Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Effect of Benzo[a]pyrene-DNA Adduct in Cord Blood on the Neurodevelopment of 12-Month-Old Infants in Qingdao City
Authors Zhang Y, Yang Y, Zhang Q, Cui J, Rahaman A , Huang XR, Su Y, Gao R, Wang B , Baloch Z
Received 12 June 2019
Accepted for publication 4 November 2019
Published 2 December 2019 Volume 2019:15 Pages 3351—3357
DOI https://doi.org/10.2147/NDT.S219244
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yong Zhang,1,* Yuehua Yang,2,* Qian Zhang,3,* Jing Cui,4 Abdul Rahaman,5 Xiao-Rong Huang,6,7 Ya Su,4 Ruqin Gao,4 Bingling Wang,4 Zulqarnain Baloch6
1Department of Paediatric Surgery, Qingdao Women and Children’s Hospital, Qingdao, People’s Republic of China; 2Department of Obstetrics, The Affiliated Central Hospital of Qingdao University, Qingdao, People’s Republic of China; 3Department of Child Health Care, Huangdao Maternity and Child Health Care Hospital of Qingdao, Qingdao, People’s Republic of China; 4Department of Environmental Health, Qingdao Centers for Disease Control and Prevention/Qingdao Institute for Preventive Medicine, Qingdao, People’s Republic of China; 5School of Food Science and Engineering, South China University of Technology, Guangzhou 510640, People’s Republic of China; 6Biomedical Research Center, Northwest Minzu University, Lanzhou 730030, People’s Republic of China; 7College of Veterinary Medicine, South China Agricultural University, Guangzhou, 51062, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zulqarnain Baloch
Biomedical Research Center, Northwest Minzu University, Lanzhou 730030, People’s Republic of China
Tel/Fax +8618344564625
Email [email protected]
Bingling Wang
Department of Environmental Health, Qingdao Institute for Preventive Medicine, No. 175 Shandong Road, Qingdao 266033, People’s Republic of China
Tel +86 532 85621975
Fax +86 532 85623909
Email [email protected]
Introduction: The study was designed to explore the possible adverse effects of prenatal polycyclic aromatic hydrocarbons (PAHs) on the neurodevelopment of the infants at the age of 12 months in a birth cohort in Qingdao of China. Benzo[a]pyrene (BaP)-DNA adduct level in umbilical cord blood was measured by enzyme immunoassay.
Methods: Child neurodevelopment was assessed at both 6 months and 12 months of age using the Gesell Development Inventory (GDI).
Results: This study results reveal that multivariate linear analysis, cord BaP-DNA adduct level was inversely associated with developmental quotient score in the adaptive domain [β = −0.08; 95% CI: (−0.16, −0.003); p = 0.04], gross motor domain [β = −0.10; 95% CI: (−0.20, −0.01); p = 0.02], fine motor domain [β = −0.15; 95% CI: (−0.25, −0.05); p = 0.01], language domain [β = −0.12; 95% CI: (−0.21, −0.03); p = 0.02], and personal–social domain [β = −0.13; 95% CI: (−0.22, −0.04); p<0.01]. Further, multivariate logistic regression analysis showed increased cord BaP-DNA adduct levels associated with increased odds of delayed in language domain.
Conclusion: In conclusion, the study suggested that prenatal PAH exposure monitored by umbilical cord blood BaP-DNA adducts may adversely affect the neurodevelopment of the infants at 12 months of age.
Keywords: neurodevelopment, PAH-DNA adducts, infant, polycyclic aromatic hydrocarbons, China
Introduction
Our previously reported cross-sectional studies have found that prenatal polycyclic aromatic hydrocarbons (PAHs) widely exist in the urban residential house dust with a high order of magnitudes in house dust and that significant association with the behavior and neurodevelopment of preschool-aged children.1 Guo et al compared the concentrations of 12 hydroxylated polycyclic aromatic hydrocarbons in 306 urine samples collected from seven Asian countries (China, India, Japan, Korea, Kuwait, Malaysia, and Vietnam) and found that the concentrations of 1-hydroxypyrene in Chinese participants' urine samples were 4 to 6 times higher than the other country participants.2 Another biomarker of PAH exposure levels, 2-hydroxyfluorene, was also reported higher in Chinese participants than other reported countries.2,3 All these reported data indicated that people in China are exposed to great concentrations of PAHs as compared to other countries.
