Back to Journals » Clinical Ophthalmology » Volume 15
Effect of Cyclosporine 0.09% Treatment on Accuracy of Preoperative Biometry and Higher Order Aberrations in Dry Eye Patients Undergoing Cataract Surgery
Authors Hovanesian JA , Berdy GJ, Epitropoulos A , Holladay JT
Received 3 July 2021
Accepted for publication 23 August 2021
Published 1 September 2021 Volume 2021:15 Pages 3679—3686
DOI https://doi.org/10.2147/OPTH.S325659
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Video abstract presented by John A Hovanesian.
Views: 1038
John A Hovanesian,1 Gregg J Berdy,2 Alice Epitropoulos,3 Jack T Holladay4
1Harvard Eye Associates, Laguna Hills, CA, USA; 2Ophthalmology Associates, St. Louis, MO, USA; 3Ophthalmic Surgeons & Consultants of Ohio, Columbus, OH, USA; 4Baylor College of Medicine, Houston, TX, USA
Correspondence: John A Hovanesian
Harvard Eye Associates, 23961 Calle Magdalena, Suite 300, Laguna Hills, CA, 92653, USA
Tel +1 949 951 2020
Email [email protected]
Purpose: To determine the effect of topical cyclosporine 0.09% on ocular surface regularity and the predictive accuracy of preoperative corneal power measurements in patients undergoing cataract surgery.
Setting: Private practice.
Design: Open-label, multicenter, prospective study.
Methods: Seventy-five patients (75 eyes) who presented for cataract surgery evaluation with signs of dry eye disease were prescribed topical cyclosporine 0.09% for 28 days BID. Corneal curvature measurements, slit lamp exam, and Standardized Patient Evaluation of Eye Dryness (SPEED) questionnaire were evaluated at the initial and follow-up visits. Cataract surgery occurred 1 to 3 weeks after the second biometry visit. Refraction and corrected distance visual acuity measurements were performed 1-month post-surgery. The primary outcome was the difference in absolute prediction error of 1-month spherical equivalent refractive outcome before and after cyclosporine treatment. Secondary outcomes included the effect of topical cyclosporine 0.09% on ocular surface irregularity.
Results: Sixty-four patients completed the study. The absolute prediction error of 1-month spherical equivalent refractive outcome was 0.39 ± 0.30 D vs 0.33 ± 0.25 D (P < 0.03) before and after treatment, respectively. The proportion of eyes that achieved the target refraction was greater based on measurements after topical cyclosporine 0.09% than would have occurred using pre-treatment measurements.
Conclusion: Cataract surgery patients with dry eye who are prescribed topical cyclosporine 0.09% BID for 28 days pre-surgery showed a statistically significant improvement in the prediction error of the spherical equivalent outcome of surgery. Other measures of dry eye severity showed significant improvements after treatment.
Keywords: cataract surgery, topical cyclosporine 0.09%, dry eye, higher order aberrations
Introduction
Patient satisfaction after cataract surgery in today’s world relies upon minimizing the refractive error and optimizing the quality of the visual image.1,2 Ocular surface irregularity adversely impacts the consistency of corneal measurements preoperatively and can continue to affect visual quality post-surgery.3–5 These inconsistent preoperative corneal measurements directly impact the ability of cataract surgeons to choose the proper intraocular lens (IOL) implant. It follows that improving the ocular surface in patients with dry eye disease (DED) preoperatively will lead to greater refractive accuracy in lens selection.6–8
This study was designed to determine if prescribing topical cyclosporine 0.09% (Cequa, Sun Pharmaceuticals) in patients presenting for cataract surgery for 28 days pre-surgery could improve surface regularity and the predictive accuracy of preoperative corneal power measurements. This study also examines the impact of topical cyclosporine 0.09% on irregularity of the ocular surface as measured by higher order aberrations (HOAs), corneal staining, tear breakup time (TBUT), and ocular redness.
