Back to Journals » Journal of Experimental Pharmacology » Volume 12
Effect of Aqueous Extract of F. capensis Leaves and Its Combination with C. aconitifolius Leaves on Essential Biochemical Parameters of Phenylhydrazine-Induced Anemic Rats
Authors Ezeigwe OC , Nzekwe FA , Nworji OF , Ezennaya CF, Iloanya EL, Asogwa KK
Received 23 March 2020
Accepted for publication 7 June 2020
Published 1 July 2020 Volume 2020:12 Pages 191—201
DOI https://doi.org/10.2147/JEP.S254003
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bal Lokeshwar
Obiajulu Christian Ezeigwe,1 Favour Amarachi Nzekwe,1 Ogechukwu Frances Nworji,1 Chidinma Felicia Ezennaya,2 Ebele Lauretta Iloanya,1 Kingsley Kelechi Asogwa1
1Department of Applied Biochemistry, Faculty of Biosciences, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria; 2Department of Chemistry/Biochemistry and Molecular Biology, Faculty of Science, Alex Ekwueme Federal University, Ndufu-Alike Ikwo, Ebonyi State, Nigeria
Correspondence: Obiajulu Christian Ezeigwe Tel +2348034623581
Fax +2347034432437
Email [email protected]
Background: Ficus capensis Moraceae and Cnidoscolus aconitifolius Euphorbiaceae leaves have been used separately in traditional medical practice to treat different ailments, of which anemia is one. This study aims to evaluate the effect of F. capensis and C. aconitifolius on hepatic, renal parameters and oxidative stress of phenylhydrazine-induced anemic rats.
Methods: Thirty-five rats were randomized into seven groups (A–G) of five rats each. Groups A and B served as the normal control and anemic control, respectively, while Groups C, D, E, F, and G were treated with a standard drug (vitamin B12), 200mg/kg bw. aqueous extract of F. capensis, 400mg/kg b.w. aqueous extract of F. capensis, 200mg/kg bw. of a combination of aqueous extract of F. capensis and C. aconitifolius and 400mg/kg bw. of a combination of aqueous extract of F. capensis and C. aconitifolius, respectively. The biochemical analysis (liver and kidney function analysis, oxidative stress) was carried out using standard diagnostic techniques.
Results: The results showed that there was significant decrease (p< 0.05) in the values obtained for Aspartate Transaminase (AST), alanine Transaminase (ALT), Alkaline Phosphatase (ALP), total bilirubin, urea, creatinine, potassium ion, Total Cholesterol (TCHOL), Low-density Lipoprotein (LDL-C), Triglycerides (TRIG), Very Low-density Lipoprotein (VLDL-C) and Malondialdehyde (MDA) and a significant increase (p< 0.05) in obtained values for High-density Lipoprotein (HDL) in all the extract-treated groups compared with the anemic-untreated. The values obtained for most of these biochemical parameters in the extract-treated groups were in the range of the normal control showing that the extract did not, in any way, alter the biochemical parameters. There was a significant increase (p< 0.05) in the glutathione peroxidase (Glut. Perox.) enzyme activity of the groups treated with the aqueous extract of F. capensis and its combination with C. aconitifolius compared with the anemic-untreated.
Conclusion: These results suggest that the aqueous extracts of F. capensis and C. aconitifolius leaves may promote liver function parameters, maintain normal serum electrolyte level and kidney function indices, stimulate reduction of “bad cholesterols” and increase “good cholesterol” and reduce oxidative stress.
Keywords: anemia, phenylhydrazine, Ficus capensis, Cnidoscolus aconitifolius, aspartate transaminase (AST), creatinine
Introduction
Nature has been a source of medicinal treatments for many years and plant-based systems play an essential role in the primary health care of 80% of the world’s developing countries.1 Among the wealth of nature’s medicinal plants, this study is interested in two unique plants and their anti-anemic relevance: Ficus capensis and Cnidoscolus aconitifolius. However, we are careful to ensure that they are not toxic even though they are medicinal. The rise or fall of certain biochemical parameters serve as markers indicative of injury or other effects of certain substances on the organs where they play their roles. For example, any kind of liver injury can cause a rise in Alanine Transaminase (ALT).2 In this same way, so many other biochemical parameters related to other organs, electrolytes, antioxidant enzymes, and can help a scientist indicate the possible toxicity of certain substances administered as medicinal decoctions.