PAH exposure levels measured by airborne PAHs or biomarkers such as PAH-DNA adducts have been reported associated with the child’s neurodevelopment. In a cohort study in the USA, higher prenatal exposure to airborne PAHs was associated with decreased scores on the mental developmental index of the Bayley Scales of Infant Development-Revised at age 36 months.4 Further, they measured the bulky hydrophobic DNA adducts by the 32P-postlabelling assay in umbilical cord blood and found the symptoms associated with anxiety/depression and attention problems at the age of 5 and 7 years.5 In an earlier China cohort near a coal-fired power, higher benzo[a]pyrene (BaP)-DNA adducts were determined by the high-performance liquid chromatography (HPLC) in umbilical cord blood and it was found with decreased scores on the Gesell test at age of 24 months.6,7 The period of rapid brain growth spans from the 3rd trimester of pregnancy to at least 2 years after birth.8 Some of the studies have been reported the prenatal PAH exposure influence on the neurodevelopment of the children less than 2 years old. Therefore, in the present analysis, we evaluated the effects of BaP-DNA adduct in umbilical cord blood, a biomarker of prenatal exposure of PAHs,9–12 on the neurodevelopment of children at the age of 12 months. Instead of HPLC and 32P-post-labeled global PAH-DNA adduct detection, a quick and convenient enzyme immunoassay method was used for the measurement of PAH-DNA adduct.
Materials and Methods
Ethical Statement
This study was approved by the Ethics Committee and Institutional Review Board of Qingdao Centers for Disease Control and Prevention. The authors declare that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Study Subjects
We performed a birth cohort study in Qingdao city of China in 2014 to find the possible adverse neurodevelopmental effects of prenatal and postnatal semi-volatile organic compound exposure on the children. The pregnant women at the obstetrics of the hospitals in Qingdao city were invited to sign the informed consent for participation in this prospective cohort study. The eligible women were free from diabetes, known HIV, and known neuropsychiatric disease; they will reside in the area for at least 3 years together with their infants. The eligible children were free from birth problems, such as delivery injuries or low birth weight; neonatal problems, such as asphyxia; intracranial hemorrhage, hypoxic–ischemic encephalopathy, and severe jaundice; acquired disabilities, including encephalitis, meningitis, encephalopathy, cerebral trauma, and cerebral injuries following convulsions; and developmental dysplasia or other developmental defects influenced by inherited metabolic diseases. In this study, the 12-month-old 348 subjects were recruited who had valid BaP-DNA adduct levels in the cord blood, of which 211 had Gesell test scores for both 6 and 12 months of age.
BaP-DNA Adduct Measurement
The umbilical cord blood was collected at the time of delivery. BaP-DNA adducts (ng/mL) were determined in extracted white blood cell DNA by OxiSelect™ BPDE DNA Adduct ELISA Kits (Cell Biolabs, Inc., San Diego, CA) according to the manufacturer’s product manual.
Measures of Child Neurodevelopment
The Gesell Development Inventory (GDI) was selected in the present study for the measurement of child neurodevelopment. The GDI, was adopted by the Chinese Pediatric Association, has been validated against a Chinese reference population and widely used for assessing the child development in China.13 There was a significant correlation between developmental assessment at 6 to 12 months on the GDI and mental development at 6 to 7 years on the Chinese version of the Wechsler Intelligence Scales for Children.14 The GDI has been translated and standardized by the Beijing Children’s Health Care Institute and consists of five behavioral domains: adaptive, gross motor, fine motor, language, and personal–social behaviors.15 Each child was assigned a developmental quotient (DQ) in each of the five specific domains. The standardized mean (±SD) of the DQ is 100±15, and a score <85 indicates a developmental delay.16,17 All subjects were tested by a professional doctor at the Department of Child Health Care of Huangdao Maternity and Child Care Hospital of Qingdao.
