Back to Journals » Infection and Drug Resistance » Volume 11
Effect of ethanol pretreatment in Acanthamoeba keratitis: a long-term follow-up study
Authors Lin IH, Tseng SH, Huang FC, Huang YH
Received 9 March 2018
Accepted for publication 1 May 2018
Published 5 July 2018 Volume 2018:11 Pages 937—943
DOI https://doi.org/10.2147/IDR.S167775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
I-Huang Lin,1 Sung-Huei Tseng,1 Fu-Chin Huang,1 Yi-Hsun Huang1,2
1Department of Ophthalmology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan, Republic of China; 2Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan, Republic of China
Purpose: The aim of this study was to evaluate the long-term outcomes of ethanol pretreatment in Acanthamoeba keratitis (AK).
Patients and methods: This single-center, retrospective, interventional study included 22 patients (24 eyes) who developed AK and underwent ethanol pretreatment between 2009 and 2015. Samples for smears, polymerase chain reaction, and culture for evidence of Acanthamoeba were collected. After ethanol pretreatment, the patients were treated with corneal epithelial debridement, topical 0.02% polyhexamethylene biguanide, and 0.1% propamidine isethionate. The primary outcomes were a clinically stable ocular surface, complete recovery from corneal infection, and acceptable corneal haze. The secondary outcome measure was improvement in best-corrected visual acuity. Complications and predictors of the visual outcome were also recorded.
Results: Ethanol pretreatment was successful in 20 (83.3%) of the 24 eyes, and no further optical keratoplasty was required. Four eyes required rescue therapeutic keratoplasty because of rapid progression of AK. Patients in whom ethanol pretreatment was successful achieved good final visual outcomes regardless of sex, age, or causative Acanthamoeba species. Patients with worse initial best-corrected visual acuity and rigid gas permeable lens-related AK had better improvement in vision.
Conclusion: Ethanol as a pretreatment for AK is safe and effective. Combined with corneal epithelial debridement, ethanol pretreatment may preclude the need for optical and therapeutic keratoplasty. This technique is suitable for all stages of AK presenting within 3 weeks of symptom onset and achieves favorable results especially in early AK.
Keywords: Acanthamoeba keratitis, Acanthamoeba, ethanol pretreatment, corneal infection, corneal epithelial debridement
Introduction
Acanthamoeba keratitis (AK) is a potentially sight-threatening infection caused by several types of amoeba of the genus Acanthamoeba.1 In recent years, AK has been increasing in prevalence, and outbreaks attributable to the use of certain types of contact lenses and cleaning solutions have been recorded.2,3 The traditional treatments for AK are topical medications such as biguanides (0.02% chlorhexidine or 0.02% polyhexamethylene biguanide [PHMB]) and diamidines (0.1% propamidine isethionate); for those who fail topical treatments, surgical interventions such as therapeutic penetrating keratoplasty (PKP) or deep anterior lamellar keratoplasty are undertaken.4,5 Although numerous studies have been performed in an effort to improve the diagnosis and treatment of AK,6,7 courses of topical anti-amoebic agents for up to 12 months remain the mainstay of treatment.8 Unfortunately, because of drug resistance,9 failure of topical medications is not uncommon, and PKP is still required in at least 25% of cases.10 Therefore, novel treatments to enhance drug efficacy are needed to decrease the morbidity associated with AK.
Mechanical epithelial debridement has been established as an effective adjuvant therapy for AK.11 We have modified this mechanical debridement procedure by pretreating the cornea with ethanol.12 Using a method similar to laser-assisted subepithelial keratomileusis (LASEK), we soaked the cornea with ethanol for 30 seconds and performing full-thickness debridement of the corneal epithelium to remove the whole layer of epithelium, thereby encouraging penetration of topical anti-amoebic agents. Our preliminary results in four AK cases showed that ethanol pretreatment combined with epithelial debridement was a successful technique.12
The aim of this study was to report the long-term effect of ethanol pretreatment in AK. We found that ethanol pretreatment not only produced favorable results in patients with early AK but also achieved satisfactory outcomes in some patients with advanced recalcitrant AK and deep stromal involvement.
Patients and methods
Study approval, design, and subjects
The protocol for this retrospective study was approved by the institutional review board of National Cheng-Kung University Hospital, which granted a waiver of the need for informed consent because the data source used maintained patient anonymity. We reviewed the medical records of patients at National Cheng-Kung University Hospital from December 1, 2009, to December 31, 2015, and identified the patients with positive results for both culture and polymerase chain reaction (PCR) examinations for inclusion in the study. Patients who could not be followed up for 1 year were excluded.