Ficus capensis, locally called “akororo” in igbo, “uwaryara” in hausa, “opoto” in Yoruba, “rimabichehi” in Fulani and “obada” in Edo, belongs to the family Moraceae and has been considered an underutilized plant.3 The leaves of this plant have been found to be abundant in dry season as a result of the plant’s resilience, adaptation and tolerance to adverse climatic conditions,4 making it a good substitute to help with the cases of reduced consumption of green leafy vegetables experienced in the dry seasons. It is one of the plants used in traditional medicine in Nigeria, for treating various diseases and promotes vascular health.5 The leaves of F. capensis are commonly used as a vegetable in foods with a substantial blood boosting effect,6 and possess the ability to prevent the sickling of red blood cells.7 In Nigeria, decoctions and aqueous extract of F. capensis are said to be used traditionally in the treatment of anemia, tuberculosis, pains, convulsions and wounds.8 Oral administration of aqueous extract of F. capensis increased haemoglobin concentration, packed cell volume and red blood cells of albino rats.9
On the other hand, Cnidoscolus aconitifolius, which belongs to the family Euphorbiaceae is an evergreen, drought-deciduous shrub up to 6m in height with alternate palmate lobed leaves and milky sap.10 C. aconitifolius is commonly referred to as “obarandu” or “akwukwo nri ohurun” depicting its perceived blood boosting effect. It is however called “chaya tree spinach” (Mexico), “iyana ipaja” (Yoruba), and “hospital too far” in Niger Delta areas of Nigeria.11 The plant is believed to possess various therapeutic and nutritive value.12 Despite the many claims to the effectiveness of C. aconitifolius in the management of many diseases, there is insufficient information on the toxicological implications of the aqueous extract of the plant leaves.13 Studies on C. aconitifolius leaves reported that the leaves have higher concentrations of sodium than potassium, which is unfavorable because potassium depresses blood pressure while the sodium in these leaves may cause hypertension and atherosclerosis when consumed.14,15 Administration of the raw and boiled leaf juice of C. aconitifolius favorably increased the hematological parameters of anemic rats.16
The combination of both plant leaves produced greater and improved hematological results when administered to anemic rats.17 However, medicinal plants can have adverse effects if wrong plant parts or wrong concentrations are administered.18 Nowadays, toxicity and safety of medicinal plants are among the most discussed topics as herbal products have become popular worldwide.13 In the contemporaries, no research has reported or documented the effect of the combination of F. capensis and C. aconitifolius leaves on essential biochemical indices of phenylhydrazine-induced anemic rats. This research seeks to study the effects of the aqueous extract of F. capenis leaves and its combination with C. aconitifolius leaves on essential biochemical parameters of anemic rats and thus determine the possible toxic effects the components of the leaves may possess when administered.
Methods and Materials
Sample Collection and Identification
The leaves of Ficus capensis were collected at Nike, Enugu East Local Government Area of Enugu State. The leaves of Cnidoscolus aconitifolius were collected at Umueze town, Nkanu West Local Government Area of Enugu State. The samples were identified by a taxonomist in the Department of Botany, Faculty of Biosciences, Nnamdi Azikiwe University, Awka. The voucher number of F. capensis and C. aconitifolius as deposited in the herbarium of the Department of Botany, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria is 164 and 168 respectively.
Preparation of the Aqueous Extracts of F. capensis and C. aconitifolius
The leaves were thoroughly washed and air dried at room temperature for four weeks. The dried leaves were ground into powder using a Corona manual grinding machine. Exactly 300g of the ground leaves powder of F. capensis and C. aconitifolius were each soaked in 1 litre of distilled water for 24 hrs. The aqueous extractions were sieved using a muslin cloth and filtered using Whatman no. 1 (125mm) filter paper. The filtrates were separately lyophilized (freeze dried) using a lyophilizer (freeze dryer). The aqueous extract combination of F. capensis and C. aconitifolius were reconstituted with distilled water in the ratio of 1:1 before administration.
Test Animals
A total of 35 male wistar albino rats weighing between 130–140g were purchased from Chris Animal Farms and Research Laboratory, Awka, Anambra State and used for the experiment. They were maintained and housed in cages under standard environmental conditions (27°C±3°C, 12-hour light/dark cycle) in the Department of Applied Biochemistry Laboratory, Nnamdi Azikiwe University, Awka. They were allowed to acclimatize with the environment for one week before use. The animals were fed Vital grower’s mash pellets purchased from Vital Feed Distributor at Awka, Anambra state and fed ad libitum. At the end of the one-week acclimatization period, the animals were weighed, grouped and labeled.
Study Design
The animals were randomized into 7 groups of 5 rats. After the induction of anemia with phenylhydrazine, the animals were treated for 14 days after which blood was collected by cardiac puncture under ketamine anesthesia and used for biochemical analysis. They were grouped as follows: Group A – Control, Group B – Anemic control (Induced but not treated), Group C – Standard drug (Induced and treated with Emzoron), Group D – 200mg/kg bw. aqueous extract of Ficus capensis (AEFC), Group E – 400mg/kg bw. aqueous extract of Ficus capensis (AEFC), Group F – 200mg/kg bw. of a combination of aqueous extract of F. capensis and C. aconitifolius (AEFC + AECA), and Group G – 400mg/kg bw. of a combination of aqueous extract of F. capensis and C. aconitifolius (AEFC + AECA).