Statistical Analysis
According to the product manual, the BaP-DNA adducts levels ≤0 ng/mL was regarded as no detection and 0 was assigned. The Shapiro–Wilk test showed that the BaP-DNA adducts were not a normal distribution, while the GDI scores could be regarded as normal distribution. Spearman’s rho correlation coefficients were calculated for the BaP-DNA and the scores at age of both 6 and 12 months. The main effects of BaP-DNA adduct levels on the DQs at the age of 12 months were analyzed by multiple linear regressions with adjusted for sex, birth weight, gestational age, and maternal education. Binary logistic regression was also adopted to find the possible risk of high BaP-DNA adduct levels >90th percentile (1.945 ng/mL) on child development. DQs at age of 6 months were entered the multivariate regression model together with BaP-DNA adduct levels because of the correlation between DQs at 6 and 12 months of age. All the analyses were done with IBM SPSS Statistics version 21 (IBM Corp).
Results
The demographic characteristics, cord BaP-DNA adducts levels, and the DQ scores are shown in Table 1. The detection rate for the cord BaP-DNA adduct (> 0 ng/mL) was only 52.3% (182/348) and the median level was 0.68 ng/mL. The average scores for all DQ domains of both 6 and 12 months old were all over 90. The scores of each DQ domain were significantly correlated between 6 and 12 months of age except fine motor domain. For the language and personal–social domains, the scores indicated a significant correlation with those of all other domains (Table 2). Adduct levels showed inverse correlation with the DQ scores at the age of 12 months, but no such correlation was found for the DQ scores at the age of 6 months (data not shown).
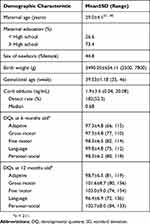 |
Table 1 Demographic Characteristics of the Study Sample (n = 348) |
 |
Table 2 Spearman’s Rho Correlation Coefficients Between Adduct Levels and DQs at 6 Months and 12 Months Old |
The results of the multiple linear regression analysis are shown in Table 3. Increased cord BaP-DNA adduct level was inversely associated with DQ score decreased in the adaptive domain [β = −0.08; 95% CI: (−0.16, −0.003); p = 0.04], gross motor domain [β = −0.10; 95% CI: (−0.20, −0.01); p = 0.02], fine motor domain [β = −0.15; 95% CI: (−0.25, −0.05); p = 0.01], language domain [β = −0.12; 95% CI: (−0.21, −0.03); p = 0.02], and personal–social domain [β = −0.13; 95% CI: (−0.22, −0.04); p<0.01], after adjusted for the same domain score at the age of 6 months, sex, gestational age, birth weight, and maternal education.
 |
Table 3 Results of Multiple Linear Regression Analyses of DQ Scores at 12 Months of Age and Cord BaP-DNA Adducts (n = 211)a |
The frequency of developmental delay in adaptive, gross motor, fine motor, language, and the personal–social domain at the age of 12 months was 4.8%, 4.8%, 6.1%, 3.8%, and 8.2%, respectively (data not shown). Multivariate logistic regression analysis showed that increased cord BaP-DNA adduct levels associated with increased odds of delayed in language domain with OR = 5.99 (95% CI: 1.88–19.02, p<0.01) (Table 4). For all domains, DQ scores at 6-month old showed positive association with 12-month-old DQs with P<0.05.
