Back to Journals » Journal of Inflammation Research » Volume 15
Effect of Alcohol Intake on Inflammatory Status and Prognosis in Cancer Patients
Authors Liu X, Zhang W, Ji W, Zheng K , Zhao Y, He Y, Cui J, Li W
Received 13 June 2022
Accepted for publication 30 September 2022
Published 11 October 2022 Volume 2022:15 Pages 5815—5826
DOI https://doi.org/10.2147/JIR.S376248
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xiangliang Liu,1,* Wenxin Zhang,2,* Wei Ji,1,* Kaiwen Zheng,1 Yixin Zhao,1 Yuwei He,1 Jiuwei Cui,1 Wei Li1
1The First Hospital of Jilin University, Changchun, People’s Republic of China; 2Zhongnan Hospital, Wuhan University, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiuwei Cui; Wei Li, Jilin University, Xinmin St No. 126, Changchun, 130021, People’s Republic of China, Tel +8613844095420 ; +8613206282295, Fax +86 431-85619254, Email [email protected]; [email protected]
Purpose: To explore the effect of alcohol on the inflammatory status and prognosis of patients with malignancy.
Patients and Methods: The clinical data of patients with malignant tumor who were admitted to the First Hospital of Jilin University from November 2011 to December 2018 were collected including basic clinical information, anthropometric indicators, body composition analysis, and serological indicators. Univariate and multivariate Cox proportional hazards models were performed to find predictors of survival. A nomogram was generated to indicate the interrelationships between the variables in the prediction model and the extent to which alcohol consumption affects prognosis. The C-index and calibration curves were used to verify the predictive accuracy of the scoring system.
Results: A total of 2929 cancer patients were included in this study, of which 552 (18.8%) patients had alcohol consumption habits and 2377 (81.2%) patients had no alcohol history. Patients in the Alcohol group had significantly shorter overall survival (OS) than the Non-Alcohol group, and further sub-analysis indicated alcohol consumption was significantly associated with prognosis of advanced breast cancer (HR = 4.617, 95% CI 1.361– 15.664, p=0.014), and early stage hepatocellular carcinoma (HCC) without viral hepatitis (HR = 8.933, 95% CI 1.444– 55.275, p = 0.019). Multivariate Cox regression models also demonstrated that daily alcohol intake was a risk factor of survival benefits (HR = 1.256, 95% CI 1.016– 1.554, p = 0.036). Finally, a nomogram was constructed and the c-index of this scoring system is 0.751. The 3- and 5-year calibration curves of the model show a high agreement between the predicted probability and the actual observed survival rate.
Conclusion: Alcohol intake was demonstrated associates with patient outcomes. A human component-prognosis scoring system was established to predict the survival benefits of cancer patients.
Keywords: alcohol, inflammation, cancer, prognosis
Introduction
In 2020, the International Agency for Research on Cancer (IARC) led several research institutions to publish a significant study on alcohol drinking and cancer.1 The study results that alcohol consumption is one of the critical risk factors for the global disease burden causally associated with various injuries and diseases, including cancer, and no safe dose exists.2 The 2018 white paper “Moderate Drinking Status of Chinese Drinking Population” shows that the Chinese drinking population is as high as 600 million. Moreover, for about 36% of the East Asian people, there is a common loss-of-function variant in the ALDH2 gene on chromosome 12 (rs671), which significantly reduces the rate of acetaldehyde decomposition and aggravates the damage of acetaldehyde to various tissues and organs.3 At present, there is a lot of evidence indicating that alcohol consumption is strongly associated with the risk of the occurrence of multiple cancers,4,5 but the extent of harm of alcohol consumption in patients with different cancers remains to be explored, and there are many missing data, especially in China. Therefore, the aim of this large retrospective cohort study was to deeply investigate the effect of alcohol consumption on the prognosis of cancer patients.