Induction of Anemia
Anemia was induced intraperitoneally in the rats using 20mg/kg b.w. of phenylhydrazine for five consecutive days. The animals were confirmed to be anemic on the 6th day before the commencement of treatment. Blood was collected by retro orbital sinus for hematological analysis before and after the induction of anemia to monitor the animals for the symptoms of anemia before the commencement of treatment.
Determination of Weight
The weight of the experimental subjects was checked using an electronic weighing scale. The weight of the rats was monitored before, during, and after the experiment to know whether the extracts and its combination have an effect on the body weight of the experimental rats.
Determination of Random Glucose Levels
The blood glucose levels of the rats were checked before the induction of anemia, during, and after treatment using One Touch Glucometer (Life Scan, USA) and test strips based on the method of Trinder.19
Kidney Function Test
Urea and creatinine were analysed using Randox test kits. The procedures were carried out according to the manufacturer’s instructions.
Electrolytes
Electrolytes such as Potassium ion (K+), Sodium ion (Na+), Chloride ion (Cl−), Bicarbonate ion (HCO3−), Total calcium (Tcal) and Ionized calcium (Ncal) were measured using routine diagnostic techniques, autoanalyser, Selectra Junior manufactured by Vital Scientific B. V., the Netherlands.
Liver Function Test
Serum biochemical indices routinely estimated for liver functions including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and Bilirubin were determined using Randox diagnostic kits. The procedures used were carried out according to the manufacturer’s instruction.
Lipid Profile
The lipid profile (Total Cholesterol [TCHOL], Triglycerides [TRIG], high-density lipoprotein [HDL], low-density lipoprotein [LDL] and very low-density lipoprotein [VLDL-C]) were determined using Randox test kits.20,21 Low density Lipoprotein-Cholesterol (LDL-C) was calculated using a standard formula from.22 The procedure used was carried out according to the manufacturer’s instructions.
Antioxidant Enzymes Assay
The following antioxidant enzyme activities were determined spectrometrically as follows:
Catalase Activity Determination
Serum catalase (CAT) activity was determined according to the method of Beers and Sizer as described by Uso et al23 by measuring the decrease in absorbance at 240nm due to the decomposition of H2O2 in a UV recording spectrophotometer. The reaction mixture (3mL) contain 0.1mL of serum ina phosphate buffer (50mM, pH 7.0) and 2.9mL of 30mMH2O2 in a phosphate buffer pH 7.0. An extinction coefficient at 240nmH2O2 of 40.0M−1cm−1 was used for the calculation.24 The specific activity of CAT was expressed as moles of H2O2 reduced per minute per mg protein.
Calculation
SOD/CAT = ∆A/min x VT/∑ x Vs
∆A = change in absorbance
VT = Total volume
Vs = Sample volume
∑ = Molar extinction coefficient
Determination of Superoxide Dismutase Activity
Superoxide Dismutase (SOD) activity was determined by its ability to inhibit the auto-oxidation of epinephrine determined by the increase in absorbance at 480nm as described by Sun and Zigma.25 The reaction mixture (3mL) containing a 2.95mL 0.05M sodium carbonate buffer pH 10.2; 0.02mL of liver homogenate and 0.03 mL of epinephrine in 0.005N HCl was used to initiate the reaction. The reference cuvette contains 2.95mL buffer, 0.03mL of substrate (epinephrine) and 0.02mL of water. Enzyme activity was calculated by measuring the change in absorbance at 480nm for 5 mins.
Determination of Glutathione Peroxidase Activity
This was determined by the method of Beutter and Kelly as adapted by Anthony and Rajamohan.26 Hydrogen peroxide (H2O2) is reduced by oxidizing reduced glutathione (GSH) to form GSSG. The reaction mixture contained 1mL of 0.3M phosphate buffer (pH 7.4), 0.3mL of 10mM (GSH), 0.3mL of 15mMH2O2 and 1.37mL distilled water. Exactly 0.1mL serum was added to the mixture in the cuvette, shaken and absorbance was read at 340nM.Extinction co-efficient of 1.622 x 10−3M−1CM−1 was used to calculate enzyme activity which was expressed in unit mg protein.
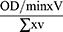
Enzyme activity was calculated using the formula:
OD = Optical Density
V = Total volume of reaction mixture
v = volume of the sample
∑ = Molar extinction coefficient
Determination of Lipid Peroxidation
Lipid peroxidation was determined by the thiobarbituric acid-reacting substances (TBARS) assay method of Buege.27 The reaction depends on the formation of complex between malondialdehyde (MDA) and thiobarbituric acid (TBA). 0.4mL of serum is collected into the test tubes; 1.6mL of 0.25N HCl was added to 0.5mL of 15% trichloroacetic acid and 0.5mL of 0.375% of TBA and then mixed thoroughly.