Efficacy outcome measures
The primary efficacy outcome measure was the success of ethanol pretreatment in eyes with AK, which was defined as a clinically stable condition, complete recovery from infection, and acceptable corneal haze. Failure was defined as progressive AK and requirement for PKP. The secondary outcome measure was the change in best-corrected visual acuity (BCVA) at each follow-up visit.
Safety outcome measures
The safety outcome measures were the intraoperative and postoperative complications of ethanol pretreatment.
Surgical technique of alcohol-assisted epithelial debridement
We performed ethanol pretreatment by placing a 7-mm corneal well (Asico, Westmont, IL, USA) over the clinically involved area, filling the well with 20% ethanol, withdrawing the ethanol after 30 seconds by adsorbing it into a cellulose sponge, irrigating the ocular surface with 10 mL of balanced salt solution, and then peeling off the well-delineated corneal epithelium with a blunt spatula. The debrided full-thickness epithelium was divided into several portions that were sent for Gram staining, acid-fast staining, histopathologic examination, PCR, and culture in non-nutrient agar overlain with Escherichia coli.
Care protocol
After ethanol pretreatment, all patients received corneal epithelial debridement and topical combination therapy consisting of 0.02% PHMB and 0.1% propamidine isethionate. The topical medications were to be applied hourly for at least 1 week and then tapered weekly according to the patient’s clinical response. Ethanol pretreatment was repeated if the clinical response was inadequate. The study eyes were examined daily in patients who had been admitted to the hospital. Patients who were being followed up in the outpatient department were seen on days 1, 3, 7, 14, 21, and 35, and at 1-month intervals thereafter.
Collection of data
Data were collected at each visit in a predesigned format, and the completed form was filed in the medical record. These data included patient age and sex, Snellen BCVA, details of the ethanol pretreatment procedure, any intraoperative or postoperative complications, microbiology results, duration of follow-up, and slit-lamp findings for the ocular surface.
Statistical analyses
SPSS version 17 software (SPSS Inc., Chicago, IL, USA) was used for data analysis. Continuous parametric data are reported as mean ± SD and nonparametric data are reported as the median and range. Visual acuity was recorded using the Snellen chart and converted to logarithm of the minimum angle of resolution (log MAR) units for the statistical analyses. Fisher’s exact test was used to compare categorical data. A two-tailed P-value of <0.05 was considered statistically significant.
Results
Patient demographics and clinical characteristics
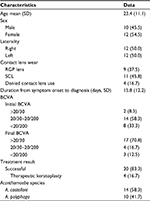
From December 1, 2009, to December 31, 2015, 24 eyes (22 patients) with AK were identified to have received ethanol pretreatment. The patient demographic and clinical characteristics are summarized in Table 1. The mean patient age was 23.4 ± 11.1 (range 13–49) years. A total of 12 patients were female and 10 were male. The infection was in the right eye in 12 cases and in the left eye in 12 cases. Nine eyes had a history of rigid gas permeable (RGP) lens use, and 11 had a history of soft contact lens (SCL) use; 4 eyes were in patients who denied any history of wearing contact lenses. A total of 22 eyes (91.7%) had an initial BCVA of <20/30 and 17 (70.8%) had a final BCVA of >20/30. The microbiology results showed that 14 eyes were infected by Acanthamoeba castellani and 10 by A. polyphaga. A total of 20 eyes were treated successfully, while the remaining 4 eyes showed rapid progression and underwent PKP for infection control.
Efficacy of ethanol pretreatment
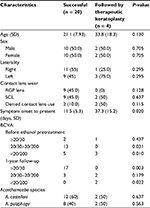
The clinical features of the successful and failed ethanol pretreatment groups are compared in Table 2. At the final follow-up visit, 20 (83.3%) of the 24 eyes were clinically stable without evidence of active lesions or recurrent infection. A total of 18 (90.0%) of the 20 eyes that responded favorably to ethanol pretreatment had trace corneal haze. There was no difference in patient age or sex, laterality, or type of contact lens use between the successful and failed ethanol treatment groups. However, the mean interval between the onset of symptoms and the intervention with ethanol pretreatment was 11.5 ± 5.3 days in the successfully treated cases and was significantly shorter (P = 0.02) than that in the treatment failure cases (37.3 ± 15.2 days). Patients who failed ethanol pretreatment also had significantly poorer initial (P = 0.01) and final (P = 0.02) BCVA than the successfully treated cases. A total of 12 of the successfully treated cases were infected by A. castellani and eight by A. polyphaga. Two of the four failed ethanol pretreatment cases were infected by A. castellani and two by A. polyphaga; the difference was not statistically significant.
