Back to Journals » International Journal of Nanomedicine » Volume 14
Effect of commercial and green synthesized ZnO NPs in murine model of chloroquine-induced pruritus
Authors Aman N , Rauf K , Khan SA, Tokhi A , Rehman NU, Yameen MA
Received 20 January 2019
Accepted for publication 27 March 2019
Published 1 May 2019 Volume 2019:14 Pages 3103—3110
DOI https://doi.org/10.2147/IJN.S202256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Nargis Aman, Khalid Rauf, Shujaat Ali Khan, Ahmed Tokhi, Naeem-Ur Rehman, Muhammad Arfat Yameen
Department of Pharmacy COMSATS University Islamabad, Abbottabad Campus, Abbottabad, KPK, Pakistan
Purpose: To investigate the effects of zinc oxide nanoparticles (ZnO NPs) on chloroquine (CQ)-induced itching, and overall behavior of mice after oral administration of ZnO NPs of various sizes and doses.
Background: With the wide-spread use of ZnO NPs in pharmaceuticals and cosmetics, concerns about their safety and toxicity are also increasing. Multiple aspects of ZnO NPs regarding cytotoxicity and tolerability are under investigation globally. Still, a clear conclusion about their safety has not been reached. Chloroquine phosphate is an antimalarial with known side effects of itching in humans and animals. In this study, CQ was used to induce itching in mice, and the effects of ZnO NPs on scratching and other neurological behavior of mice were observed.
Methods: Female BALB/c mice were divided into eleven groups of six mice each. ZnO NPs of various sizes and doses were administered orally 1 hour before CQ (32 mg/kg body weight) was administered subcutaneously. The effect of ZnO NPs on CQ-induced pruritus was observed for the next 30 minutes. Simultaneously, overall behavioral changes (socialization and locomotion) were also recorded using a video camera.
Results: A significant reduction (P˂0.001) in scratching bouts was observed at all three doses of ZnO NPs (particle sizes 100, 30 nm, and green synthesized 30 nm). Locomotion was reduced significantly (P˂0.001) in ZnO NPs-treated groups in comparison to normal saline and CQ group, additionally, a significant increase in socialization (P˂0.05) was observed in ZnO NP-treated groups as compared to CQ group.
Conclusion: ZnO NPs, instead of aggravating the dermatological condition, ameliorated the pruritus. All sizes of ZnO NPs used significantly improved socialization among mice and reduced locomotion activity.
Keywords: ZnO NPs, chloroquine-induced pruritus, CQ, green synthesized ZnO NPs, mice
Introduction
Today, in the field of advanced research, nanotechnology is undergoing extensive growth.1 Specifically, particles smaller than 100 nm are consistently reported to exhibit diverse, therapeutic, and bio-imaging properties.2 Nanomaterials have become more attractive since it was established that they can be used as a target-specific carrier with some modification.3 Nano-sized particles have a higher surface area to volume ratio.4 Their nano size imparts specific properties that have been different from their bulk.5,6 Recently, some of the metallic nanomaterials including gold, copper, aluminum, titanium, silver, and zinc oxide (ZnO) have been used in textiles as embedding agents.7
ZnO nanoparticles (NPs) have been investigated thoroughly due to their distinctive features. They have good stability and are capable of being used as a diagnostic tool in cancer diagnosis and therapy.8 Globally, a lot of research is underway to explore their potential candidacy as drug delivery tool and behavior toward biological systems.9–14
Topical applications with ZnO as active moiety have been reported for their various antibacterial, anti-inflammatory, and astringent properties.15–17 These wide-spread application trends gave rise to safety concerns about ZnO NPs, and toxicity studies have been carried out in response. In vivo testing has been carried out in animals at doses ranging from 250–2,000 mg/kg body weight. Chronic exposure at 250 mg/kg affected body weight and serum glutamic-pyruvic transaminase activity.18 ZnO NPs at oral doses of 300 and 2,000 mg/kg (short-term exposure) in Swiss mice resulted in reduced sperm count, aberrant morphology, and genotoxicity in a dose-dependent manner,19 while in basal diet they caused developmental toxicity at 5,000 mg/kg.20
