Back to Journals » Journal of Pain Research » Volume 15
Effect of Acute Brief Social Isolation on Visceral Pain
Authors Zhang C, Zhou X, Zhou X
Received 12 June 2022
Accepted for publication 5 October 2022
Published 9 November 2022 Volume 2022:15 Pages 3547—3553
DOI https://doi.org/10.2147/JPR.S378244
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qi Fang
Chenjing Zhang,1 Xiaolu Zhou,1 Xuelong Zhou2
1Department of Gastroenterology, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, 310014, People’s Republic of China; 2Department of Anesthesiology and Perioperative Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, 210029, People’s Republic of China
Correspondence: Xuelong Zhou, Department of Anesthesiology and Perioperative Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, 210029, People’s Republic of China, Tel +86-021-83714511, Email [email protected]
Objective: The sensitivity of somatic pain could be affected by social isolation; however, few studies have examined the impact of social isolation on visceral pain. In the present study, the effect of acute brief social isolation on visceral pain response was investigated.
Methods: Adult male rats were either reared individually or grouped for 2 hours or 24 hours. Colorectal distention (CRD)-induced abdominal withdrawal reflex (AWR) score and pain threshold were used to assess visceral pain sensitivity. The amount of fecal bolus was used to determine the stress level.
Results: Acute brief isolation rearing for 2 hours significantly increased AWR score and reduced visceral pain threshold in rats when compared to group rearing. Similarly, acute isolation for 24 hours resulted in visceral hypersensitivity, as indicated by an increase in the AWR score and a decrease in the visceral pain threshold. Furthermore, the amount of fecal bolus in acute isolation rearing (2 or 24 hours) rats was considerably higher than in the control group rearing rats.
Conclusion: Acute short-term social isolation enhances visceral pain sensitivity, which could be related to an increase in stress levels.
Keywords: social isolation, visceral pain, stress
Introduction
Social contact is an essential need of social animals, much like eating and sleeping. Appropriate social interaction has numerous advantages, including the acquisition of partners, cooperative defence, and the upbringing of the next generation.1 Lack of social connection or social support, on the other hand, has a variety of negative consequences for social individuals’ health, including depression,2 cognitive impairment,3 and even life cycle shortening.4 Given the rising prevalence of social isolation in today’s society,5 it’s critical to investigate and examine the behavioral changes that occur when an animal is isolated from its social environment, as well as the mechanisms that cause them.
Previous studies have found that social isolation induces aberrant pain perception in rodents. When compared to group-housed rats, the pain sensitivity of isolation housed rats’ feet to noxious heat and electric shock is dramatically altered.6,7 Social isolation rearing decreases mechanical pain sensitivity in mice in a chronic inflammatory condition caused by complete Freund’s adjuvant (CFA).8 In addition, the endogenous opioid and serotonin (5-HT) systems are involved in the changes in somatic pain sensitivity caused by social isolation.9,10 The impact of social isolation on pain perception has primarily been studied in the context of somatic pain. However, few studies have examined the impact of social isolation on visceral pain. In the present study, we aimed to clarify the effect of social isolation on visceral pain sensitivity. By measuring visceral pain sensitivity in rats, we found that acute short-term social isolation increases visceral pain response.
Materials and Methods
Animal
Adult male Sprague Dawley rats weighing 220g ~ 250g were used. They were collected from the experimental animal center of Hangzhou Medical College. The temperature of the housing room is kept at 22 ± 2 °C with a 12 hours light-dark cycle. All experimental procedures were approved by the animal ethics committee of the Affiliated People’s Hospital of Hangzhou Medical College and followed the Guide for the care and use of laboratory animals (National Institutes of Health, 1996).
Acute Brief Isolation Rearing
Five rats were housed in each group for at least one month after they were obtained. Partial cage rats were randomly allocated and housed individually for 2 or 24 hours. The rats in the remaining cages were still housed in groups. Figure 1 depicts the experimental paradigm. The sensitivity to visceral pain was tested before and after social isolation rearing.
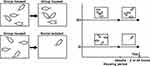 |
Figure 1 Schematic diagrams of the experimental protocols for experiments. Abbreviations: GH, group housing; SI, social isolation. |
Visceral Pain Measurement
Colorectal distention (CRD)-induced abdominal withdrawal reflex (AWR) score and pain threshold were used to assess visceral pain sensitivity.11 Briefly, the rats were fasting for 12 hours and placed in a self-made transparent box for 15 minutes. A balloon was rapidly inflated to a desired pressure (20, 40, 60, or 80 mmHg) in the descending colorectal region for 20 seconds, followed by a 2-minute rest. The AWR was given a score of: 0: no response; 1: brief head movement with no obvious body movement; 2: abdominal muscle contraction; 3: abdominal lifting; 4: abdominal arching. A stimulus intensity that elicited an AWR score of 3 was established as the visceral pain threshold. Starting at 10 mmHg, CRD was applied in 10 mmHg increments. All of the measurements were carried out in a blind manner.