Materials and Methods
This open-label, multicenter, prospective study included 75 eyes (75 patients) who had presented for cataract surgery evaluation with signs of DED, including corneal staining with fluorescein and a TBUT of ≤ 10 seconds (Figure 1). Three centers participated in the study, and 3 surgeons performed all procedures (JAH, GJB, AE). Each patient underwent the same set of presurgical diagnostics before and after treatment with cyclosporine 0.09%. Patients who were scheduled to have both eyes undergo cataract surgery had their first eye enrolled in the study. The study is listed on ClinicalTrials.gov (NCT04342988), and the protocol followed the Declaration of Helsinki and the US Food and Drug Administration’s good clinical practices, and all patients signed informed consent paperwork to participate in the study before enrollment was confirmed. This study was overseen by Aspire IRB (Santee, California, USA). De-identified data from this study will be provided in response to reasonable requests from the corresponding author for 5 years following publication.
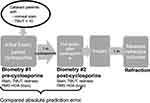 |
Figure 1 Study design. |
The inclusion/exclusion criteria and study protocol have been previously published as this group has evaluated other DED treatments.8 Briefly, exclusion criteria included previous ocular surgery within the past 3 months, ocular inflammation or corneal scarring, corneal dystrophy, or other defect or abnormality of the ocular surface. Subjects were permitted to continue unchanged any baseline dry eye treatments (lubricant drops, warm compresses) but not make any modifications, add any treatments, or perform any procedures beyond the addition of topical cyclosporine 0.09%.
At the 1-month visit following cyclosporine 0.09% treatment, patients were asked to report on their compliance, and noncompliant patients were excluded.
Assessments
Corneal curvature measurements were performed at both the initial visit and after 28 days of cyclosporine 0.09% using an IOL Master 500 or 700 (Carl Zeiss Meditec, California, US). Corneal topography was collected with a Zeiss Atlas 900 or later topographer, and root-mean-square (RMS) HOAs were recorded in the central 6.0 mm of the cornea for each visit. Other assessments included slit lamp examination (conjunctival hyperemia by the Schulze scale,3,5 corneal staining by the Oxford grading scale, and TBUT). The Standardized Patient Evaluation of Eye Dryness (SPEED) survey was also administered, and scores > 10 were considered abnormal.3,6 Following the initial evaluation, patients were prescribed 28 days of cyclosporine 0.09% BID. At the end of the 28-day cycle, biometry and exam measurements were repeated. Cataract surgery was performed 1 to 3 weeks after the second evaluation using the intraocular lens (IOL) power suggested by the biometry measurements from the latter evaluation.
Approximately 1 month postoperatively, a refraction and corrected distance visual acuity (CDVA) measurement was performed.
Data Analysis
Each set of the biometry measurements was used to generate an IOL power calculation for the study eye using the Barrett Universal II Formula; the predicted manifest refraction spherical equivalent of the IOL selected for surgery was calculated for each set of measurements. These were tested for normality using the Shapiro–Wilk test. These predicted spherical equivalents were then compared against the actual final manifest refraction spherical equivalent measured 30 days after surgery to determine each prediction’s absolute prediction error. The absolute prediction error of the pre- and post-treatment measurements were compared for each eye and tested for significance with a paired t-test.
SPEED questionnaire scores, conjunctival hyperemia scores, corneal staining, and TBUT were recorded for each visit and entered into a database for comparison before and after surgery. Paired t-testing was used to evaluate the difference for statistical significance with 95% confidence. Paired t-tests were also used to compare RMS HOAs measurements before and after cyclosporine 0.09% treatment.
Results
A total of 75 patients were enrolled. Of these, 8 (11%) withdrew because of a renewed SARS-CoV-2–related stay-at-home order by cancelling their surgery or withdrawing from study participation to reduce clinic visits. Two (3%) cancelled due to insurance or scheduling conflicts, and 1 (1%) withdrew because the study medication was not tolerable. Of the 64 patients who completed the study, 34 (53%) were female and 30 (47%) were male. A total of 36 patients (56%) had their right eyes studied and 28 (44%) had their left. The mean age was 70.5 ± 7.4 years (range 50 to 84). Patients were enrolled from Harvard Eye Associates (n = 27), Ophthalmology Associates (n = 20), and Ophthalmic Surgeons and Consultants of Ohio (n = 17).