Materials and Methods
Clinical Data Collection
We collected clinical data from November 2011 to December 2018 from the Department of Oncology, Cancer Center, First Hospital of Jilin University. Inclusion criteria: (1) Clear diagnosis of malignancy in pathological specimens. (2) Age ≥ 18 years. (3) No nutritional support treatment prior to nutritional assessment and laboratory testing. Exclusion criteria: (1) Death within 30 days of hospital admission. (2) Mixed types of tumor. (3) Patients who declined to participate. Finally, 2929 patients were involved. Patients with multiple hospitalizations were considered as one case, and data from the first investigation were analyzed. All patients were regularly followed up by telephone interviews or outpatient visits. All pathological staging was defined according to the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system. Overall survival (OS) was defined as the time period from diagnosis to mortality for any cause.
Research Methods
All patients received anthropometry, laboratory biochemical tests and nutritional assessment within 24 hours of hospital admission. Smoking history, alcohol consumption, comorbidities and diagnostic information were recorded. The nutritional assessment including the nutritional risk screening 2002 score (NRS 2002), patient-generated subjective global assessment (PG-SGA), Karnofsky performance status (KPS) and quality of life questionnaire-core 30 (QOL-C30). Patients were classified into Alcohol group once they had alcohol consumption habit and those who never drank were classified into Non-Alcohol group.
Drinking Volume of Alcohol Intake
Drinking volume of alcohol intake (g) = Drinking volume (mL) × alcoholic concentration (%) × 0.8 (alcohol density). The alcohol concentration of beer, wine, and liquor in China is calculated according to 4%, 12%, and 50% of the white paper “Moderate Drinking Status of Chinese Drinking Population”.
Hand Grip Strength (HGS)
HGS was examined by Jamar hydraulic grip dynamometer (Sammons Preston Rolyan, Illinois, USA). Patients seated upright with their elbows flexing at 90°, forearms and wrists in a neutral position. Then, patients were instructed to grip the dynamometer three times with their maximum strength and there was a 1-minute interval for rest each time. The maximum reading was recorded.
Body Composition
Visceral fat area (VFA) was assessed by the Inbody S10 equipment using bioelectrical impedance analysis technology. Electrode pads were attached to the ipsilateral upper and lower extremities in the supine position, and all procedures are performed according to the manufacturer’s recommendations.
Laboratory Testing Indicators
Venous blood samples were collected on an empty stomach and sent to the hospital laboratory for testing. The parameters recorded included albumin, prealbumin, transferrin, total protein (TP), C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR).
Statistical Analysis
Data were analyzed by SPSS for Windows version 26.0 (IBM SPSS Statistics, IBM Corp., Armonk, NY) and R version 4.0 (R Foundation for Statistical Computing, Vienna, Austria). The Kolmogorov–Smirnov test was used to confirm normal distributions of continuous data. Independent t-tests were used for normally distributed data. Counting data were examined by using the chi-square test. Univariate Cox proportional hazards models were performed for all potential baseline predictors to calculate hazard ratios (HR) and their 95% confidence intervals (CI). Variables that were significant in univariate analysis (P < 0.05) and those with known prognostic values were selected to build multivariate Cox proportional models with a stepwise forward selection of variables. A nomogram was generated to indicate the interrelationships between the variables in the prediction model and the extent to which alcohol consumption affects prognosis. Then, the C-index and calibration curves were adopted to verify the predictive accuracy of the scoring system.
Results
A total of 2929 cancer patients were included in this study, including 1271 (43.4%) male and 1658 (56.6%) female. There were cancers of lung 898 (30.7%) cases, digestive tract 539 (18.4%) cases, biliary tract and pancreas 77 (2.6%) cases, leukemia 315 (10.8%) cases, breast 701 (23.9%) cases, liver 100 (3.4%) cases, esophageal 58 (2.0%) and others 241 (8.2%) cases. 552 (18.8%) patients had previous alcohol history and 2377 (81.2%) patients had no alcohol history.