 |
Table 4 Results of Multivariate Logistic Regression Analyses of DQ Scores at 12 Months of Age and Cord BaP-DNA Adducts (n = 211)a |
Discussion
In this study, we found that the BaP-DNA adduct level in the newborn umbilical cord blood was associated with the reductions of the DQ scores at the age of 12 months. After adjusted for the DQ scores at 6 months and some other covariates, increased BaP-DNA adduct levels were significantly associated with reduced DQs at 12 months in all five domains. Our findings are consistent with the laboratory and field observations. Laboratory studies exposing experimental animals to PAHs during the prenatal period have reported impairments of memory and learning,18,19 as well as anxiety and depression behavior effects.20–22 Epidemiological studies in the USA,4,5,23 China,7,24 and Poland25 had indicated neurodevelopment effects of PAHs in humans. It has been well accepted with evidence that prenatal exposure to PAHs may have an adverse effect on the neurodevelopment of children aged 2 to 8 years. Perera et al estimated the effects of prenatal exposure to airborne PAHs on neurodevelopment in children of 12 months through 36 months among inner-city children by a general estimated equation. At 36 months, the adverse effects on a child’s cognitive development were significantly greater for high-exposed (>4.16 ng/m3) compared to low-exposed (≤4.16 ng/m3) children.4 Edwards et al found in a cohort of pregnant, healthy, nonsmoking women in Poland that higher (above the median of 17.96 ng/m3) prenatal exposure to airborne PAHs associated with decreased Raven Colored Progressive Matrices Scores at the age of 5 years.25 As the biomarker of airborne PAHs exposure, cord blood BaP-DNA adduct measured by HPLC showed positive association with symptoms of anxious/depressed and attention problems at the age of 6 to 7 years.23 In another earlier study in china, cord blood BaP-DNA measured by HPLC indicated an association with the delayed development in the motor and language domain of the children aged 2 years.7 To the best of our knowledge, the association between prenatal PAH exposure and the child’s neurodevelopment at 12 months had not been detected. Perera et al investigated the effects of prenatal airborne PAH exposure on the child’s neurodevelopment at 12, 24, and 36 months. However, no effects of PAH on cognitive development at 12 and 24 months were found.4 Thus, we are the first to report the possibly significant effect on a child’s development at the age of 12 months.
We adopted the enzyme immunoassay in this study for the rapid detection of BaP-DNA adducts. This is the first time to use this method to detect BaP-DNA adducts as the PAH exposure measure to investigate the possible PAH effect on the neurodevelopment, although this method has been successfully used recently in some studies like for cell,26,27 swine,28 or human.29 It seemed that the adverse effects found in 12 months of age in our study were earlier than those reported in other studies. However, it is difficult to compare our BaP-DNA adduct levels with other studies by HPLC or 32P-post-labeled global PAH-DNA adduct. The unit ng/mL is used in the enzyme immunoassay, while adduct count/108 nucleotides are used in HPLC and 32P-post-labeled analysis. It was inappropriate to convert from one unit to another, especially for 32P-post-labeled global PAH-DNA adduct which formed by a range of hydrophobic aromatic hydrocarbons in addition to PAH, such as nitro-aromatic compounds30 and heterocyclic amines.31 A strong reason for our enzyme immunoassay using is the convenience of its commercial ELISA kit compared to the HPLC complication and the safety compared to the possible radioactivity of 32P-post-labeled analysis. The BaP-DNA standard in the kit was created in vitro by incubating calf thymus DNA with BPDE-I (NCI Carcinogen Repository MRI 477). The producer pointed out that it is difficult to identify how many BPDE modifications per DNA molecule. Therefore, the OD values cannot be converted to pmol/µg DNA. Thus, the unit ng/mL was used in the present study. However, the detection rates are still comparable between studies although the units are different. The current study suggested a lower detection rate of BaP-DNA adduct compared with another Chinese report for the Tongliang children living near to a coal-fired power plant (52% vs 80%).7 But our detection rate is comparable to that of a cohort without special exposure in the USA (45~50%).32,33
The main limitation of the present study was that we lacked air PAH monitoring data for all three trimesters. The umbilical cord blood PAH-DNA adduct from the prenatal PAH exposure is influenced by the mother’s metabolic and detoxifying enzymes and dietary habit. Therefore, we were not able to identify the predominant exposure of these three periods. We also did not measure postnatal personal air PAH exposure and were unable to adjust directly for postnatal PAH exposure. We also lacked other known prenatal and postnatal neurotoxicant exposure from breast milk, infant complementary foods, or other ambient environment media. Future studies should continue to examine the influence of the postnatal PAH and other possible neurotoxicant exposure and to confirm the present findings.