The reaction mixture was then placed in 100°C boiling water for 15 mins, allowed to cool and centrifuged at 3000 rpm for 10 mins. The supernatant was collected and the optical density recorded at 532nm against reagent blank containing distilled water.
The lipid peroxidation activity was calculated using the formula:
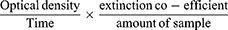
Where the extinction coefficient value is 1.56 x 10−5M−1CM−1
The unit is expressed as umol/MDA/mg of protein.
Data Analysis
Data obtained from the experiments were analyzed using the Statistical Package for Social Sciences software for windows version 23 (SPSS Inc., Chicago, Illinois, USA). All the data collected were expressed as Mean ± SEM. Statistical analysis of the results obtained were performed by using ANOVA Tests to determine if significant difference exists between the mean of the test and control groups. The limit of significance was set at p<0.05.
Results
Effect of Extract on Weight of Anemic Rats
The weights of the rats were recorded on day 0 (before the induction of anemia), day 6 (after five days of consecutive induction of anemia), day 13 (after seven days treatment) and day 20 (after 14 days of treatment) (Table 1). The induction of anemia did not show any significant difference in the weight of the rats when the test groups were compared with the control groups. However, as treatment progressed a significant (p<0.05) increase in weight was observed in both the test and control groups on days 13 and 20 compared to days 0 and 6. The weight of the rats increased normally in the course of treatment. The gain in weight cannot be attributed to the treatment regimen, as can be seen from the results comparing the anemic-untreated with the normal rats and the test groups. These results are also represented in Figure 1.
 |
Table 1 Weight of Phenylhydrazine-Induced Anemic Rats Treated with F. capensis and its Combination with C. aconitifolius Expressed as Mean ± SEM |
 |
Figure 1 Weight of phenylhydrazine-induced anemic rats treated with F. capensis and its combination with C. aconitifolius expressed as mean ± SEM. |
Effect of Extract on Random Blood Glucose Levels
The random blood glucose levels were checked to know the effect of treatment with aqueous extract of F. capensis and its combination with C. aconitifolius on random blood glucose levels. The random glucose levels of the rats were recorded on day 0 (before the induction of anemia), day 6 (after 5 days of consecutive induction of anemia), day 13 (after 7 days treatment) and day 20 (after 14 days of treatment) (Table 2). The rats maintained normal blood glucose levels before, during, and after treatment. The results of the random blood glucose levels are explained in Figure 2.
 |
Table 2 Random Blood Glucose Levels of Phenylhydrazine-Induced Anemic Rats Treated with F. capensis and its Combination with C. aconitifolius Expressed as Mean ± SEM |
 |
Figure 2 Random blood glucose levels of phenylhydrazine-induced anemic rats treated with F. capensis and its combination with C. aconitifolius expressed as mean ± SEM. |
Effect of Extract on Liver Function Parameters
The result of the effect of administration of aqueous extract of F. capensis and its combination with C. aconitifolius to the experimental subjects on liver function parameters is represented in Table 3. The induction of anemia with phenylhydrazine elevated the liver function parameters (ALP, AST, ALT, total bilirubin, and direct bilirubin levels). Treatment with the aqueous extract of F. capensis and its combination with C. aconitifolius significantly (p<0.05) reduced the liver function parameters in all the treatment groups compared to the anemic-untreated. The value of the ALP, AST, ALT, T.BIL, and D.BIL of the groups treated with graded doses of the extract was close to that of the normal control group that was not induced. However, a better reduction in the liver function parameters was recorded in the groups that were treated with 200 and 400mg/kg bodyweight of aqueous extract of a combination of F. capensis and C. aconitifolius compared with the groups that were administered 200 and 400mg/kg bodyweight of aqueous extract of F. capensis alone. The group that was treated with 400mg/kg bodyweight of aqueous extract of a combination of F. capensis and C. aconitifolius was far better compared to other test groups and the group treated with a standard antianemic drug.
 |
Table 3 Effect of Treatment with Aqueous Extract of F. capensis and its Combination with C. aconitifolius on Liver Function Parameters of Phenylhydrazine-Induced Anemic Rats Expressed as Mean ± SEM |
Effect of Extract on Kidney Function Parameters
The result of the effect of administration of aqueous extract of F. capensis and its combination with C. aconitifolius to the experimental subjects on kidney function parameters is represented in Table 4. The induction of anemia with phenylhydrazine caused a significant (p<0.05) increase in the urea and creatinine levels in all the rats induced except the normal control group which was not induced. Treatment with the aqueous extract of F. capensis and its combination with C. aconitifolius significantly (p<0.05) decreased the urea and creatinine levels in all the treatment groups compared to the anemic-untreated. The urea and creatinine levels of the groups treated with graded doses of the extract were close to that of the normal control group that was not induced. However, a better reduction in urea and creatinine levels were recorded in the groups that were treated with 200 and 400mg/kg bodyweight of aqueous extract of a combination of F. capensis and C. aconitifolius compared to the groups that were administered 200 and 400mg/kg bodyweight of aqueous extract of F. capensis alone. The group that was treated with 400mg/kg bodyweight of aqueous extract of a combination of F. capensis and C. aconitifolius fared better compared to other test groups and the group treated with a known standard drug.