Characteristics of Involved Patients Stratified by Alcohol Intake
According to the statistics displayed in Table 1, it was men drank much more prevalent than women (P < 0.001). And a person who drank was more possible being a smoker (P < 0.001). No statistical significance was revealed in comorbidities between Non-Alcohol group and Alcohol group (P = 0.025). The proportion of patients with alcohol consumption history was higher in lung cancer (43.5% vs 27.7%, P < 0.05), digestive tract cancer (25.9% vs 16.7%, P < 0.05) and esophageal cancer (6.5% vs.0.9%, P < 0.05). Besides, TNM stages were more advanced in Alcohol group, which was 30.5% vs 25.0% of the III stage and 24.1% vs 19.2% of the IV stage (P < 0.05).
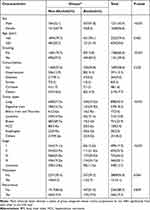 |
Table 1 Characteristics of Involved Patients Stratified by Alcohol Intake |
Relationship Between Alcohol Consumption and Inflammatory Factors
KPS scores were higher in the Alcohol group (90.67 vs 88.8, P = 0.003) than patients in the Non-Alcohol group in lung cancer. TP was higher patients without alcohol consumption than those with alcohol consumption in lung cancer (68.05g/L vs 67.13g/L, P = 0.050), biliary tract and pancreas cancer (65.23g/L vs 60.36g/L, P = 0.017) and breast cancer (69.33g/L vs 66.45g/L, P = 0.026). Prealbumin in Alcohol group was higher in leukemia (0.23g/L vs 0.21g/L, P = 0.041) and breast cancer (0.25g/L vs 0.25g/L, P = 0.037) than those in Non-Alcohol group. In digestive tract tumors, CRP was significantly higher in Alcohol group patients compared with the Non-Alcohol group (31.91 mg/L vs 21.80 mg/L, P = 0.041). Notably, patients in the Alcohol group had higher HGS in lung cancer (30.66kg vs 24.53kg, P < 0.001), digestive tract cancer (30.74kg vs 23.53kg, P < 0.001), biliary tract and pancreas cancer (30.71kg vs 22.19kg, P = 0.006) and leukemia (31.20kg vs 24.51kg, P < 0.001) than those in the Non-Alcohol group (Table 2).
 |
Table 2 Basic Clinical Information for Cancer Patients Stratified by Alcohol Intake |
Effect of Alcohol Consumption on the Prognosis of Different Cancer Types Stratified by Stage
Table 3 and Figure 1 showed the relationship between alcohol consumption and specific cancer types stratified by stage. Patients in the Alcohol group had significantly shorter OS than those in the Non-Alcohol group no matter the stages were. And patients in the advanced stage had a relatively lower HR (HR = 1.445, 95% CI 1.147–1.821, P = 0.002) than that in the early stage (HR = 1.705, 95% CI 1.329–2.187, P < 0.001). Subgroup analysis found that alcohol consumption was significantly associated with prognosis of advanced breast cancer (HR = 4.617, 95% CI 1.361–15.664, P = 0.014). Moreover, there seemed a correlation between alcohol consumption and prognosis of hepatocellular carcinoma (HCC) in early stage (HR = 2.367, 95% CI 0.886–6.318, P = 0.086) although the results were not statistically significant.
 |
Table 3 Risk of Mortality in Drinking Patients Stratified by Stage |
 |
Figure 1 Kaplan–Meier curves of overall survival in different cancer types stratified by stage. Note: E for early stage, A for advanced stage. |
Further Study on the Combination of Viral Hepatitis in HCC Patients
Given that viral hepatitis was a main cause of HCC in China, we further stratified HCC patients by viral hepatitis. The results were surprising. Alcohol consumption was significantly associated with prognosis of early HCC patients without viral hepatitis (HR = 8.933, 95% CI 1.444–55.275, P = 0.019). However, the relation was not observed neither in HCC with viral hepatitis nor advanced HCC without viral hepatitis (P > 0.05) (Table 4, Figure 2).
