Back to Journals » OncoTargets and Therapy » Volume 13
Effect of Dose Adjustments on the Safety and Efficacy of Afatinib in Chinese Patients with EGFR-Mutated Non-Small Cell Lung Cancer Who Participated in the LUX-Lung Clinical Trial Program
Received 19 September 2020
Accepted for publication 30 October 2020
Published 7 December 2020 Volume 2020:13 Pages 12539—12547
DOI https://doi.org/10.2147/OTT.S273866
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Hai-Yan Tu, Yi-Long Wu
Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital & Guangdong Academy of Medical Sciences, Guangzhou 510080, People’s Republic of China
Correspondence: Yi-Long Wu
Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital & Guangdong Academy of Medical Sciences, 106 Zhongshan Er Road, Guangzhou 510080, People’s Republic of China
Tel +86 20 8387 7855
Email [email protected]
Background: Post hoc analysis of the LUX-Lung 3 and 6 (LL3/6) Phase III trials showed that tolerability-guided dose-adjustments of afatinib reduced treatment-related adverse events (TRAEs) without affecting progression-free survival (PFS) in patients with epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer (NSCLC). The current post hoc analysis evaluated outcomes of tolerability-guided dose adjustments of afatinib in patients enrolled in the LL3/6/7 trials in Chinese centers.
Patients and Methods: Patients enrolled in LL3/6/7 had advanced EGFR mutation-positive NSCLC. LL3 and LL7 recruited patients globally (including China) and LL6 enrolled Asian patients from China, Thailand, and South Korea. In LL3 and LL6, patients were randomized to afatinib 40 mg/day or cisplatin-based chemotherapy. In the Phase IIb LL7 trial, patients were randomized to afatinib 40 mg/day or gefitinib. Tolerability-guided dose adjustments were permitted for TRAEs, and PFS was the primary endpoint. This post hoc analysis pooled data from patients enrolled in Chinese centers in LL3/6/7 and analyzed the frequency and severity of TRAEs before and after afatinib dose reductions during the first 6 months. PFS and overall survival (OS) were compared for patients who had a dose reduction in the first 6 months and those who did not.
Results: Overall, 299 patients were enrolled in Chinese centers; 68 (23%) had afatinib dose reductions to < 40 mg/day in the first 6 months. Prior to dose reduction, 55/68 patients (81%) experienced grade ≥ 3 TRAE versus 13/68 (19%) after dose reduction. Grade ≥ 3 TRAEs were much more common in patients with than in those without dose reduction. Median PFS was 11.0 months in both groups, and median OS did not differ significantly: 23.1 months in patients with a dose reduction and 26.9 months in those without a dose reduction.
Conclusion: Tolerability-guided afatinib dose adjustment is an effective strategy to reduce TRAEs without affecting efficacy in Chinese patients.
Keywords: afatinib, efficacy, tolerability, dose-adjustment
Introduction
Globally, first-line treatment options for epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer (NSCLC) include the use of first-generation reversible EGFR tyrosine kinase inhibitors (TKIs) erlotinib, gefitinib, and icotinib (in China), the second-generation irreversible TKIs of human epidermal growth factor (ErbB) family receptors, afatinib and dacomitinib, and the third-generation irreversible EGFR TKI osimertinib.1–3 In randomized, controlled studies of patients with advanced NSCLC, first-line treatment with these agents demonstrated improvements in clinical outcome, including progression-free survival (PFS) and objective response rate compared with standard care.4–14 Furthermore, analyses of data from the global Phase III LUX-Lung 3 (LL3) study and the LUX-Lung 6 (LL6) study in Asian patients, whose tumors harbored common EGFR Del19 mutations demonstrated a significant improvement in overall survival (OS) for afatinib-treated patients compared with cisplatin-based chemotherapy.15 Data from the LUX-Lung 7 (LL7),16 ARCHER 1050,13 and FLAURA trials14 suggest that afatinib, dacomitinib and osimertinib, respectively, are more effective than first-generation TKIs; there were no statistically significant differences in PFS or OS between the first-generation agents erlotinib and gefitinib in the CTONG 0901 trial.17 To date, there are no head-to-head studies comparing second- and third-generation TKIs, and both are approved as first-line therapy in this setting.