 |
Table 4 Effect of Treatment with Aqueous Extract of F. capensis and its Combination with C. aconitifolius on Kidney Function Parameters of Phenylhydrazine-Induced Anemic Rats Expressed as Mean ± SEM |
Effect of Extract on Electrolyte Levels
Induction of anemia increased the potassium ion (K+), sodium ion (Na+), chloride ion (Cl−), bicarbonate ion (BCO3−), total calcium (Tcal) and ionized calcium (ncal) in all the groups except the normal control that was not induced (Table 5). However, the increase was not statistically significant (p>0.05). The electrolyte levels of the test groups decreased compared to the anemic-untreated group. The value of the electrolyte tests recorded for the group that was treated with a combination of aqueous extract of F. capensis and C. aconitifolius at a dose of 200mg/kg body weight was close to the value recorded for the normal control group. The administration of the extract combination at a dose of 200mg/kg bodyweight was observed to be better than the other treatment groups and the group treated with a standard drug (Table 5).
 |
Table 5 Effect of Treatment with Aqueous Extract of F. capensis and its Combination with C. aconitifolius on electrolytes of Phenylhydrazine-Induced Anemic Rats Expressed as Mean ± SEM |
Effect of Extract on Lipid Profile
The lipid profile analysis was carried out to know the effect of the continuous administration of the aqueous extract of F. capensis and its combination with C. aconitifolius in the anemic rats. The induction of anemia significantly (p<0.05) increased the TCHOL, (LDL-C, TRIG and VLDL while it significantly (p<0.05) decreased HDL-C in all the groups except the normal control group (Table 6). The groups treated with the aqueous extract of F. capensis and C. aconitifolius showed a significant (p<0.05) decrease in the TCHOL, LDL, TRIG and VLDL levels compared with the anemic-untreated group. A better reduction was observed in the group treated with 400mg/kg bodyweight of the extract combination for TCHOL, LDL and VLDL which was close to the values recorded for the normal control group. There was a significant (p<0.05) increase in the HDL-C of the groups administered 200 and 400mg/kg bodyweight of the aqueous extract of a combination of F. capensis and C. aconitifolius compared with the groups treated with 200 and 400mg/kg bodyweight of the aqueous extract of F. capensis alone.
 |
Table 6 Effect of Treatment with Aqueous Extract of F. capensis and its Combination with C. aconitifolius on Lipid Profiles of Phenylhydrazine-Induced Anemic Rats Expressed as Mean ± SEM |
Effect of Extract on Antioxidant Enzymes
CAT, SOD and glutathione peroxidase (Glut. Perox.) activity of the experimental subjects were assayed to know the effect of the administration of F. capensis and its combination with C. aconitifolius on the antioxidant enzyme activity (Table 7). The extract combination increased the CAT and SOD activity in the test groups although the increase was more noticeable in the groups administered 200 and 400mg/kg bodyweight of the combination of F. capensis and C aconitifolius. There was a significant (p<0.05) increase in the Glut. Perox. activity of all the test groups compared with the anemic-untreated group. The increase was found to be highest in the group administered 400mg/kg bodyweight of aqueous extract of F capensis and C. aconitifolius compared with the group administered aqueous extract of F. capensis alone.
 |
Table 7 Effect of Treatment with Aqueous Extract of F. capensis and its Combination with C. aconitifolius on Antioxidant Enzymes of Phenylhydrazine-Induced Anemic Rats Expressed as Mean ± SEM |
Effect of Extract on Lipid Peroxidation (Malondialdehyde)
Induction of anemia caused a significant (p<0.05) increase in the MDA level of all the groups except the normal control which was not induced (Table 8). Administration of 200 and 400mg/kg bodyweight of aqueous extract of F. capensis and its combination with C aconitifolius significantly (p<0.05) decreased the MDA level in all the test groups compared with the anemic-untreated group. The highest decrease was observed in the group treated with 400mg/kg bodyweight of a combination of aqueous extract of F. capensis and C. aconitifolius. The animals that received the combination of the extract at a dose of 400mg/kg body weight fared better than the group given the same dose of aqueous extract of F. capensis alone.