Generally, shape and size are considered determinant factors in the toxicity of ZnO NPs,21 while their composition affects stability and biocompatibility.22 It has already been documented that these NPs are capable of reaching various organs after oral administration.23 Mechanistically, ZnO NPs impart their toxic effects due to generation of Zn2+ ions and free radicals after dissolution.24 In animal studies, ZnO NPs have exhibited their toxic effects on all organs including the nervous system, GIT, lungs, cardiovascular system, genitourinary system, and blood (in mice).25 Shrivastava et al, in 2014, reported neurotoxic effects of ZnO NPs upon oral administration, which resulted in an upsurge of norepinephrine and dopamine in the cerebral cortex.26 Although zinc, in trace amounts, has a positive impact on overall central nervous system functioning,27 in higher concentrations, it leads to oxidative stress and chronic exposure resulting in a higher level of malondialdehyde.28 Chronic exposure leads to a decline in cognitive performance by enhancing, mainly, apoptosis of pyramidal neurons.29,30 Additionally, it has also been established that ZnO NPs induced synaptic plasticity by enhancing neuronal excitability in an animal model of mood disorder.31 Currently, researchers are trying to diminish the toxic effects of NPs through coating,32 simultaneously, green synthesis is another approach to combat toxic effects of the chemicals involved in production of NPs.33 Now, chemical methods are being replaced by eco-friendly and cost-effective green synthesis techniques3 providing a wide range of sizes and shapes.34 ZnO NPs have successfully been synthesized using various plant extracts as reducing agents.35 Yet, toxicities and bio-hazards of ZnO NPs are conflicting.36
Chloroquine phosphate (CQ) is primarily used for treating malaria, rheumatoid arthritis, systemic lupus erythematosus, and some viral infections. One of the side effects associated with CQ use in humans is pruritus.37 CQ-induced pruritus is a “sharp biting” sensation which is observed after CQ administration.37–39 As pruritus is one of the symptoms of dermatological malfunctioning,40 this side effect of CQ has been used to study pruritus in mice.37,38
The current study was designed to explore the toxic effects, as well as other behavioral parameters of chemically and green synthesized ZnO NPs on CQ-induced pruritus using a murine model.
Material and methods
Animals
Female BALB/c mice with weight range 20–30 g were purchased from National Institute of Health, Islamabad, Pakistan. Animals were kept under controlled temperature ranging from 22ºC–25ºC, were provided with hygienic environment with saw bedding (which was changed on alternative days), and 12/12 hour light-dark cycles starting from 08:00 am to 08:00 pm. Animals were provided free access to food and water ad libitum. All the experimental protocols and animal handling procedures were carried out according to regulations guided by the UK 1986 Animals (scientific procedures) Act.43 Ethical approval for the work was obtained from Research Ethics Committee COMSATS University Islamabad, Abbottabad Campus, Abbottabad, Pakistan under the certificate number PHM.Eth/CS-M01/18–002.
Chemicals
Chemicals used were ZnO NPs (100 nm) (Sigma-Aldrich Co., St Louis, MO, USA), ZnO NPs (20–30 nm) (Alfa Aesar USA), ZnO NPs (25–30 nm, green synthesized in the lab using Aloe vera extract),44 and chloroquine phosphate ampule (322.5 mg/5 mL) (Shanxi Shuguang Pharma Pvt. Ltd, China).
Characterization of ZnO NPs
Scanning electron microscopy (SEM) was carried out to observe the surface morphology of ZnO NPs (purchased and green synthesized ZnO NPs) using JSM-IT100 scanning electron microscope. The size and crystallinity of all the samples were evaluated using X-ray powder diffraction (XRD) (JDX 3532, JEOL, Tokyo, Japan) technique at CuKa (1.5418Å) and 2 theta-range was 20–70°.
Drug administration
For pruritus induction, CQ was diluted to 3.2 mg/mL with saline and administered subcutaneously (SC) at a dose of 32 mg/kg body weight. Samples of ZnO NPs were dispersed in saline. They were sonicated for 30 minutes and properly vortexed each time before use. ZnO NPs were administered orally (PO) at a dose of 250, 500, and 750 mg/kg body weight.18–20
Behavioral experiments
Animals (according to each size and dose of ZnO NPs) were divided into eleven groups of six mice each. These were: control (normal saline), CQ-treated animals, ZnO NP-treated (250 mg/kg), ZnO NP-treated (500 mg/kg), and ZnO NP-treated (750 mg/kg). ZnO NPs were administered orally to the mice. After 1 hour of ZnO NPs' administration, pruritus was induced using CQ (32 mg/kg) in 0.3 mL SC injection at the nape of the neck of the mouse to observe the scratching behavior.45 Each mouse was used once.