EGFR TKIs have well-defined adverse event (AE) profiles, consistent with their mode of action. EGFR-class treatment-related AEs (TRAEs) include diarrhea, rash/acne, stomatitis, and nail effects, which are predictable and manageable with established protocols for dose modification (afatinib and erlotinib) and dose interruption (gefitinib) according to tolerability.18 Analyses of data from the LL3 and LL6 studies demonstrated that tolerability-guided dose adjustment of afatinib was effective in reducing TRAEs without impacting PFS.19
The frequency of EGFR mutations in tumors from Asian patients and particularly Chinese patients is high – approximately 50% compared with 10–15% in Caucasian patients.20 Therefore, the choice of first-line EGFR TKI in Chinese patients is important and should take into consideration efficacy, tolerability and cost. Subgroup analysis of the FLAURA trial showed a consistent benefit of osimertinib over first-generation TKIs with respect to PFS in Asian versus non-Asian patients.14 Post-hoc data with the pan-ErbB TKI afatinib would be of interest to provide more information regarding PFS and OS in Chinese patients. Subgroup analysis in Chinese patients from LL6 showed that afatinib treatment was well tolerated with few discontinuations (6%) and resulted in longer PFS than treatment with chemotherapy.21 Additional data are also available from a pooled analysis of LL3 and LL6 with afatinib,15 which unlike the FLAURA,14 and ARCHER 1050 trials13 also included patients with uncommon EGFR mutations. We present the results of a post hoc analysis conducted to assess outcomes of tolerability-guided afatinib dose adjustment in patients enrolled in the LL3, LL6, and LL7 clinical trials at study centers in China.
Methods
Study Design and Patients
Study designs and eligibility criteria for the LL3, LL6, and LL7 trials have been previously reported in detail.11,12,16 LL3 and LL6 were open-label, randomized Phase III trials, and LL7 was an open-label, randomized Phase IIb trial. All were international, multicenter trials; LL3 and LL7 recruited patients globally (including China) and LL6 was an Asian study enrolling patients from China, Thailand, and South Korea.
Briefly, eligible patients had stage IIIB/IV, EGFR mutation-positive NSCLC, measurable disease by Response Evaluation Criteria in Solid Tumor version 1.1 (RECIST v1.1), and had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0/1 with adequate organ function.
The primary endpoints of LL3 and LL6 were PFS (by independent review); co-primary endpoints of LL7 included PFS (by independent review), time to treatment failure and OS. Secondary endpoints in the trials included objective response and disease control, OS (LL3 and LL6), patient-reported outcomes and safety. Pharmacokinetic analyses were performed in LL3 and LL6.
The studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice as defined by the International Conference on Harmonisation, and the protocol was approved by independent ethics committees or institutional review boards at each participating center (Table S6–S8). All patients provided written informed consent.
Treatment
In both the LL3 and LL6 trials, patients were randomized (2:1) to oral afatinib 40 mg/day or up to six cycles of cisplatin-based chemotherapy (dosing and schedule previously reported)11,12 stratified by EGFR mutation-type (Del19/L858R/other) and by race in LL3 (Asian/non-Asian). In the LL7 trial, patients were randomized (1:1) to afatinib 40 mg/day or gefitinib 250 mg/day, stratified by EGFR mutation type (Del19/L858R) and the presence of baseline brain metastases (presence vs absence) as reported previously.16 Dose escalation to 50 mg/day afatinib was permitted after the first cycle in LL3 and LL6, and after 4 weeks in LL7 in the absence of grade >1 TRAEs.
Tolerability-guided dose adjustments were permitted for TRAEs. Afatinib treatment was interrupted for up to 14 days until the reduction in TRAE severity to grade 1 or less (or the grade present at baseline) in case of the following: any grade ≥3 TRAE, prolonged grade 2 diarrhea (lasting ≥2 days in LL7), grade 2 nausea or vomiting for ≥7 days despite best supportive care, and grade ≥2 worsening renal function. Afatinib dosing was then resumed at a lower dose, being reduced in 10 mg decrements to a minimum of 20 mg/day. Treatment was permanently discontinued in patients who did not recover to grade 1 or less, or baseline grade, within 14 days.
Outcomes and Assessments
AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) v16.1 for LL3 and LL6, and 19.0 for LL7 and graded by the Common Terminology Criteria for Adverse Events (CTCAE) v3. MedDRA preferred terms for AEs of a similar nature were grouped; grouped terms included rash/acne, stomatitis, nail effects, fatigue (Table S1). Relatedness to treatment was determined by the investigator.