In this study, we showed that prenatal PAH exposure monitored by umbilical cord blood BaP-DNA adduct at levels recently encountered in the infants at the age of 12 months of Qingdao City may adversely affect the neurodevelopment of the infants. Our findings should be carefully interpreted and require further confirmation but of potential concern because of the quick and convenient performance at the clinics with an ELISA kit for the BaP-DNA adduct measure and the widely used Gesell Development Inventory for the neurodevelopment assessment in China. We are continuing to follow this cohort to determine the long-term effects of prenatal PAH exposure and the possible confounding influences of the postnatal PAH exposure on the neurodevelopment of the children.
Author Contributions
Yong Zhang, Yuehua Yang, and Qian Zhang are considered first authors. Zulqarnain Baloch and Bingling Wang designed the research and supervised the whole process; Yong Zhang, Yuehua Yang, Qian Zhang, and Jing Cui finished the lab work, as well as statistical analysis, and were responsible for manuscript writing and revising. Abdul Rahaman, Xiao-Rong Huang, YaSu, and Ruqin Gao were responsible for data and sample collection and take part in lab work. All authors contributed to data analysis, drafting, and revising the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Wang BL, Pang ST, Zhang XL, et al. Levels and neurodevelopmental effects of polycyclic aromatic hydrocarbons in settled house dust of urban dwellings on preschool–aged children in Nanjing, China. Atmos Pollut Res. 2014;5(2):292–302. doi:10.5094/APR.2014.035
2. Guo Y, Senthilkumar K, Alomirah H, et al. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Technol. 2013;47(6):2932–2938. doi:10.1021/es3052262
3. Fan R, Wang D, Mao C, et al. Preliminary study of children’s exposure to PAHs and its association with 8-hydroxy-2ʹ-deoxyguanosine in Guangzhou, China. Environ Int. 2012;42(4):53–58. doi:10.1016/j.envint.2011.03.021
4. Perera FP, Rauh V, Whyatt RM, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114(8):1287–1292. doi:10.1289/ehp.9084
5. Perera FP, Wang S, Vishnevetsky J, et al. Polycyclic aromatic hydrocarbons-aromatic DNA adducts in cord blood and behavior scores in New York city children. Environ Health Perspect. 2011;119(8):1176–1181. doi:10.1289/ehp.1002705
6. Perera F, Li TY, Zhou ZJ, et al. Benefits of reducing prenatal exposure to coal-burning pollutants to children’s neurodevelopment in China. Environ Health Perspect. 2008;116(10):1396–1400. doi:10.1289/ehp.11480
7. Tang D, Li TY, Liu JJ, et al. Effects of prenatal exposure to coal-burning pollutants on children’s development in China. Environ Health Perspect. 2008;116(5):674–679. doi:10.1289/ehp.10471
8. Rice D, Barone S
9. Poirier MC, Beland FA. DNA adduct measurements and tumor incidence during chronic carcinogen exposure in animal models: implications for DNA adduct-based human cancer risk assessment. Chem Res Toxicol. 1992;5(6):749–755. doi:10.1021/tx00030a003
10. Perera FP. Molecular epidemiology: on the path to prevention? J Natl Cancer Inst. 2000;92(8):602–612. doi:10.1093/jnci/92.8.602
11. Veglia F, Matullo G, Vineis P. Bulky DNA adducts and risk of cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2003;12(2):157–160.
12. Srogi K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ Chem Lett. 2007;5(4):169–195. doi:10.1007/s10311-007-0095-0
13. Song J, Zhu Y. Children’s Neuropsychological Tests.
14. Zhou X, Luo Y, Liang J, et al. Follow-up study of mental developments in high-risk children. J Zhejiang Univ Med Sci. 2004;33(5):449–451.
15. Zhu Y, Lu S, Tang C, Wang Z, Song J. Application of DDST in China: retrospective and prospective era. J Clin Pediatr. 1983;1:129–132.