 |
Table 4 Risk of Mortality Stratified by Stage and Viral Hepatitis in Drinking Patients Suffering HCC |
Establish and Validate Nomogram for Prognosis-Related Influences
As the OS was the endpoint event, the univariate Cox regression model results showed that the following nine factors associated with the OS: Sex, age, BMI, TNM stage, daily alcohol intake, smoking packs per week, NLR, PLR, and VFA (P < 0.05). Multivariate Cox regression models further confirmed that daily alcohol intake more than 30g was a risk factor of prognosis of cancer patients among all the included variables (HR = 1.256, 95% CI 1.016–1.554, P = 0.036) (Table 5).
 |
Table 5 Univariable and Multivariable Cox Regression of Factors Related to Overall Survival of Cancer Patients |
A nomogram was established based on the results of the multivariate regression model. Thus, a prognostic scoring system was developed as displayed in Figure 3, where the daily alcohol intake more than 30g got 1.1 points. The C-index of this scoring system is 0.751. The 3- and 5-year calibration curves of the model showed a high agreement between the predicted probability and the actual observed survival rate (Figure 4A and B).
Discussion
Epidemiologic evidence has shown that alcohol is a risk factor of cancer occurrence and development. It is reported that approximately 4% of cancers worldwide are caused by alcohol consumption, and cancers of the esophagus (26%), liver (21%), and breast (13%) represent the most alcohol-attributable cases of cancer globally.6 Herein, the proportion of patients with alcohol consumption history was higher in lung cancer, digestive tract cancer and esophageal cancer, which agreed with previous studies.7,8 Although the mechanisms of alcohol inducing cancers are not totally clear, several aspects have been demonstrated interacted between alcohol consumption and cancer including genetic polymorphism, oxidative stress, retinoic acid metabolism and immune modulation.9
Alcohol mainly metabolites in liver through cytochrome P-450 2E1 (CYP2E1) systems. Alcohol consumption leads to oxidative stress.10,11 When ethanol administration in vivo, CYP2E1 is activated which generates large amounts of reactive oxygen species (ROS), and oxidizes to acetaldehyde. Acetaldehyde, in turn, stimulates specific white blood cells (monocytes and macrophages) to produce inflammatory cytokines and downstream ROS.6 This is consistent with the results that prealbumin and CRP were higher in the Alcohol group than in the Non-Alcohol group in the pan-cancerous patients included in our study. However, the elevated HGS of patients in the Alcohol group is not consistent with the poor prognosis because the number of males in the Alcohol group is as high as 507, accounting for 91.8% of the total alcohol consumption. Gender can behave a large impact on HGS12 and bias the final results.
Alcohol consumption has negative effects on tumor patients of different stages as displayed in Table 3. Alcohol increases the metabolic burden of the liver and affects its health status.13 However, when we analyzed the patients with HCC based on alcohol consumption, the results were not ideal. This alerts us to - viral hepatitis, another major interference factor affecting HCC.14,15 According to a comparative risk study led by the National Cancer Center/Peking Union Medical College Cancer Hospital of the Chinese Academy of Medical Sciences, approximately 76.3% and 65.9% of HCC among males and females over 20 years old in Jilin Province were attributable to viral hepatitis.8 Among the 100 cases of HCC included, 78 cases combined viral hepatitis, accounting for 78% of the total HCC patients. Therefore, we further stratified the discussion and found that alcohol consumption was significantly associated with the prognosis of early HCC without viral hepatitis but not with the prognosis of advanced HCC without viral hepatitis and HCC with viral hepatitis. This reflects the impact of viral hepatitis on the prognosis of HCC and also confirms that the larger impact of alcohol consumption on the prognosis of early-stage patients than that of advanced patients, which is consistent with the hazard ratio of pan-cancer patients (Advanced: HR = 1.445 vs Early: HR = 1.705). This may be due to the fact that advanced patients often have metastases, combined with multiple organ failures and a shorter survival period,16 thus concealing the effect of alcohol consumption on prognosis. For advanced breast cancer patients, alcohol consumption often predicts a poor prognosis and a higher risk, which can be explained by two aspects: 1. Breast cancer is closely related to hormone levels in the body and is thus more susceptible to alcohol;17 2. Fewer early breast cancer people with alcohol consumption were included in the study (2.2% of total early breast cancer patients).