Tumors were assessed using computed tomography or magnetic resonance imaging and reviewed by an independent central imaging group, until disease progression or the start of a new therapy. PFS was defined as the time from random assignment to progression or death. EGFR mutation analyses were performed at central laboratories using standardized allele-specific quantitative real-time polymerase chain reaction methodologies.
Statistics
This post hoc analysis was performed on individual patient data pooled from patients enrolled and treated with at least one dose of afatinib at centers in China participating in the LL3, LL6, and LL7 studies.
In patients with dose reductions, the frequency and severity of TRAEs pre- and post-reduction from 40 mg/day in the first 6 months of treatment were analyzed.
PFS and OS were analyzed and compared in patients who experienced a dose reduction from 40 mg/day within the first 6 months of treatment and those who did not. Kaplan–Meier estimates and 95% confidence intervals (CI) for the median survival distribution were calculated. A Cox proportional-hazard model was used to derive hazard ratios (HRs) and 95% CIs comparing patients with or without dose reduction.
Results
Patients and Treatment Exposure
In total, 299 (48%) of 628 patients treated with afatinib in the LL3, LL6, and LL7 studies were enrolled at Chinese centers (Table 1). All were of Taiwanese or Chinese origin. Dose reductions in the first 6 months to <40 mg/day afatinib occurred in 68 (23%) patients and accounted for 15% of the total number of patients who had dose reductions (68/460) in the three studies. Of the 68 patients with dose reductions, 62 were treated with 30 mg/day and six received 20 mg/day. Of the 299 patients, 231 (77%) remained on afatinib ≥40 mg/day (Table 1). At 6 months, 207 patients remained on afatinib 40 mg/day and a further 24 patients had their dose increased to 50 mg/day. Key patient and disease characteristics at baseline are shown in Table 2. In the overall group of Chinese patients, there was a higher proportion of female (61%) than male patients (39%), 31 (10%) patients had brain metastases at baseline and the majority (270, 90%) had tumors harboring common EGFR mutations. Compared with the group that did not reduce their dose, there were more females in the dose reduction group (69% vs 58%), and more patients had a body weight of <50 kg (22% vs 9%).
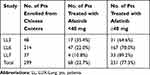 |
Table 1 Number of Patients Enrolled at Chinese Centers from Each Trial, According to Afatinib Dosage <40 mg/Day or ≥40 mg/Day at 6 Months |
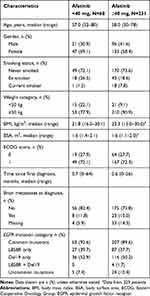 |
Table 2 Patient Demographics and Baseline Characteristics |
Median duration of treatment was 13.8 months (range 1.4–45.9 months) for the 68 patients with dose reductions and 11.5 months (range 0.1–48.4 months) for the 231 patients without dose reductions (p=0.8268).
TRAEs
All (100%) patients in the Chinese population experienced TRAEs. The most common TRAEs of any grade and grade ≥3 were diarrhea (91%) and rash (12%), respectively (Table S2). TRAEs occurring prior to and after dose reduction from 40 mg/day are summarized in Figure 1 (detailed in Table S3).
 |
Figure 1 Most common TRAEs pre-and post-afatinib dose reduction from 40 mg/day. Abbreviations: LL, LUX-Lung; TRAEs, treatment-related adverse events. Note: *Grouped term. |
Prior to dose reduction, 55/68 (81%) experienced grade ≥3 TRAEs. The most common grade ≥3 TRAEs reported prior to dose reduction were rash/acne (43%), stomatitis (21%), and diarrhea (16%). Following dose reduction, 13/68 (19%) experienced grade ≥3 TRAEs.
TRAEs of grade ≥3 were much more frequently reported in patients who had a dose reduction from 40 mg afatinib than in those who did not, irrespective of patient subgroup category (Table S4). The frequency of grade ≥3 TRAEs ranged from 60% to 83% depending on the subgroup in patients who had a dose reduction compared with 31% to 43% across the subgroups in patients who did not have a dose reduction.
Progression-Free Survival
The median follow-up period was 9.3 months (range 0–44.0 months) for patients who had a dose reduction during the first 6 months, and 9.6 months (range 0–41.3 months) for those who did not.