The mice were taken out of the cage briefly for oral gavage and injection, and returned to the same cage. The behavior was recorded using a CAT-1 video camera. In order to avoid any distraction, the camera was adjusted in an unmanned condition.42 The number of scratching bouts near the site of injection were then counted. Each bout was instigated by moving paw to the site of injection and ended when paw was returned back to the floor or mouth.37,41 After CQ administration the scratching bouts were counted during 30 minutes and simultaneous behavioral changes like socialization and locomotion were also monitored. The number of itching bouts the mouse experienced was revealed by how frequently the mouse scratches itself.45–47 Locomotion was evaluated by the number of lines crossed by the mice in 30 minutes in open field locomotion box.48
Results
Characterization
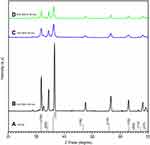
SEM results are shown in Figure 1A–C. From the images, spherical appearance of NPs was confirmed. Standard XRD pattern for ZnO according to the Joint Committee on Powder Diffraction Standards (JCPDS) is shown in Figure 2A. The peaks for ZnO NPs (100 nm), as shown in Figure 2B, were at positions 31.85°, 34.40°, 36.50°, 47.60°, 56.65°, 63.00°, 68.05°, and 69.25° corresponding to (100), (002), (101), (102), (110), (103), (112), and (201) planes respectively. Similarly for ZnO (30 nm) (shown in Figure 2C) the peaks at 2θ =31.67°, 34.28°, 36.36°, 47.80°, 56.63°, 63.13°, 68.06°, and 69.10°, correspond to (100), (002), (101), (102), (110), (103), (112), and (201). For green synthesized ZnO NPs (Figure 2D) peaks were observed at 31.94°, 34.53°, 36.35°, 47.79°, 56.88°, 63.12°, 68.05°, and 69.10°, corresponding to (100), (002), (101), (102), (110), (103), (112), and (201). The relevance of sharp diffraction peaks for ZnO NPs indicated the purity of particles.49–52 Diameters of the ZnO NPs were evaluated using Debye-Scherrer formula.53,54
 | Figure 1 Characterization of ZnO NPs by SEM. (A) ZnO NPs (100 nm), (B) ZnO NPs (30 nm) and (C) ZnO NPs (G 30 nm). Abbreviations: ZnO NPs, Zinc oxide nanoparticles;G, Green synthesized. |
d = 0.89 λ/β cosθ
Where θ is the Bragg diffraction angle, β is full width at half maximum of the diffraction peak, 0.89 is Scherrer’s constant, and λ is the wavelength of X-rays. It was indicated that the particles were in nanometer range confirming the average particle size of 100 nm, 30 nm, and 30 nm respectively (green synthesized ZnO NPs).54
Scratching bouts
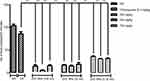
Scratching bouts were recorded in animals of all groups. In normal saline group, animals lacked itching. Onset of scratching was within 5 minutes of SC injection and lasted for almost 30 minutes. There were 133 scratching bouts recorded during 30 minutes in CQ-treated mice. When ZnO NPs were PO administered before SC injection of CQ, it was observed that the number of scratching bouts significantly reduced to 14, nine, and three as a result of ZnO NPs (100 nm) at doses of 250, 500, and 750 mg/kg body weight (Figure 3).
 | Figure 3 Scratching bouts/30 minutes with CQ and effect of ZnO NPs (100, 30 nm and G 30 nm)Abbreviations: N/S; normal saline, CQ, Chloroquine; ZnO NPs, Zinc oxide nanoparticles; G, Green synthesized. |
There was a further reduction in number of scratching bouts to nine, nine, and eight as a result of ZnO NPs (30 nm) at respective doses of 250, 500, and 750 mg/kg body weight (Figure 3). Furthermore, the experiment was repeated at the same PO doses of ZnO NPs (green synthesized 30 nm). Observations were recorded and showed four, three, and three scratching bouts respectively for doses of 250, 500, and 750 mg/kg body weight (Figure 3).