Safety of ethanol pretreatment
No intraoperative and postoperative complications were detected in the eyes pretreated with ethanol. The epithelial defect generated after ethanol pretreatment healed within 2 weeks. Therapeutic PKP was performed uneventfully in the four eyes that did not initially respond to the treatment after the ethanol pretreatment.
Visual prognostic factors
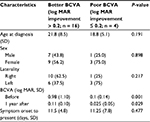
We divided the successfully treated eyes into two subgroups, i.e., those with more BCVA improvement (log MAR >0.2) and those with less improvement (log MAR ≤0.2) to determine the visual prognostic factors (Table 3). There was no significant difference in the age or sex distribution between the two subgroups; however, patients with worse initial BCVA showed significant improvement (P = 0.001). To determine whether the type of contact lens use would affect the outcome of ethanol pretreatment, we compared the demographic data for the RGP and SCL groups (Table 4). A total of 18 of the 20 successfully treated eyes had a history of wearing contact lenses (RGP, n = 9; SCL, n = 9). The patient age was 20.3 ± 10.8 years in the RGP group and 22.0 ± 5.1 years in the SCL group; the difference was not significant. However, patients with a history of wearing RGP lenses had worse initial BCVA (P = 0.02) but had more improvement in vision (P = 0.01).
Discussion
Early and accurate diagnosis is the key factor in the prognosis and final visual outcome of AK.13 Previously, the use of a spatula or blade for mechanical epithelial debridement had satisfactory results in the diagnosis and treatment of early-stage AK.14 However, extensive epithelium debridement down to Bowman’s layer can be difficult to complete, especially in young contact lens wearers, in whom adherence of the basal epithelial cells to the basement membrane is stronger. Soaking the cornea with a dilute solution of ethanol (20%) before debridement, as described in this study, is a well-established procedure for refractive surgery, such as photorefractive keratectomy and LASEK.15 Furthermore, ethanol has been found to have a cytotoxic effect on some infectious forms of Acanthamoeba.16,17 Therefore, soaking the cornea for 30 seconds in ethanol before debridement not only allows ophthalmologists to easily separate the full thickness of the epithelial layer from the basement membrane in both young and older patients with AK, but also had some parasiticidal effects. Moreover, the larger specimens harvested during debridement ensure not only a higher chance of diagnosing AK but also a better chance of penetration of topical anti-amoebic agents.
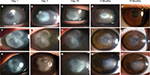
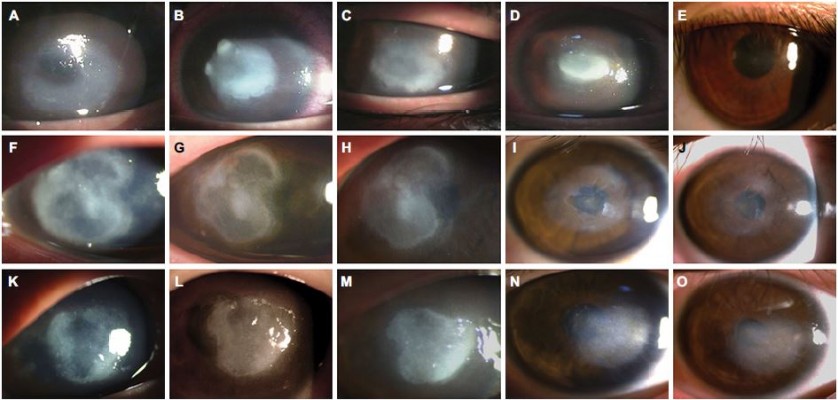
In a previous study, we described four cases of early AK which were successfully treated by pretreating the cornea with ethanol.12 Long-term follow-up of these cases showed that not only early-stage AK but also advanced late-stage AK could be successfully treated. Figure 1 shows three eyes with involvement of the corneal stroma in which dense ring-shaped infiltrations and corneal thinning were found on slit-lamp examination. Based on the previous studies, these eyes were classified as stage III–V AK.13,18 In such cases, up to 58.3% of patients may require corneal surgery.18 Ethanol pretreatment and corneal epithelial debridement were performed soon before topical administration of 0.02% PHMB and 0.1% propamidine isethionate. The topical medications were prescribed hourly for at least 1 week and then tapered weekly according to the clinical condition of the eye. For these severe AK patients, ethanol pretreatments were repeated for three times, and no toxicity was noticed due to ethanol application. During admission, the eyes were examined daily. After discharge, the patients were followed up in the outpatient department on days 3, 7, 14, 21, and 35, and at 1-month intervals thereafter. After 18 months of follow-up, there were no signs of recurrent AK, the final BCVA was as high as 20/20, and none of the eyes needed PKP.