Median PFS was 11.0 months both in patients who had a dose reduction from 40 mg/day in the first 6 months (95% CI: 8.3–14.0) and in those who remained on afatinib ≥40 mg/day (95% CI: 9.6–13.1; HR 1.15 [95% CI: 0.84–1.58], p=0.39; Figure 2). In the dose reduction group, 49 (72%) of patients progressed or died, compared with 180 (78%) of patients who remained on afatinib ≥40 mg/day. Median PFS in subgroups of patients who reduced the afatinib dose compared with those who did not are presented in Table S5. Of note, some of these subgroups included small numbers of patients.
 |
Figure 2 Kaplan–Meier curves showing PFS (independent review) by dose reduction status in afatinib-treated patients. Abbreviations: CI, confidence interval; PFS, progression-free survival. |
Overall Survival
Afatinib dose reduction did not have an impact on the median OS compared with no dose reduction. Median OS was 23.1 months in the patients who had an afatinib dose reduction from 40 mg/day in the first 6 months (95% CI: 19.3–32.9) and 26.9 months (95% CI: 23.2–30.4) in those who remained on ≥40 mg/day: HR 0.96 (0.69–1.34); p=0.80 (Figure 3).
 |
Figure 3 Kaplan–Meier curves showing OS (months) by dose reduction in status in afatinib-treated patients. Abbreviations: CI, confidence interval; OS, overall survival. |
Discussion
Patients treated at Chinese centers comprised 48% of afatinib-treated patients from the LL3, LL6, and LL7 studies.11,12,16 In this pooled analysis of data from those studies, 23% of patients had a dose reduction to <40 mg/day in the first 6 months. In comparison, dose reductions from 40 mg/day were previously reported in 20% and 45% of non-Asian patients in the LL3 and LL7 study, respectively.11,22 Furthermore, in the LL3 study, a higher proportion of patients recruited from Japanese sites had dose reductions than did not (31% and 17%).11 This may suggest ethnic differences in dose reduction rates from starting doses of 40 mg/day afatinib; however, pharmacokinetic data have suggested that ethnicity does not affect exposure to afatinib.23
More female patients and those with a lower body weight (<50 kg) had a dose reduction to below 40 mg/day. This may be due to increased afatinib plasma exposure in these patients, as both gender and body weight have been identified as covariates for exposure to afatinib.23,24 The frequency of patients having dose reductions was similar in other patient subgroups, including those whose tumors contained EGFR common and uncommon mutations and those with or without brain metastases at baseline.
The safety profile of afatinib was as expected, with diarrhea, rash, and stomatitis being the most common TRAEs. This was in line with the parent studies.11,12,16 As would be expected, patients who went on to reduce their afatinib dose experienced a much higher frequency of grade ≥3 TRAEs than those patients who maintained their afatinib dose. Among the 68 patients with a dose reduction of afatinib, grade ≥3 TRAEs were reported prior to the dose reduction in 55 patients (81%) and after the dose reduction in only 13 (19%) patients. This finding is expected and reassuring, demonstrating that the tolerability-guided dose-adjustment strategy developed for afatinib is effective and applicable to Chinese patients.
The effectiveness of tolerability-guided dose adjustment at reducing TRAEs has been demonstrated previously for the overall populations of LL3 and LL6,19 and LL7.22 It has also been shown in a non-interventional, real-world, global study of EGFR-mutation-positive NSCLC patients treated with first-line afatinib (RealGiDo).25 In this study, 67.1% of patients who started on afatinib 40 mg/day had a dose reduction. This reduced the overall frequency of grade 3/4 AEs from 30.1% to 13.7%, and the incidence of grade ≥3 diarrhea and rash from 13.7% and 9.6%, respectively, to 1.4% and 2.7%. Similarly, in a Phase IIIb study conducted in Asian patients with EGFR-mutation-positive NSCLC, dose reduction of afatinib decreased the rates of grade ≥3 diarrhea, rash/acne, and stomatitis from 27%, 24%, and 11%, respectively, to 4%, 11%, and 5%, respectively.26
Median PFS was 11.0 months in the patients with or without dose adjustment of afatinib in the first 6 months of treatment in this analysis. Similarly, median PFS was no different when dose adjustments occurring after the first 6 months of treatment were considered compared with no dose adjustment. The median PFS values seen here are nearly identical to those observed in the overall studies,11,12,16 suggesting that afatinib is similarly effective in Chinese patients as it is in all other patients. In addition, median OS did not differ significantly between the two groups: 23.1 months in patients with a dose reduction and 26.9 months in those without a dose reduction.