Socialization
Socialization with each other was observed after administration of ZnO NPs as compared with normal saline and CQ-treated group (Figure 4). All animals showed improved socialization behavior after ZnO NPs' administration. The effect persisted even after CQ was administered to the mice.
Locomotion
Locomotion was significantly decreased in ZnO NP (100 and 30 nm)-treated mice as compared to normal saline and CQ treated mice. Locomotion was enhanced in mice administered green synthesized ZnO NPs as compared to commercially available ZnO NPs of 100 and 30 nm sizes (Figure 5).
Discussion
Data obtained from XRD analysis were in agreement with the JCPDS PDF no 36–1451 for the standard hexagonal polycrystalline structure of ZnO.55 The results for green synthesized ZnO NPs were in good agreement with commercially available ZnO NPs (30 nm). When scratching behavior was evaluated for these NPs, a significant (P≤0.001) decrease in intensity of bouts was observed after PO administration of ZnO NPs in all doses and particle sizes (as shown in Figure 3). When the decrease in itching was recorded among different particle sizes and doses, it was observed that there was no significant difference among ZnO NPs of 100 nm and 30 nm, chemically and green synthesized.
The induction of itching by using CQ (32 mg/kg) was consistent with previous studies.56 CQ is known to activate Mrgpr A3 receptors (G protein-coupled receptors), that primarily contribute to scratching desire.41 These acute scratching bouts can be inhibited by blockers (eg, anti-depressants, nalfurafin, and N-methyl-D-aspartate receptor antagonist) of TRPA1/TRPV1.57 However, CQ-induced pruritus is also mediated by increased production of NO (linked to cGMP).41 In in vivo studies, ZnO NPs have been reported to reduce NO level.36,41 Here, the reduction in scratching intensity could be attributed to the ZnO NPs’ NO lowering property. ZnO NPs are reported to induce toxicological response systemically, but in this study it was observed that CQ-induced itching was relieved by ZnO NPs.
ZnO NPs were previously reported to improve memory impairment, anxiety, and oxidative stress in mice.58 In this study, it was established that ZnO NPs significantly improved socialization in mice as compared to the control group. Earlier, Sultana and Najam (2012) reported that Aloe vera caused reduction in locomotion and sedation when independently tested PO for depression, locomotion, and anxiety in mice.59,60 ZnO NPs induced improved socialization further need neuro-endocrine profiling. In this study, administration of ZnO NPs had a calming effect in comparison to the CQ group. However, mice that were given green synthesized ZnO NPs showed significantly (P˂0.001) improved locomotion in comparison to 100 and 30 nm (commercially available) ZnO NPs. Aloe vera has already been reported to have an anti-depressive effect.60 The results of this study support the concept that green synthesis successfully ameliorates the harmful effects of intervening chemicals used during synthesis.61,62 Here, the effect of green synthesized NPs could have been related to the synthesis method of ZnO NPs. This aspect of green synthesis might be a contributing factor to the improved locomotion in mice observed with green synthesized ZnO NPs. More studies are warranted to explore ZnO NPs' effect on the role of gender, as this study was conducted using female mice only.
Conclusion
Instead of aggravating the dermatologic condition, ZnO NPs, overall, imparted a significant protective effect in a murine model of CQ-induced pruritus. Additionally, socialization was improved significantly at a lower dose (250 mg/kg) with green synthesized NPs, which showed a less depressive effect, unlike commercially available ZnO NPs. Studies using non-toxic doses of ZnO NPs are warranted to explore their effect on CQ-induced pruritus, socialization, and locomotion.