 |
Table 8 Effect of Treatment with Aqueous Extract of F. capensisand its Combination with C. aconitifolius on MDA Levels of Phenylhydrazine-Induced Anemic Rats Expressed as Mean ± SEM |
Discussion
Some essential biochemical indices which serve as toxicity markers were assayed to investigate the safety in administering aqueous extracts of F. capensis and its combination with C. aconitifolius. The weight gained by the experimental animals in all the test groups showed no significant difference from that of the normal control group. Thus, the administration of aqueous extracts of F. capensis and its combination with C. aconitifolius have no significant effect on the weight of the animals. The blood glucose levels of the wistar albino rats after the administration of F. capensis leaf extract alone and with its combination with C. aconitifolius, increased slightly but without a significant difference (p>0.05) which is not above the normal maximum range for blood glucose in normal wistar rats given to be 50–135mg/dl.28
Liver function parameters used to detect the presence of liver disease or potential harm to the liver include the serum level of the enzymes AST, ALT and Alkaline phosphatase. Bilirubin levels are also useful in this case. Usually, any kind of liver injury can cause a rise in ALT,2 and the release of ALT and AST from the cytosol occurs when there is injury to hepatocytes, especially in membrane damage.29 The results of our analysis showed that there was a significant increase (p<0.05) in the levels of ALP, AST, and ALT in the anemic-untreated group compared to the values of the normal control group. But the groups treated with different doses of only F. capensis extract as well as different doses of the combination of F. capensis and C. aconitifolius were observed to have a very significant reduction (p<0.05) in the levels of these enzymes bringing their values closer to that of the normal control. The administration of 400mg/kg of combination of AEFC and AECA significantly marked a restoration of the levels of these enzymes to the normal levels and to the same range as the group treated with the standard drug (Emzoron). This does not only suggest the safety of these leaf extracts in the correct doses to the liver, but also suggest their protective and restorative potential in cases of liver damage or injury.
Higher levels of blood urea nitrogen and creatinine could be a sign of an underlying condition affecting the kidneys.30 However, the levels of urea and creatinine (mg/dl) assayed for indicated no nephrotoxicity as there was a noticeable significant decrease (p<0.05) in the values of these markers as against that of the anemic control. The levels of urea and creatinine in the groups treated with AEFC and AECA were within range of the normal control group as seen in Table 4 and also within the normal range of these parameters, which ruled out the possibility of precipitated abnormalities. It was noticed, however, that the blood urea level of animals in Groups F and G which were treated with a combination of AEFC and AECA in doses 200mg/kg and 400mg/kg showed closer relativity to the values of the normal control group suggesting that the combination is more effective.
levels of serum electrolytes like potassium, sodium, bicarbonate and chloride which are too high or too low are suggestive of tubular dysfunction.31 In this study, the levels of sodium, chloride, bicarbonate and total calcium in all the groups treated with AEFC and AEFC+AECA showed a slight difference from the normal control which remained within the relevant safe range. There was a significant reduction (p<0.05) in the levels of potassium ion in Groups C to G compared to that of the anemic-untreated group, bringing the levels back within the range of the normal control, which was impressive. It also showed relativity to the values of K+ obtained with the use of a similar anti-anemic herb Waltheria indica in doses of 200mg/kg and 400mg/kg,32 which further suggests the safety of these herbs in the doses administered as there was not any value suggestive of hyper or hypokalemia, natremia, chloremia, or calcemia.
In the analysis of the effects of aqueous extracts of F. capensis and its combination with C. aconitifolius on the lipid profile of the test animals, all groups showed significant decrease (p<0.05) in TCHOL, LDL-C, TRIG and VLDL-C, and a significant increase (p<0.05) in HDL-C levels with respect to the anemic-untreated group. The increase in HDL levels was remarkably observed in Groups F and G (Table 6). This is a good indicator of the effectiveness and safety of administering the combination of AEFC and AECA on the test animals. HDL is known to be the good cholesterol in the body because it transports cholesterol to the liver to be expeled, helping the body get rid of excess cholesterol so it is less likely to end up in the arteries to form plaques, facilitating the prevention of cardiovascular risk factors.32 However, LDL-C, TRIGS and VLDL-C are better at lower levels in the body, and our experiments showed aqueous extracts of both plants to be very effective in this regard, especially as seen with the combination of both plant extracts in Groups F and G (Table 6).