There is now a wealth of data demonstrating that tolerability-based dose adjustment of afatinib does not reduce PFS, and may even improve outcomes, and this analysis adds to the evidence base. In LL3, median PFS was 11.3 months in those who dose reduced and 11.0 months in those who did not (p=0.175); while in LL6, median PFS was 12.3 months in dose reducers and 11.0 months in patients who did not (p=0.982).19 In LL7, median PFS was 12.8 months in those who dose reduced and 11.0 months in those who did not (p=0.144).22 In the Phase IIIb study in Asian patients, median PFS was improved in patients who dose reduced (14.1 months) compared with those who did not (11.3 months; p=0.041).26 In real-world studies, such as RealGiDo, median time to progression was 29.0 months in those who remained on afatinib ≥40 mg/day and 20.0 months in those who had a dose reduction to <40 mg/day; the difference was not significant (p=0.392).25 A literature review of real-world studies of afatinib for the treatment of NSCLC found six studies, all from Asia.27 In four of these, PFS or time to treatment failure was not affected by dose reductions, while PFS was slightly shorter in dose reducers in one study, and was improved in dose reducers in another. In a retrospective study of 125 patients who received treatment with afatinib at an Asian cancer treatment center, the median PFS was longer in patients with brain metastases who started treatment with afatinib 40 mg/day (n=17) than in 25 patients who initiated 30 mg/day.28
Pharmacokinetic analysis of patients in LL3 found that afatinib trough plasma concentrations were higher on day 22 in patients who subsequently had a reduction in dose from 40 mg/day to 30 mg/day due to TRAEs than in those who remained on 40 mg/day. Subsequently, on day 43, afatinib trough plasma concentrations in the patients now on 30 mg/day were similar to those in patients who had remained on 40 mg/day.19 This was thought to be due to higher initial afatinib exposure, possibly as a result of female gender or lower body weight; the tolerability-guided dose modification reduced excessive exposure, providing a level of EGFR inhibition that was adequate for clinical efficacy, but reducing the risk of TRAEs caused by off-target effects.
In conclusion, tolerability-guided dose reduction of afatinib for Chinese patients treated in the LL3, LL6, and LL7 studies led to decreased incidence of TRAEs without affecting median PFS or median OS. Chinese patients can be initiated on afatinib 40 mg/day and subsequently have the dose reduced if tolerability becomes a problem, while remaining confident that they are receiving adequate afatinib exposure for efficacy.
Data Sharing Statement
To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to all relevant material, including participant-level clinical study data, and relevant material as needed by them to fulfill their role and obligations as authors under the ICMJE criteria.
Furthermore, clinical study documents (eg study report, study protocol, statistical analysis plan) and participant clinical study data are available to be shared after the publication of the primary manuscript in a peer-reviewed journal and if regulatory activities are complete and other criteria met per the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data: https://trials.boehringer-ingelheim.com/transparency_policy.html
Prior to providing access, documents will be examined, and, if necessary, redacted and the data will be de-identified, to protect the personal data of study participants and personnel, and to respect the boundaries of the informed consent of the study participants.
Clinical Study Reports and Related Clinical Documents can be requested via this link:https://trials.boehringer-ingelheim.com/trial_results/clinical_submission_documents.html
All such requests will be governed by a Document Sharing Agreement.
Bona fide, qualified scientific and medical researchers may request access to de-identified, analysable participant clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a Data Sharing Agreement, data are shared in a secured data-access system for a limited period of 1 year, which may be extended upon request.
Researchers should use https://trials.boehringer-ingelheim.com to request access to study data.
Acknowledgments
We would like to thank the patients and their families and the investigators who participated in the LUX-Lung studies. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Greg Plosker and Caroline Perry of GeoMed, an Ashfield company, part of UDG Healthcare plc, during the development of this manuscript. The authors were responsible for all content and editorial decisions.
Author Contributions
Both authors contributed to the conception, data acquisition and preparation of the first draft. Both authors contributed to manuscript development through critical review and revision of the manuscript, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The studies and analysis presented in this manuscript were funded by Boehringer Ingelheim.
Disclosure
H-YT declares no potential conflicts of interest. Y-LW reports having received honoraria from AstraZeneca, Roche, Boehringer Ingelheim, Pfizer, BMS, MSD, and Eli Lilly, institutional grants and personal fees from AstraZeneca and Boehringer Ingelheim, and personal fees as a speaker from BMS, Eli Lilly, MSD, Pfizer, Roche, and Sanofi, outside the submitted work, and reports no other potential conflicts of interest for this work.