Primary Outcome Measure
The prediction error of 1-month spherical equivalent refractive outcome was tested and found to follow a normal distribution for biometry performed both before and after treatment with cyclosporine 0.09% (P value 0.99, Shapiro–Wilk test). The absolute prediction error was 0.39 ± 0.30 D vs 0.33 ± 0.25 D based on biometry performed before and after treatment with cyclosporine 0.09%, respectively. This difference was statistically significant (P < 0.03, paired t-test). The proportion of eyes that would have achieved the target refraction was greater after cyclosporine 0.09%: 41% vs 47% within 0.25 D (P < 0.05, McNemar’s Chi-squared test), 72% vs 73% within 0.5 D (P < 0.31), and 88% vs 95% within 0.75 D (P < 0.03). These differences were statistically significant for accuracy within 0.25 D and 0.75 D (Figure 2).
 |
Figure 2 Predictive accuracy of corneal power measurements performed after cyclosporine was significantly higher than when performed before cyclosporine (N = 64, P < 0.03, paired t-test). |
Secondary Outcomes
Cyclosporine 0.09% treatment caused changes in total HOAs measured within the central 6.0 mm of the cornea with improvement by a mean of 0.28 ± 0.27 µ in 28 (44%) of eyes, no change in 18 (28%), and worsening by a mean of 0.17 ± 0.15 µ in 18 (28%) of eyes (Figure 3). These differences were statistically significant, favoring improvement (P < 0.0001, McNemar’s Chi-squared test). The overall mean magnitude of corneal HOAs was also significantly improved by cyclosporine 0.09% with a value of 0.68 ± 0.32 µ before treatment and 0.60 ± 0.22 µ after (P < 0.02, paired t-test).
 |
Figure 3 Significantly more patients had improvement than a decline in RMS HOA after 28 days of cyclosporine (N=64, P < 0.0001, McNemar’s Chi-squared test). |
The presence of total corneal HOAs greater than 0.5 µ before surgery are associated with suboptimal subjective patient perceptions postoperatively. Using this cutoff as a measure of probability for success with a multifocal IOL, 25 (39%) patients before and 29 (45%) patients after cyclosporine 0.09% treatment would be considered candidates for a multifocal IOL. This difference was statistically significant (P < 0.05, McNemar’s Chi-squared test).
SPEED scores significantly improved after cyclosporine 0.09% treatment, with a mean score of 7.9 ± 6.2 before and 5.2 ± 5.3 after (P < 0.00001, paired t-test). Scores of ≤ 5 were observed in 25 (39%) patients before and 40 (63%) patients after cyclosporine 0.09% treatment, and scores < 10 were noted in 48 (75%) patients before and 56 (88%) patients after cyclosporine 0.09% treatment (Figure 4). These differences were statistically significant (P < 0.007 and P < 0.04, respectively, McNemar’s Chi-squared test).
 |
Figure 4 SPEED scores improved significantly after 28 days of treatment with cyclosporine (P < 0.00001, paired t-test). |
Corneal staining, measured by the Oxford scale, significantly improved from a mean grade of 1.6 ± 0.56 before cyclosporine 0.09% to 0.5 ± 0.62 after treatment (P < 0.000001, paired t-test). All eyes had at least grade 1 staining before treatment: 36 (56%) improved to grade 0 (absence of stain), 24 (38%) improved to grade 1, and 4 (6%) finished the study with grade 2 staining. Only 2 (3%) eyes showed no improvement in corneal staining, remaining at grade 2 after treatment (Figure 5).
TBUT improved significantly from a mean of 5.2 ± 2.2 seconds before treatment to 7.0 ± 2.9 seconds after (P < 0.000001, paired t-test). Mean improvement was 2.6 ± 2.4 seconds. TBUT was 0 to 5 seconds in 37 (59%) and 23 (36%) eyes before and after cyclosporine 0.09% treatment, respectively (P < 0.002, McNemar’s chi-squared test), and 6 to 10 seconds in 26 (41%) and 33 (52%) patients before and after cyclosporine 0.09% treatment, respectively (P < 0.03, McNemar’s chi-squared test; Figure 6).
 |
Figure 6 Tear breakup time was measured before and after 28 days of treatment with cyclosporine and improved significantly following treatment (N=64, P<0.00001, paired t-test). |
Conjunctival erythema, measured by the Schulze scale, also significantly improved with cyclosporine 0.09% treatment (mean score 19.1 ± 8.9 and 15.3 ± 6.7 before and after treatment, respectively; P < 0.002, paired t-test). The lowest grading of 10 was observed in 23 (36%) eyes before and 36 (56%) after treatment (P < 0.02, McNemar’s chi-squared test). Other values for conjunctival redness are shown in Table 1.
