesartan relative to other angiotensin II receptor antagonists: an overview of randomized controlled studies. Fundam Clin Pharmacol. 2007;21(2):181–190. doi:10.1111/j.1472-8206.2007.00464.x
17. Smith DH. Comparison of angiotensin II type 1 receptor antagonists in the treatment of essential hypertension. Drugs. 2008;68(9):1207–1225. doi:10.2165/00003495-200868090-00003
18. Kario K. Perfect 24-h management of hypertension: clinical relevance and perspectives. J Hum Hypertens. 2017;31(4):231–243. doi:10.1038/jhh.2016.65
19. Hermida RC, Ayala DE, Mojon A, Smolensky MH, Portaluppi F, Fernandez JR. Sleep-time ambulatory blood pressure as a novel therapeutic target for cardiovascular risk reduction. J Hum Hypertens. 2014;28(10):567–574. doi:10.1038/jhh.2014.1
20. Yi JE, Shin J, Ihm SH, et al. Not nondipping but nocturnal blood pressure predicts left ventricular hypertrophy in the essential hypertensive patients: the Korean ambulatory blood pressure multicenter observational study. J Hypertens. 2014;32(10):1999–2004. doi:10.1097/HJH.0000000000000272
21. Kario K. Systemic hemodynamic atherothrombotic syndrome and resonance hypothesis of blood pressure variability: triggering cardiovascular events. Korean Circ J. 2016;46(4):456–467. doi:10.4070/kcj.2016.46.4.456
22. Ushijima K, Nakashima H, Shiga T, et al. Different chronotherapeutic effects of valsartan and olmesartan in non-dipper hypertensive patients during valsartan treatment at morning. J Pharmacol Sci. 2015;127(1):62–68. doi:10.1016/j.jphs.2014.09.004
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