In addition, we also establish a nomogram for patient basic clinical information and inflammatory factors to further study the influence of alcohol consumption on patient prognosis. We found that when the patients with daily alcohol intake exceed 30g, the impact on the prognosis is equivalent to smoking more than one pack per week. Like smoking, drinking alcohol will not only increase the cancer rate but also affect the prognosis of patients,18 and this harm may not disappear with quitting smoking and drinking.
It is also noted that comorbidities may have an impact on prognosis as a confounding factor. Patients with cardiovascular diseases may have higher cardiac load and lower tolerance to cardiotoxicity of chemo- and immuno-therapeutic drugs,19 such as anthracycline and some immune check point inhibitors. This tends to limit patients’ treatment options, and even cause death from treatment related cardiotoxicity. Long-term metformin administration has been shown to achieve inhibition of tumor growth through activation of AMP-activated protein kinase (AMPK) in some diabetic patients.20 Besides, alcohol metabolites interrupt the balance of protein anabolism catabolism, cause inhibition of that protein’s function and/or an immune response, and results in multi comorbidities and impairs nutrition status.21,22 However, some studies refer that light to moderate alcohol consumption does have beneficial effects on cardiovascular system, diabetes, some autoimmune diseases and etc.23 Rodrigues et al reported that types of alcohol beverage drinks also differ.24 Similarly, it was liquor but not wine or beer found a risk factor of prognosis herein with HR 1.738 (95% CI 1.460–2.070, P < 0.001). However, no comorbidities or scores reflecting nutritional status were involved in the nomogram. The clue appeared where there no difference of comorbidities and nutritional status scores between Alcohol group and Non-Alcohol group in Tables 1 and 2. Although the debates are ongoing, what needs to be emphasize is that alcohol is definitely not recommended because the risk-benefit ratio of alcohol remains negative, particularly in the perspective of the carcinogenic effects.
There were limitations. As a real world study, 2929 cases is some kind of a large sample size. However, small case number of some cancer types limited the representation of conclusions. And, several cancer types are not listed and discussed separately whose case number less than 50. Therefore, perspectives need to be cautiously interpreted like “alcohol tends to affect prognosis of HCC in early stage” due to only 22 cases of HCC combined alcohol consumption habit.
In conclusion, we found that drinking patients tended to have poorer health status and higher levels of inflammation in overall tumor types. This may be due to acetaldehyde, a primary product of alcohol metabolism. Meanwhile, alcohol can also promote cancer development and metastasis by inhibiting DNA methylation.25 Since there is a lot of evidence that alcohol consumption is closely related to the risk of cancer, researches are still in an initial stage on the effect of alcohol consumption on the prognosis of cancer patients. Therefore, we believe further investigation imperative.
Data Sharing Statement
Materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of the first affiliated hospital of Jilin University (2017-362), and all the patient data in this research was approved by this committee. All study participants have filled out written informed consent for participation.
Funding
This work was supported by National Key R&D Program of China (2016YFC1303804).