Generally, antioxidant enzymes mop up Reactive Oxygen Species (ROS) into less harmful products. SOD converts superoxide radical to hydrogen peroxide and molecular oxygen which is in turn converted to water by CAT and Glut. Perox. and in the case of CAT to oxygen and water.33 From our analysis, there was no significant difference in the activities of these enzymes in the test animals treated with AEFC and AEFC + AECA with respect to the normal control group. However, the test animals in Group E, in which 400mg/kg of AEFC was administered, showed a noticeable decrease in the activity of CAT when compared to the normal control, although the decrease was not statistically significant. A decrease in CAT is reported to be correlated with the carcinogen-initiated emergence of the malignant phenotype in mouse keratinocytes.34
MDA is a product of lipid peroxidation and indicates most representatively oxidative stress in the body.35 There was a significant increase (p<0.05) of MDA level in the anemic-untreated group compared with the normal control. Interestingly, other groups treated with a standard drug, AEFC (200mg/kg and 400mg/kg) and AEFC + AECA (200mg/kg and 400mg/kg) reflected a significant decrease (p<0.05) in the level of MDA with respect to the anemic-untreated. The MDA level of all the groups treated with the graded doses of the aqueous extract of F. capensis and its combination with C. aconitifolius were close to that of the normal control group. This suggests that the administration of aqueous extracts of F. capensis and its combination with C. aconitifolius in the doses used in this study does not induce lipid peroxidation.
Conclusion
Previous studies show that F. capensis and C. aconitifolius leaves have a blood boosting effect without studying the effect of these extracts on essential biochemical parameters of anemic rats. However, from this study it can be inferred that the combined extracts of F. capensis and C. aconitifolius does not have any adverse effect on the essential biochemical parameters of phenylhydrazine induced-anemic rats. The results of the biochemical analysis revealed that aqueous extracts of F. capensis and C. aconitifolius did not exhibit any form of adverse effect or toxicity to the organs and metabolic processes marked by these parameters with respect to the doses administered and duration of study. Our findings also revealed that the combination of AEFC and AECA elicits better ameliorative effects from the disruptions caused by the induction of phenylhydrazine compared to the administration of the extracts singly. This study not only authenticates the folklore use of the leaves of F. capensis and C. aconitifolius, but also suggests its inclusion as a better remedy in the treatment of anemia.
Ethical Approval
All authors hereby declare that “Principles of laboratory animal care” were followed. All experimental protocols were examined and approved by the Animal Research Ethics Committee of Nnamdi Azikiwe University, Awka, Nigeria in line with the recommendations for Animal Care and Use in Research, Education and Testing (ACURET).
Acknowledgment
We are immensely grateful to the management of Chris Experimental Animal Farm and Research Laboratory, Awka, for providing the rats used for this research and to the Laboratory Technologists, Department of Applied Biochemistry, Nnamdi Azikiwe University, Awka, for their technical assistance. We wish to thank Dr. (Mrs.) B. O. Aziagba for assisting in the plant authentication and identification.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zolfaghari B, Ghannadi A. Research in medical sciences. J Isfahan Uni Med Sci. 2000;6:1–6.
2. Shivary G, Prakash BD, Hull V, Avinash AKM, Senal NV, Shruthi SK. A review on laboratory liver function tests. The Pan Afri Med J. 2009;3(17):17.
3. Njoku-Oji NN, Nwike CV, Dimkpa U, et al. Hematological changes following oral administration of Aqueous leaf extract of Ficus capensis in albino rats. Intl Blood Res Rev. 2016;5(1):1–7.
4. Otitoju GTO, Nwamarah JU, Otitoju O, Iyeghe LU. Nutrient composition of some lesser known green leafy vegetables in Nsukka L.G.A of Enugu state. J Biodiv Environs Sci. 2014;4(4):233–239.
5. Njoku UO, Ogugofor MO, Nwodo OFC. Treatment with methanol extract of Ficus capensis stem bark protects against changes in biomarker levels of carbontetrachloride-induced cardiotoxicity of Rats. J App Sci. 2017;17(8):407–414. doi:10.3923/jas.2017.407.414
6. Owolabi OJ, Nworgu ZA, Falodun A, Ayinde BA, Nwako CN. Evaluation of tocolytic activity of ethanol extract of the stem bark of F. capensisthunb. (Moraceae). Acta Pol Pharm. 2009;66:293–296.
7. Uzoekwe NM, Mohammed JJ. Phytochemical, proximate and mineral contents of leaves and bark of Ficus capensis. J App Sci Environs Mgt. 2015;19:633–637.
8. Olowokudejo JD, Kadri AB, Travih VA. An ethnobotanical survey of herbal markets and medicinal plants in Lagos state of Nigeria. Ethnobot.Leafl. 2018;12:851–865.
9. Njoku-Oji NN, Nwike CV, Dimkpa U, et al. Haematological changes following oral administration of aqueous leaf extract of Ficus capensisin Albino rats. Int Blood Res Rev. 2016;5(1):1–7.
10. Fagbohun ED, Egbebi AO, Lawal OU. Phytochemical analysis and in-vitro antimicrobial activities of methanolic extract of Cnidoscolusaconitifoliusleaves. Intl J Pharm Sci Rev Res. 2012a;13(1):1–5.