Acknowledgment
The authors acknowledge the support of Ms Mehreen Arif, Department of Pharmacy, COMSATS University Islamabad, Abbottabad Campus, Abbottabad, KPK, Pakistan.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vasantharaj S, Sathiyavimal S, Saravanan M, et al. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: characterization of antibacterial activity and dye degradation potential. J Photochem Photobiol B. 2019;191:143–149. doi:10.1016/j.jphotobiol.2018.12.026
2. Suganthy N, Ramkumar VS, Pugazhendhi A, Benelli G, Archunan G. Biogenic synthesis of gold nanoparticles from Terminalia arjuna bark extract: assessment of safety aspects and neuroprotective potential via antioxidant, anticholinesterase, and antiamyloidogenic effects. Environ Sci Pollut Res. 2017;10418–10433. doi:10.1007/s11356-017-9789-4
3. Shanmuganathan R, MubarakAli D, Prabakar D, et al. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ Sci Pollut Res. 2018;25(11):10362–10370. doi:10.1007/s11356-017-9367-9
4. Oves M, Aslam M, Rauf MA, et al. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater Sci Eng C. 2018;89:429–443. doi:10.1016/j.msec.2018.03.035
5. Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi:10.1038/sj.clpt.6100400
6. Saratale RG, Saratale GD, Shin HS, et al. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: current knowledge, their agricultural and environmental applications. Environ Sci Pollut Res. 2018;25(11):10164–10183. doi:10.1007/s11356-017-9912-6
7. Sathiyavimal S, Vasantharaj S, Bharathi D, et al. Biogenesis of copper oxide nanoparticles (CuONPs) using sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of gram negative and gram positive bacteria. J Photochem Photobiol B. 2018;188:126–134. doi:10.1016/j.jphotobiol.2018.09.014
8. Wang J, Gao S, Wang S, Xu Z, Wei L. Zinc oxide nanoparticles induce toxicity in CAL 27 oral cancer cell lines by activating PINK1/Parkin-mediated mitophagy. Int J Nanomedicine. 2018;13:3441. doi:10.2147/IJN.S177627
9. Sarkar S, Makhal A, Baruah S, Mahmood MA, Dutta J, Pal SK. Nanoparticle-sensitized photodegradation of bilirubin and potential therapeutic application. J Phys Chem C. 2012;116(17):9608–9615. doi:10.1021/jp301316e
10. Hanley C, Layne J, Punnoose A, et al. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology. 2008;19(29):295103. doi:10.1088/0957-4484/19/29/295103
11. Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011;77(7):2325–2331. doi:10.1128/AEM.02149-10
12. Fakhar-e-Alam M, Rahim S, Atif M, et al. ZnO nanoparticles as drug delivery agent for photodynamic therapy. Laser Phys Lett. 2013;11(2):025601. doi:10.1088/1612-2011/11/2/025601
13. Umar H, Kavaz D, Rizaner N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int J Nanomedicine. 2019;14:87. doi:10.2147/IJN.S186888
14. Wang R, Song B, Wu J, Zhang Y, Chen A, Shao L. Potential adverse effects of nanoparticles on the reproductive system. Int J Nanomedicine. 2018;13:8487. doi:10.2147/IJN.S177627
15. Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract. 2014;2014:709152. doi:10.1155/2014/709152
16. Aksoy B, Atakan N, Aksoy HM, et al. Effectiveness of topical zinc oxide application on hypertrophic scar development in rabbits. Burns. 2010;36(7):1027–1035. doi:10.1016/j.burns.2010.01.020
17. Bhagwat D, Glassman D, Glassman BP. Topical pharmaceutical base with anti-pruritic and/or anti-inflammatory drugs. Patent No: 6,495,602 B1. 2002 Dec 17. Google Pat. Fairfield (NJ): Bradley Pharmaceuticals, Inc.; 2002.