Disclosure
Xiangliang Liu, Wenxin Zhang, and Wei Ji are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. Rumgay H, Shield K, Charvat H, et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol. 2021;22(8):1071–1080. doi:10.1016/S1470-2045(21)00279-5
2. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi:10.1016/S0140-6736(20)30752-2
3. Manthey J, Shield KD, Rylett M, et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modeling study. Lancet. 2019;393(10190):2493–2502. doi:10.1016/S0140-6736(18)32744-2
4. LoConte NK, Brewster AM, Kaur JS, et al. Alcohol and cancer: a statement of the American Society of Clinical Oncology. J Clin Oncol. 2018;36(1):83–93. doi:10.1200/JCO.2017.76.1155
5. Hendriks HFJ. Alcohol and human health: what is the evidence? Annu Rev Food Sci Technol. 2020;11:1–21. doi:10.1146/annurev-food-032519-051827
6. Rumgay H, Murphy N, Ferrari P, et al. Alcohol and cancer: epidemiology and biological mechanisms. Nutrients. 2021;13(9):3173. doi:10.3390/nu13093173
7. Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7(2):149–156. doi:10.1016/S1470-2045(06)70577-0
8. Chen W, Xia C, Zheng R, et al. Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: a comparative risk assessment. Lancet Glob Health. 2019;7(2):e257–e269. doi:10.1016/S2214-109X(18)30488-1
9. Ratna A, Mandrekar P. Alcohol and cancer: mechanisms and therapies. Biomolecules. 2017;7:61. doi:10.3390/biom7030061
10. Gao B, Ahmad MF, Nagy LE, et al. Inflammatory pathways in alcoholic steatohepatitis. J Hepatol. 2019;70(2):249–259. doi:10.1016/j.jhep.2018.10.023
11. Kang H, Lee Y, Bae M, et al. Astaxanthin inhibits alcohol-induced inflammation and oxidative stress in macrophages in a sirtuin 1-dependent manner. J Nutr Biochem. 2020;85:108477. doi:10.1016/j.jnutbio.2020.108477
12. Sutta C, Haddock B, Alcazar J, et al. The Copenhagen Sarcopenia Study: lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. J Cachexia Sarcopenia Muscle. 2019;10(6):1316–1329. doi:10.1002/jcsm.12477
13. Ganne-Carrié N, Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol. 2019;70(2):284–293. doi:10.1016/j.jhep.2018.10.008
14. Lim CJ, Lee YH, Pan L, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut. 2019;68(5):916–927. doi:10.1136/gutjnl-2018-316510
15. Jia L, Gao Y, He Y, et al. HBV induced hepatocellular carcinoma and related potential immunotherapy. Pharmacol Res. 2020;159:104992. doi:10.1016/j.phrs.2020.104992
16. Peixoto da Silva S, Santos JMO, Costa E, et al. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia, and asthenia. J Cachexia Sarcopenia Muscle. 2020;11(3):619–635. doi:10.1002/jcsm.12528
17. Li N, Deng Y, Zhou L, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol. 2019;12(1):140. doi:10.1186/s13045-019-0828-0
18. Ko H, Chang Y, Kim HN, et al. Low-level alcohol consumption and cancer mortality. Sci Rep. 2021;11(1):4585. doi:10.1038/s41598-021-84181-1
19. Carrillo-Estrada M, Bobrowski D, Carrasco R, et al. Coronary artery disease in patients with cancer: challenges and opportunities for improvement. Curr Opin Cardiol. 2021;36(5):597–608. doi:10.1097/HCO.0000000000000878
20. Wu D, Hu D, Chen H, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559(7715):637–641. doi:10.1038/s41586-018-0350-5
21. Key TJ, Bradbury KE, Perez-Cornago A, et al. Diet, nutrition, and cancer risk: what do we know and what is the way forward? BMJ. 2020;368:m51. doi:10.1136/bmj.m51
22. Chen HL. The Investigation and Analysis of Alcoholic Liver Cirrhosis Nutritional Status. Shangxi Medical University; 2018.
23. Le Daré B, Lagente V, Gicquel T. Ethanol and its metabolites: update on toxicity, benefits, and focus on immunomodulatory effects. Drug Metab Rev. 2019;51(4):545–561. doi:10.1080/03602532.2019.1679169
24. Rodrigues P, Santos-Ribeiro S, Teodoro T, et al. Association between alcohol intake and cardiac remodeling. J Am Coll Cardiol. 2018;72(13):1452–1462. doi:10.1016/j.jacc.2018.07.050
25. Sina AA, Carrascosa LG, Trau M. DNA methylation-based point-of-care cancer detection: challenges and possibilities. Trends Mol Med. 2019;25(11):955–966. doi:10.1016/j.molmed.2019.05.014
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