References
1. Shi Y, Sun Y, Ding C, et al. China experts consensus on icotinib for non-small cell lung cancer treatment (2015 version). Ann Transl Med. 2015;3(18):260. doi:10.3978/j.issn.2305-5839.2015.10.30
2. Wu YL, Planchard D, Lu S, et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(2):171–210. doi:10.1093/annonc/mdy554
3. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):iv192–iv237. doi:10.1093/annonc/mdy275
4. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, Phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi:10.1016/S1470-2045(11)70184-X
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi:10.1016/S1470-2045(11)70393-X
6. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the Phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883–1889. doi:10.1093/annonc/mdv270
7. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi:10.1056/NEJMoa0810699
8. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi:10.1056/NEJMoa0909530
9. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi:10.1016/S1470-2045(09)70364-X
10. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443–2450. doi:10.1093/annonc/mdx359
11. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi:10.1200/JCO.2012.44.2806
12. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. doi:10.1016/S1470-2045(13)70604-1
13. Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466. doi:10.1016/S1470-2045(17)30608-3
14. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi:10.1056/NEJMoa1713137
15. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–151. doi:10.1016/S1470-2045(14)71173-8
16. Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589. doi:10.1016/S1470-2045(16)30033-X
17. Yang JJ, Zhou Q, Yan HH, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer. 2017;116(5):568–574. doi:10.1038/bjc.2016.456
18. Califano R, Tariq N, Compton S, et al. Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs. 2015;75(12):1335–1348. doi:10.1007/s40265-015-0434-6
19. Yang JC, Sequist LV, Zhou C, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol. 2016;27(11):2103–2110. doi:10.1093/annonc/mdw322
20. Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: which tyrosine kinase inhibitor and when? Future Oncol. 2018;14(11):1117–1132. doi:10.2217/fon-2017-0636
21. Wu YL, Xu CR, Hu CP, et al. Afatinib versus gemcitabine/cisplatin for first-line treatment of Chinese patients with advanced non-small-cell lung cancer harboring EGFR mutations: subgroup analysis of the LUX-Lung 6 trial. Onco Targets Ther. 2018;11:8575–8587. doi:10.2147/OTT.S160358
22. Schuler M, Tan EH, O’Byrne K, et al. First-line afatinib vs gefitinib for patients with EGFR mutation-positive NSCLC (LUX-Lung 7): impact of afatinib dose adjustment and analysis of mode of initial progression for patients who continued treatment beyond progression. J Cancer Res Clin Oncol. 2019;145(6):1569–1579. doi:10.1007/s00432-019-02862-x
23. Wind S, Schnell D, Ebner T, Freiwald M, Stopfer P. Clinical pharmacokinetics and pharmacodynamics of afatinib. Clin Pharmacokinet. 2017;56(3):235–250. doi:10.1007/s40262-016-0440-1
24. Freiwald M, Schmid U, Fleury A, Wind S, Stopfer P, Staab A. Population pharmacokinetics of afatinib, an irreversible ErbB family blocker, in patients with various solid tumors. Cancer Chemother Pharmacol. 2014;73(4):759–770. doi:10.1007/s00280-014-2403-2
25. Halmos B, Tan EH, Soo RA, et al. Impact of afatinib dose modification on safety and effectiveness in patients with EGFR mutation-positive advanced NSCLC: results from a global real-world study (RealGiDo). Lung Cancer. 2019;127:103–111. doi:10.1016/j.lungcan.2018.10.028
26. Wu Y, Tu H, Feng J, et al. P1.01-98 A phase IIIb trial of afatinib EGFRm+ NSCLC: analyses of outcomes in patients with brain metastases or dose reductions. J Thorac Oncol. 2018;13(10):S501. doi:10.1016/j.jtho.2018.08.654
27. Park K, Wan-Teck Lim D, Okamoto I, Yang JC. First-line afatinib for the treatment of EGFR mutation-positive non-small-cell lung cancer in the ‘real-world’ clinical setting. Ther Adv Med Oncol. 2019;11:1758835919836374. doi:10.1177/1758835919836374
28. Tan WL, Ng QS, Lim C, et al. Influence of afatinib dose on outcomes of advanced EGFR-mutant NSCLC patients with brain metastases. BMC Cancer. 2018;18(1):1198. doi:10.1186/s12885-018-5110-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