18. Wang C, Cheng K, Zhou L, et al. Evaluation of long-term toxicity of oral zinc oxide nanoparticles and zinc sulfate in mice. Biol Trace Elem Res. 2017;178(2):276–282. doi:10.1007/s12011-017-0934-1
19. Srivastav AK, Kumar A, Prakash J, et al. Genotoxicity evaluation of zinc oxide nanoparticles in Swiss mice after oral administration using chromosomal aberration, micronuclei, semen analysis, and RAPD profile. Toxicol Ind Health. 2017;33(11):821–834. doi:10.1177/0748233717717842
20. Wang C, Lu J, Zhou L, et al. Effects of long-term exposure to zinc oxide nanoparticles on development, zinc metabolism and biodistribution of minerals (Zn, Fe, Cu, Mn) in mice. PLoS One. 2016;11(10):e0164434. doi:10.1371/journal.pone.0164434
21. Salata OV. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2(1):3. doi:10.1186/1477-3155-2-3
22. Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539(1–2):104–111. doi:10.1016/j.ijpharm.2018.01.034
23. Baek M, Chung H-E, Yu J, et al. Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. Int J Nanomedicine. 2012;7:3081. doi:10.2147/IJN.S30631
24. Ng CT, Yong LQ, Hande MP, et al. Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int J Nanomedicine. 2017;12:1621. doi:10.2147/IJN.S124403
25. Vandebriel RJ, De Jong WH. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol Sci Appl. 2012;5:61. doi:10.2147/NSA
26. Shrivastava R, Raza S, Yadav A, Kushwaha P, Flora SJ. Effects of sub-acute exposure to TiO2, ZnO and Al2O3 nanoparticles on oxidative stress and histological changes in mouse liver and brain. Drug Chem Toxicol. 2014;37(3):336–347. doi:10.3109/01480545.2013.866134
27. Zahra J, Iqbal S, Zahra K, et al. Effect of variable doses of zinc oxide nanoparticles on male albino mice behavior. Neurochem Res. 2017;42(2):439–445. doi:10.1007/s11064-016-2090-y
28. Attia H, Nounou H, Shalaby M. Zinc oxide nanoparticles induced oxidative DNA damage, inflammation and apoptosis in rat‘s brain after oral exposure. Toxics. 2018;6:2. doi:10.3390/toxics6020029
29. Darbandi N, Momeni H. Effect of zinc oxide nanoparticles on memory retrieval, hippocampal CA1 pyramidal neurons and some serum oxidative stress factors in male wistar rats. URMIA MED J. 2018;29(6):450–463.
30. Tian L, Lin B, Wu L, et al. Neurotoxicity induced by zinc oxide nanoparticles: age-related differences and interaction. Sci Rep. 2015;5:16117. doi:10.1038/srep16117
31. Zhao J, Xu L, Zhang T, Ren G, Yang Z. Influences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neurons. Neurotoxicology. 2009;30(2):220–230. doi:10.1016/j.neuro.2008.12.005
32. Hsiao I-L, Huang Y-J. Titanium oxide shell coatings decrease the cytotoxicity of ZnO nanoparticles. Chem Res Toxicol. 2011;24(3):303–313. doi:10.1021/tx1001892
33. Pugazhendhi A, Prabakar D, Jacob JM, Karuppusamy I, Saratale RG. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb Pathog. 2018;114:41–45. doi:10.1016/j.micpath.2017.11.013
34. Saratale RG, Karuppusamy I, Saratale GD, et al. A comprehensive review on green nanomaterials using biological systems: recent perception and their future applications. Colloids Surf B. 2018. doi:10.1016/j.colsurfb.2018.05.045
35. Kalpana V, Devi Rajeswari V. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg Chem Appl. 2018;2018:3569758. doi:10:1155/2018/3569758
36. Kim C-S, Nguyen H-D, Ignacio RM, et al. Immunotoxicity of zinc oxide nanoparticles with different size and electrostatic charge. Int J Nanomedicine. 2014;9(Suppl 2):195.
37. Tarrasón G, Carcasona C, Eichhorn P, et al. Characterization of the mouse model of chloroquine-induced pruritus using an automated recording system.
38. Aghahowa S, Obianwu H, Isah A, Arhewoh I. Chloroquine-induced pruritus. Indian J Pharm Sci. 2010;72(3):283. doi:10.4103/0250-474X.70471
39. Wilson SR, Gerhold KA, Bifolck-Fisher A, et al. TRPA1 is required for histamine-independent, Mas-related G protein–coupled receptor–mediated itch. Nat Neurosci. 2011;14(5):595. doi:10.1038/nn.2789
40. Bussaratid V, Walsh DS, Wilairatana P, Krudsood S, Silachamroon U, Looareesuwan S. Frequency of pruritus in Plasmodium vivax malaria patients treated with chloroquine in Thailand. Trop Doct. 2000;30(4):211–214. doi:10.1177/004947550003000410
41. Haddadi A-S, Foroutan A, Ostadhadi S, et al. Peripheral NMDA receptor/NO system blockage inhibits itch responses induced by chloroquine in mice. Acta Derm Venereol. 2017;97(5):571–577. doi:10.2340/00015555-2617
42. Ru F, Sun H, Jurcakova D, et al. Mechanisms of pruritogen-induced activation of itch nerves in isolated mouse skin. J Physiol. 2017;595(11):3651–3666. doi:10.1113/JP273795