11. Orji OU, Ibiam UA, Aloke C, et al. Biochemical assessment of ethanol leaf extract of cnidoscolusaconitifoliuson liver integrity of albino rat treated with lead. Global J Pharmacol. 2016;10(4):108–113.
12. Jensen SA. Chaya, the mayan miracle plant. J Food Sci. 1997;51:234–244.
13. Akachukwu D, Okafor PN, Ibegbulem CO. Phytochemical content of Cnidoscolusaconitifolius and toxicological effect of its aqueous leaf extract in wistar rats. J Inv Biochem. 2014;3(1):26–31. doi:10.5455/jib.20140504023102
14. Fagbohun ED, Egbebi AO, Opeyemi UL. Phytochemical screening, proximate analysis and in-vitro antimicrobial activities of methanolic extract of C. aconitifolius leaves. Intl J Pharm Sci Rev Res. 2012b;13(1):28–33.
15. Ezeigwe OC, Okpala CO, Ogana J, et al. Comparative phytochemical and nutritional profiles of Ficus capensis and Cnidoscolusaconitifolius Leaves. Int J Res Innov Appl Sci. 2020;5(1):16–21.
16. Onuoha NO, Okafor AM, Eme PE, Odo EC. Haematinic effect of raw and boiled leaf juice of Cnidoscolusaconitifolius using cyclophosphamide-treated adult male albino rats. EC Nutri. 2017;7(5):187–194.
17. Ezeigwe OC, Nwobodo VOG, Enemchukwu BN, et al. Lipid profile and its complications in phenylhydrazine-induced anemic rats. J Medic Plants Studies. 2019;7(6):161–166.
18. Fronhe D. Plant intoxicants- is there a risk from herbal preparation? J Phytotherapeut. 1999;1999(20):201–202.
19. Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1972;6:224–227.
20. Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–27. doi:10.1177/000456326900600108
21. Tietze NW, Finley PR, Pruden EL. Clinical Guide to Laboratory Tests.
22. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low–density lipoprotein cholesterol in plasma, without use of preparative ultracentrifuge. Clin.Chem. 1972;18:499–502. doi:10.1093/clinchem/18.6.499
23. Usoh IF, Akpan EJ, Etim EO, Farombi EO. Antioxidant actions of dried flower extract of Hibiscus sabdariffa L. on sodium arsenite-induced oxidative stress in rats. Pak J Nutri. 2005;4:135–141. doi:10.3923/pjn.2005.135.141
24. Aebi H. Catalase in vitro. In: Colowick SP, Kaplane NO, editors. Methods Enzymol. Vol. 105. 1984:121–126.
25. Sun M, Zigma S. An improved spectrophotometric assay of superoxide dismutase based on epinephrine antioxidant. Analytic. Biochem. 1978;90(1):81–89. doi:10.1016/0003-2697(78)90010-6
26. Anthony LL, Rajamohan T. Hepatoprotective and antioxidant effect of tender coconut water on carbon tetrachloride induced liver injury in rats. Ind J Biochem Biophy. 2003;40:354–357.
27. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310.
28. Azizi F, Abdi H. Towards ultimate care for graves’ hyperthyroidism. Intl J Endocrinol Met. 2020;18(1):e98255.
29. Chikwendu JN, Nwamarah JU, Uchegbu VA. Evaluation of effects of aqueous extract of Ficus capensis (thumb.) leaves in adult albino rats. Asian J Biol Life Sci. 2015;4(3):185–188.
30. Raj T. Creatinine Blood Level: Renal Disease in the Tropics. Manson’s Tropical Infectious Disease.
31. Hamidu JL, Ayo JO, Adelaiye AB, Abubakar MS. Effects of ethanolic extract of Waltheriaindicaaerial parts on some liver and kidney function indices in albino rats. Afri J Biotechnol. 2018;17(19):626–632. doi:10.5897/AJB2017.15974
32. Hemalatha T, Ahino MD, Saravana GA. Acute and Sub-acute toxicity study of Tremaorientalis(L.) Bl. methanol extract in rats. J Drug Deliv and Therapeut. 2019;9(1):307–311. doi:10.22270/jddt.v9i1-s.2353
33. Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase, and glutathione peroxidase in cultured cells and tissues. Nat Protocols. 2010;5(1):51–66. doi:10.1038/nprot.2009.197
34. Nishawa M, Tamada A, Kumai H, Yamashita F, Hashida M. Inhibition of experimental pulmonary metastasis by controlling biodistribution of catalase in mice. Int J Cancer. 2002;99(3):474–479. doi:10.1002/ijc.10387
35. Cheng DL, Zhu N, Cheng LL, et al. Significance of malondialdehyde, superoxide dismutase and endotoxin levels in Budd-Chiari syndrome in patients and a rat model. Exp Therap Med. 2018;16:5227–5235.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