Previously, Robaei et al10 reported that 50 (25.5%) of 196 cases diagnosed with AK finally underwent keratoplasty. A total of 24 of these 50 cases required optical keratoplasty after healing and 26 required therapeutic keratoplasty for refractory AK. The median duration of symptoms before diagnosis was 35 (25–90) days. Chew et al19 also reported that 13 eyes in 51 patients (25.5%) failed traditional medical treatment; 5 of the eyes required therapeutic keratoplasty and 8 required optical keratoplasty. Moreover, Qian et al20 reported that 8 (27.6%) out of 29 eyes underwent PK in their studies, Por et al18 showed 15 (35.7%) out of 42 eyes, Park et al21 revealed 13 (36.1%) out of 36 eyes, and Illingworth et al22 described 9 (39.1%) out of 23 eyes. In our series, the need for keratoplasty following AAED was lower (4/24, 16.7%) than that with topical treatments alone. The reason for this could be the more extensive removal of infected tissue and better penetration of topical medications into the deeper stroma. However, we found that the four eyes that failed treatment had a significantly longer interval (>3 weeks) between the onset of symptoms and ethanol pretreatment (37.3 ± 15.2 vs 11.5 ± 5.3 days, P < 0.05). Thus, despite ethanol pretreatment being an effective therapy, we believed that early and accurate diagnosis still plays a crucial role in the visual prognosis.
Nowadays, wearing contact lenses is the strongest risk factor for AK.8 In our study, 20 (83%) of 24 eyes had a history of contact lens use; 11 patients wore SCL and 9 wore RGP lenses, including orthokeratology lenses. We found that ethanol pretreatment was effective regardless of whether AK was RGP related or SCL related. Patients with a history of wearing RGP have better improvement in vision after undergoing ethanol pretreatment. Therefore, given that the wearers of RGP lenses, especially orthokeratology lenses, are younger, we suggest that ethanol pretreatment can achieve a favorable visual outcome in these patients and reduce the need for keratoplasty in the future.
Finally, there are >10 species of Acanthamoeba that can cause AK. The most common species are A. polyphaga and A. castellani,23 which were the causative pathogens isolated from all 24 eyes in our study. Ethanol pretreatment was effective for both species.
Although ethanol pretreatment was effective and safe, there are a few limitations to our study. First, there was no control group for comparison. However, since its introduction in 2009, ethanol pretreatment has proven to be not only theoretically effective but also genuinely promising in clinical practice. Therefore, in the interests of patient welfare, we have used ethanol pretreatment and corneal epithelial debridement in combination with anti-amoebic agents in all our patients with AK since 2010. Second, because AK is a relatively rare infectious disease, we could only identify 24 cases for inclusion in our study. This number is far fewer than the 196 cases treated in 1991–2012 at Moorfields Eye Hospital,10 but is close to that treated in other centers throughout the world (on average four eyes per year).20,24,25 We know that a randomized clinical trial (RCT) assigning patients to either ethanol pretreatment or standard epitheliectomy is the best way to determine better treatment for AK. Although, AK is still a rare disease, and different genotypes may lead to diverse results, it is mandatory to include controls and more cases in future studies. Therefore, it may take several years for an RCT to yield a promising conclusion. In publishing this article, we hope that sharing our experiences of ethanol pretreatment will encourage other ophthalmologists to collaborate with us in an RCT.
Conclusion
We demonstrated that ethanol pretreatment was a safe and effective procedure in patients with AK. For patients who were diagnosed within 3 weeks, ethanol pretreatment combined with corneal epithelial debridement produced favorable results even in eyes with severe recalcitrant AK. Moreover, ethanol pretreatment might preclude the need for optical and therapeutic keratoplasty and achieved more visual improvement in RGP wearers. An RCT is needed to confirm the effects of ethanol pretreatment in AK.
Acknowledgment
This study was supported by grant (NCKUH-10704003) from the National Cheng Kung University Hospital, Tainan, Taiwan.
Disclosure
The authors report no conflicts of interest in this work.