43. Hollands C. The Animals (scientific procedures) Act 1986. Lancet (London, England). 1986;2(8497):32.
44. Varghese E, George M. Green synthesis of zinc oxide nanoparticles. Int J Adv Res Sci Eng. 2015;4(1):307–314.
45. Elliott P, G’Sell M, Snyder LM, Ross SE, Ventura V. Automated acoustic detection of mouse scratching. PLoS One. 2017;12(7):e0179662. doi:10.1016/j.pain.2006.03.023
46. Zhao N, Gu M, Yang W, et al. Increased ZAP70 is involved in dry skin pruritus in aged mice. Biomed Res Int. 2016;2016:6029538. doi:10.1155/2016/6029638
47. Green AD, Young KK, Lehto SG, Smith SB, Mogil JS. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain. 2006;124(1–2):50–58. doi:10.1016/j.pain.2006.03.023
48. Gellért L, Varga D. Locomotion activity measurement in an open field for mice. Bio-protocol. 2016;6(13):e1857. doi:19.201769/BioProtoc.1857. Front Behav Neurosci. 2015.
49. Elmorsi TM, Elsayed MH, Bakr MF. Enhancing the removal of methylene blue by modified ZnO nanoparticles: kinetics and equilibrium studies. Can J Chem. 2017;95(5):590–600. doi:10.1139/cjc-2016-0456
50. Gopal VV, Kamila S. Effect of temperature on the morphology of ZnO nanoparticles: a comparative study. App Nanosci. 2017;7(3–4):75–82. doi:10.1007/s13204-017-0553-3
51. Khan W, Khan Z, Saad A, Shervani S, Saleem A, Naqvi A Synthesis and characterization of Al doped ZnO nanoparticles.
52. Dinesh V, Biji P, Ashok A, et al. Plasmon-mediated, highly enhanced photocatalytic degradation of industrial textile dyes using hybrid ZnO@ Ag core–shell nanorods. RSC Adv. 2014;4(103):58930–58940. doi:10.1039/C4RA09405K
53. Cullity BD. Elements of X-Ray Diffraction. USA: Addison-Wesley Publishing Company, Inc.; 2001.
54. Mohan AC, Renjanadevi B. Preparation of zinc oxide nanoparticles and its characterization using scanning electron microscopy (SEM) and X-ray diffraction (XRD). Procedia Technol. 2016;24:761–766. doi:10.1016/j.protcy.2016.05.078
55. Krishna Reddy G, Jagannatha Reddy A, Hari Krishna R, Nagabhushana B, Gopal GR. Luminescence and spectroscopic investigations on Gd3 + doped ZnO nanophosphor. J Asian Ceram Soc. 2017;5(3):350–356. doi:10.1016/j.jascer.2017.06.008
56. Barry DM, Yu Y-Q, Hao Y, Liu X-T, Chen Z-F. Response to comment on “molecular and neural basis of contagious itch behavior in mice”. Science. 2017;357(6347):eaan5000. doi:10.1126/science.aan5000
57. Ru F, Sun H, Jurcakova D, et al. Mechanisms of pruritogen‐induced activation of itch nerves in isolated mouse skin. J Physiol. 2017;595(11):3651–3666. doi:10.1113/JP273795
58. Hafez MH, Gad SB. Zinc oxide nanoparticles effect on oxidative status, brain activity, anxiety-like behavior and memory in adult and aged male rats. Pak Vet J. 2018;38:3.
59. Sultana N, Najam R. Anxiolytic activity of aloe vera (L.) burm. F tested in rodents. Pak J Pharmacol. 2012;29(1):7–15.
60. Salehi B, Biazar E, Jahromi MH, Romani HA. Antidepressant effects of aloe vera hydroalcoholic extract. J Paramed Sci. 2011;2:3.
61. Vasantharaj S, Sathiyavimal S, Senthilkumar P, LewisOscar F, Pugazhendhi A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: antimicrobial properties and their applications in photocatalytic degradation. J Photochem Photobiol B. 2019;192:74–82. doi:10.1016/j.jphotobiol.2018.12.025
62. Pugazhendhi A, Prabhu R, Muruganantham K, Shanmuganathan R, Natarajan S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J Photochem Photobiol B. 2019;190:86–97. doi:10.1016/j.jphotobiol.2018.11.014
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.