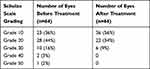 |
Table 1 Conjunctival Erythema Before and After Treatment with Cyclosporine |
Discussion
This study enrolled patients with DED to assess the impact of a new formulation of cyclosporine on the refractive accuracy of cataract surgery. We found a statistically significant improvement in the prediction error of the spherical equivalent outcome of surgery when we used the measurements 28 days after cyclosporine 0.09% treatment had been initiated when compared to measurements performed before treatment. Other measures of dry eye severity, including corneal RMS HOAs, corneal staining, TBUT, conjunctival erythema, and SPEED scores, also showed significant improvements after cyclosporine 0.09% treatment.
It is well established that the management of ocular surface disease is critical to the success of cataract surgery for several reasons. Not only can cataract surgery exacerbate dry eye signs and symptoms, even in patients who self-report as asymptomatic, but ocular surface irregularities can compromise the accuracy of presurgical biometry and corneal topography/tomography, causing inaccurate IOL calculations, suboptimal postoperative outcomes, and patient dissatisfaction.9,10 HOAs have also been linked to postoperative patient dissatisfaction, especially if multifocal IOLs were implanted. Given the generally high patient postoperative expectations,11 cataract surgeons must prioritize minimizing these issues to meet patient needs.
Significant improvement was also noted for tear breakup time, giving evidence that treatment established a more stable tear film in dry eyes approaching surgery. This is particularly important for postoperative patients because achieving satisfaction with surgery depends not only on establishing clarity but also the ability to support prolonged reading and other visually intensive tasks.
Although many pharmacologic treatments for dry eye exist, cyclosporine 0.09% is the highest dose of cyclosporine approved by the FDA. It is also the only cyclosporine approved that includes nanomicellar technology for better penetration and drug absorption.12 To our knowledge, this is the first study demonstrating improvements in refractive accuracy of cataract surgery with pretreatment cyclosporine 0.09%. Our findings add to the literature by showing cyclosporine 0.09% has added utility beyond general dry eye treatment, further stocking the cataract surgeon’s armamentarium.
This study is not without limitations, however. The number of patients who initiated therapy and completed the study was lower than originally planned due to a high number of patients canceling surgery or wishing to withdraw to avoid noncrucial office visits. One patient withdrew because of intolerance to the study medication, a rate of discontinuation slightly better than the 2.4% discontinuation rate reported in the US Food and Drug Administration Phase 3 approval study of cyclosporine 0.09%.12 Despite the lower number of participants, the primary outcome measure—an improvement in refractive accuracy—showed a statistically significant result.
This study evaluated a single treatment, cyclosporine 0.09%, for DED. It is possible that similar results could be achieved with other treatments like artificial tears, warm compresses, dietary and habit modifications, and procedural remedies for DED. In combination with cyclosporine 0.09% these treatments might offer even more benefit than was found in this study. Our study purposely excluded those other treatments to avoid confounding the results. The pivotal study of cyclosporine 0.09% demonstrated superiority of this formulation over vehicle alone in treating DED.12 It would, therefore, be reasonable to assume that the improvements in refractive accuracy shown in this study from cyclosporine 0.09% would be similarly superior to those of its vehicle.
As with DED, corneal HOAs can be influenced by many factors, including seasonality, state of bodily hydration, hormonal changes, and any other factor that affects state of ocular hydration. Each of these can act at random and cause variance in the “smoothness” of the ocular surface. This may explain why 28% of cyclosporine 009%-treated patients had a worsening of their HOAs in this study. Despite these noteworthy and random variables, a significantly greater proportion of eyes, 44%, showed improvement in this metric.
It is interesting to note that, although all patients in the study had significant DED, the majority were symptom-free at baseline. SPEED scores were ≤ 10 in 75% and < 5 in almost 40%. This corroborates earlier studies, including the PHACO study,13 that showed similar proportions of symptom-free patients, and it reinforces common clinic procedures to screen cataract surgery candidates for DED.