References
Awwad ST, Petroll WM, McCulley JP, Cavanagh HD. Updates in Acanthamoeba keratitis. Eye Contact Lens. 2007;33(1):1–8. | ||
Joslin CE, Tu EY, Shoff ME, et al. The association of contact lens solution use and Acanthamoeba keratitis. Am J Ophthalmol. 2007;144(2):169–180. | ||
Tu EY, Joslin CE. Recent outbreaks of atypical contact lens-related keratitis: what have we learned? Am J Ophthalmol. 2010;150(5):602–608.e602. | ||
Jiang C, Sun X, Wang Z, Zhang Y. Acanthamoeba keratitis: clinical characteristics and management. Ocul Surf. 2015;13(2):164–168. | ||
Sarnicola E, Sarnicola C, Sabatino F, Tosi GM, Perri P, Sarnicola V. Early deep anterior lamellar keratoplasty (DALK) for Acanthamoeba keratitis poorly responsive to medical treatment. Cornea. 2016;35(1):1–5. | ||
Ortilles A, Belloc J, Rubio E, et al. In-vitro development of an effective treatment for Acanthamoeba keratitis. Int J Antimicrob Agents. 2017;50(3):325–333. | ||
Tu EY, Joslin CE, Shoff ME. Successful treatment of chronic stromal Acanthamoeba keratitis with oral voriconazole monotherapy. Cornea. 2010;29(9):1066–1068. | ||
Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. | ||
Huang FC, Liu TS, Li SC, Shih MH, Shin JW, Lin WC. The effect of the disulfideisomerase domain containing protein in the defense against polyhexamethylene biguanide of highly tolerant Acanthamoeba at the trophozoite stage. Int J Parasitol Drugs Drug Resist. 2016;6(3):251–257. | ||
Robaei D, Carnt N, Minassian DC, Dart JK. Therapeutic and optical keratoplasty in the management of Acanthamoeba keratitis: risk factors, outcomes, and summary of the literature. Ophthalmology. 2015;122(1):17–24. | ||
Holland GN, Donzis PB. Rapid resolution of early Acanthamoeba keratitis after epithelial debridement. Am J Ophthalmol. 1987;104(1):87–89. | ||
Li ML, Shih MH, Huang FC, Tseng SH, Chen CC. Treatment of early Acanthamoeba keratitis with alcohol-assisted epithelial debridement. Cornea. 2012;31(4):442–446. | ||
Tu EY, Joslin CE, Sugar J, Shoff ME, Booton GC. Prognostic factors affecting visual outcome in Acanthamoeba keratitis. Ophthalmology. 2008;115(11):1998–2003. | ||
Brooks JG Jr, Coster DJ, Badenoch PR. Acanthamoeba keratitis. Resolution after epithelial debridement. Cornea. 1994;13(2):186–189. | ||
O’Keefe M, Kirwan C. Laser epithelial keratomileusis in 2010 – a review. Clin Experiment Ophthalmol. 2010;38(2):183–191. | ||
Aqeel Y, Rodriguez R, Chatterjee A, Ingalls RR, Samuelson J. Killing of diverse eye pathogens (Acanthamoeba spp., Fusarium solani, and Chlamydia trachomatis) with alcohols. PLoS Negl Trop Dis. 2017;11(2):e0005382. | ||
Ezz Eldin HM, Sarhan RM. Cytotoxic effect of organic solvents and surfactant agents on Acanthamoeba castellanii cysts. Parasitol Res. 2014;113(5):1949–1953. | ||
Por YM, Mehta JS, Chua JL, et al. Acanthamoeba keratitis associated with contact lens wear in Singapore. Am J Ophthalmol. 2009;148(1):7–12.e12. | ||
Chew HF, Yildiz EH, Hammersmith KM, et al. Clinical outcomes and prognostic factors associated with Acanthamoeba keratitis. Cornea. 2011;30(4):435–441. | ||
Qian Y, Meisler DM, Langston RH, Jeng BH. Clinical experience with Acanthamoeba keratitis at the cole eye institute, 1999–2008. Cornea. 2010;29(9):1016–1021. | ||
Park DH, Palay DA, Daya SM, Stulting RD, Krachmer JH, Holland EJ. The role of topical corticosteroids in the management of Acanthamoeba keratitis. Cornea. 1997;16(3):277–283. | ||
Illingworth CD, Cook SD, Karabatsas CH, Easty DL. Acanthamoeba keratitis: risk factors and outcome. Br J Ophthalmol. 1995;79(12):1078–1082. | ||
Maycock NJ, Jayaswal R. Update on Acanthamoeba keratitis: diagnosis, treatment, and outcomes. Cornea. 2016;35(5):713–720. | ||
Yamazoe K, Yamamoto Y, Shimazaki-Den S, Shimazaki J. Visual outcome in Japanese patients with Acanthamoeba keratitis. Eye. 2012;26(4):517–522. | ||
Lee MH, Abell RG, Mitra B, Ferdinands M, Vajpayee RB. Risk factors, demographics and clinical profile of Acanthamoeba keratitis in Melbourne: an 18-year retrospective study. Br J Ophthalmol. 2017;102(5):687–691. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.