16. Hudon L, Jr KJ M, Hegemier SE, et al. Long-term neurodevelopmental outcome after intrauterine transfusion for the treatment of fetal hemolytic disease. Am J Obstet Gynecol. 1998;179(4):858–863. doi:10.1016/S0002-9378(98)70178-4
17. Tang D, Lee J, Muirhead L, et al. Molecular and neurodevelopmental benefits to children of closure of a coal burning power plant in China. PLoS One. 2014;9(3):e91966. doi:10.1371/journal.pone.0091966
18. Brown LA, Khousbouei H, Goodwin JS, et al. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology. 2007;28(5):965–978. doi:10.1016/j.neuro.2007.05.005
19. Wormley DD, Ramesh A, Hood DB. Environmental contaminant-mixture effects on CNS development, plasticity, and behavior. Toxicol Appl Pharmacol. 2004;197(1):49–65. doi:10.1016/j.taap.2004.01.016
20. Saunders CR, Shockley DC, Knuckles ME. Fluoranthene-induced neurobehavioral toxicity in F-344 rats. Int J Toxicol. 2003;22(4):263–276. doi:10.1080/10915810305114
21. Saunders CR, Ramesh A, Shockley DC. Modulation of neurotoxic behavior in F-344 rats by temporal disposition of benzo(a)pyrene. Toxicol Lett. 2002;129(1–2):33–45. doi:10.1016/S0378-4274(01)00467-2
22. Saunders CR, Das SK, Ramesh A, Shockley DC, Mukherjee S. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J Appl Toxicol. 2006;26(5):427–438. doi:10.1002/(ISSN)1099-1263
23. Perera FP, Tang D, Wang S, et al. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6-7 years. Environ Health Perspect. 2012;120(6):921–926. doi:10.1289/ehp.1104315
24. Perera F, Li TY, Lin C, Tang D. Effects of prenatal polycyclic aromatic hydrocarbon exposure and environmental tobacco smoke on child IQ in a Chinese cohort. Environ Res. 2012;114(2):40–46. doi:10.1016/j.envres.2011.12.011
25. Edwards SC, Jedrychowski W, Butscher M, et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ Health Perspect. 2010;118(9):1326–1331. doi:10.1289/ehp.0901070
26. Chiu CY, Yen YP, Tsai KS, Yang RS, Liu SH. Low-dose benzo(a)pyrene and its epoxide metabolite inhibit myogenic differentiation in human skeletal muscle-derived progenitor cells. Toxicol Sci. 2014;138(2):344. doi:10.1093/toxsci/kfu003
27. Yang J, Chen W, Fan Y, Zhang H, Wang W, Zhang H. Ubiquitin protein ligase ring2 is involved in S‐phase checkpoint and DNA damage in cells exposed to benzo[a]pyrene. J Biochem Mol Toxicol. 2016;30(10):481–488. doi:10.1002/jbt.21811
28. Peters RE, Wickstrom M, Siciliano SD. Do biomarkers of exposure and effect correlate with internal exposure to PAHs in swine? Biomarkers. 2016;21(3):1–9. doi:10.3109/1354750X.2016.1138322
29. Su Y, Zhao B, Guo F, et al. Interaction of benzo[a]pyrene with other risk factors in hepatocellular carcinoma: a case-control study in Xiamen, China. Ann Epidemiol. 2014;24(2):98–103. doi:10.1016/j.annepidem.2013.10.019
30. Arlt VM, Sorg BL, Osborne M, et al. DNA adduct formation by the ubiquitous environmental pollutant 3-nitrobenzanthrone and its metabolites in rats. Biochem Biophys Res Commun. 2003;300(1):107–114. doi:10.1016/S0006-291X(02)02789-4
31. Munnia A, Saletta F, Allione A, et al. 32P-Post-labelling method improvements for aromatic compound-related molecular epidemiology studies. Mutagenesis. 2007;22(6):381–385. doi:10.1093/mutage/gem030
32. Herbstman JB, Tang D, Zhu D, et al. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA adducts, and genomic DNA methylation in cord blood. Environ Health Perspect. 2012;120(5):733–738. doi:10.1289/ehp.1104056
33. Perera FP, Tang D, Tu YH, et al. Biomarkers in maternal and newborn blood indicate heightened fetal susceptibility to procarcinogenic DNA damage. Environ Health Perspect. 2004;112(10):1133–1136. doi:10.1289/ehp.6833
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