Although tempting, we cannot compare these outcomes to those of an earlier study conducted by the same group that enrolled a similar patient population and treated dry eye with lifitegrast.8 Both studies found significantly improved predictive power for IOL implants, and both studies showed pretreatment with pharmacologics approved for DED significantly improved other tested measures of dry eye severity. Indeed, both studies help to re-confirm the importance of treating DED before performing biometry to achieve optimal results. More study is needed to directly compare two of these approved drugs to determine if one confers an additional benefit over the other.
Future studies may evaluate whether a shorter course of therapy than 28 days, with cyclosporine 0.09% alone or in combination with other therapies, could achieve similar or better results. Meanwhile, the positive findings of this study suggest this drug may have a place of value in managing the ocular surface of dry eye patients undergoing surgery.
Conclusions
The use of topical cyclosporine 0.09% BID for 28 days before cataract surgery in patients with dry eye produced a statistically significant improvement in the prediction error of the spherical equivalent outcome of surgery.
Acknowledgments
Dalton & Associates, Inc. assisted in manuscript preparation.
Funding
Funding provided by Sun Pharmaceuticals.
Disclosure
John A Hovanesian reports personal fees for consultancy from Sun Pharmaceuticals, during the conduct of the study, provisional patent application has been applied for by Sun Pharmaceutical in Dr Hovanesian’s name but under Sun Pharmaceuticals’ ownership and reports grants and personal fees from Novartis, outside the submitted work. Gregg J Berdy is a consultant for Sun Pharmaceuticals. Alice Epitropoulos is a consultant & a speaker for Sun Pharmaceuticals. Jack T Holladay is a consultant for Sun Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
1. Mönestam E. Long-term outcomes of cataract surgery: 15-year results of a prospective study. J Cataract Refract Surg. 2016;42(1):19–26. doi:10.1016/j.jcrs.2015.07.040
2. Gollogly HE, Hodge DO, St Sauver JL, Erie JC. Increasing incidence of cataract surgery: population-based study. J Cataract Refract Surg. 2013;39(9):1383–1389. doi:10.1016/j.jcrs.2013.03.027
3. Lee AC, Qazi MA, Pepose JS. Biometry and intraocular lens power calculation. Curr Opin Ophthalmol. 2008;19(1):13–17. doi:10.1097/ICU.0b013e3282f1c5ad
4. Jeong J, Song H, Lee JK, Chuck RS, Kwon JW. The effect of ocular biometric factors on the accuracy of various IOL power calculation formulas. BMC Ophthalmol. 2017;17(1):62. doi:10.1186/s12886-017-0454-y
5. Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34(3):368–376. doi:10.1016/j.jcrs.2007.10.031
6. Sheard R. Optimising biometry for best outcomes in cataract surgery. Eye. 2014;28(2):118–125. doi:10.1038/eye.2013.248
7. Sahin A, Hamrah P. Clinically relevant biometry. Curr Opin Ophthalmol. 2012;23(1):47–53. doi:10.1097/ICU.0b013e32834cd63e
8. Hovanesian J, Epitropoulos A, Donnenfeld ED, Holladay JT. The effect of lifitegrast on refractive accuracy and symptoms in dry eye patients undergoing cataract surgery. Clin Ophthalmol. 2020;14:2709–2716. doi:10.2147/opth.S264520
9. Li XM, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007;26(9Suppl 1):S16–20. doi:10.1097/ICO.0b013e31812f67ca
10. Epitropoulos AT, Matossian C, Berdy GJ, Malhotra RP, Potvin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672–1677. doi:10.1016/j.jcrs.2015.01.016
11. Addisu Z, Solomon B. Patients’ preoperative expectation and outcome of cataract surgery at Jimma University Specialized Hospital -department of ophthalmology. Ethiop J Health Sci. 2011;21(1):47–55. doi:10.4314/ejhs.v21i1.69044
12. Goldberg DF, Malhotra RP, Schechter BA, Justice A, Weiss SL, Sheppard JD. A phase 3, Randomized, Double-Masked Study of OTX-101 ophthalmic solution 0.09% in the treatment of dry eye disease. Ophthalmology. 2019;126(9):1230–1237. doi:10.1016/j.ophtha.2019.03.050
13. Trattler WB, Majmudar PA, Donnenfeld ED, McDonald MB, Stonecipher KG, Goldberg DF. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423–1430. doi:10.2147/OPTH.S120159
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