Spontaneous Visceral Pain Behaviors
Prior to measuring visceral pain sensitivity, video recording was used to observe the spontaneous visceral pain behaviors of rats in a resting state in the cage for 20 minutes. Abdominal licking, abdominal stretching, squashing of the lower abdomen, and abdominal retractions are considered as visceral pain behaviors.12,13
Fecal Bolus Collection
The rats were freely available to food and water. The number of fecal bolus deposited in the cage was counted for 2 hours.14 The number of fecal bolus deposited in the cage of group rearing rats was monitored via video recording.
Statistical Analysis
All statistical analyses and graphics were carried out by Origin Pro (2021) software. The data were presented as mean ± SD. Differences between the two groups were compared using Student’s t-test. For repeated measurements (AWR score), data were analyzed by two-way repeated-measures ANOVA. Post hoc Bonferroni’s multiple comparisons were used if there was a significant difference. Statistical significance was defined as a P < 0.05.
Result
Acute 2h Social Isolation
Acute 2h Isolation Increased Visceral Pain Caused by CRD Stimulation
To investigate the effect of social isolation on visceral pain, rats were reared individually for 2 hours, and then their visceral pain sensitivity was assessed. No significant difference in baseline visceral pain sensitivity was identified between group rearing rats and isolated rearing rats. CRD-induced AWR score and pain threshold were comparable among the two groups before social isolation (Figure 2A and B). However, as compared to group rearing rats, 2 hours of isolation rearing increased the CRD-induced AWR score and decreased the pain threshold (Figure 2E and F), implying that 2 hours of acute solation rearing causes visceral hypersensitivity in rats.
Acute Social Isolation for 2 Hours Had No Effect on Visceral Pain Behaviors
In addition to the assessment of CRD-induced visceral pain, we also detected spontaneous visceral pain in rats within 2 hours of isolation. There was no significant difference in the number of spontaneous pain behaviors between group rearing rats and isolated rearing rats before and after social isolation (Figure 2C and G), suggesting that the 2 hours of isolation had no influence on the spontaneous visceral pain.
The Number of Fecal Bolus Was Increased After a 2-Hour Period of Social Isolation
The defecation frequency of rats reflects their stress level.15,16 To determine the effect of acute isolation on stress levels, we collected fecal bolus during the 2 hours of isolation rearing. The baseline number of fecal bolus was comparable between isolation rearing rats and group rearing rats (Figure 2D). However, the number of fecal bolus was significantly increased in 2 hours of social isolation rearing rats than in group rearing rats (Figure 2H), indicating that the stress level of rats was considerably increased after 2 hours of acute social isolation.
Acute 24h Social Isolation
Acute 24h Isolation Increased CRD Stimulation-Induced Visceral Pain
Because a 24-hour isolation time is also regarded as an acute social isolation period,1 we investigated the effect of acute 24-hour social isolation on visceral pain sensitivity in rats further. Between isolation and group rearing rats, there was no significant difference in baseline visceral pain sensitivity caused by CRD (Figure 3A and B). However, as compared to group rearing rats, 24 hours of acute isolation significantly increased CRD-induced AWR score and reduced visceral pain threshold (Figure 3E and F), suggesting that isolation rearing for 24 hours produces visceral hypersensitivity in rats.
Acute 24h Isolation Did Not Affect Spontaneous Visceral Pain
The findings of the spontaneous visceral pain behaviours test revealed that there was no significant difference in baseline between rats reared in groups and rats reared in isolation (Figure 3C). In isolation rearing rats, the number of spontaneous visceral pain behaviours was comparable to that of group rearing rats (Figure 3G). These findings imply that a period of acute isolation of 24 hours had no noticeable impact on spontaneous visceral pain.
Acute 24h Isolation Increased the Number of Fecal Bolus
The results revealed that the baseline number of fecal bolus was comparable in both isolation and group rearing rats (Figure 3D). However, isolation rearing for 24 hours significantly increased the number of fecal bolus when compared to group rearing (Figure 3H), indicating that acute 24 hour isolation, equivalent to 2 hours, increased the stress level of rats.
Discussion
In the present study, we investigated the effect of acute social isolation (2 or 24 hours) on visceral pain sensitivity in rats. The main results were as follows: acute isolation increased visceral pain sensitivity in rats after CRD stimulation; acute isolation had no effect on spontaneous visceral pain; the amount of fecal bolus was increased in acute isolation rearing rats.
Many studies have shown that social isolation induces a variety of behavioural abnormalities in animals, including locomotor hyperactivity,17 learning and memory impairment,3 and an excessive aggressive defense reaction to external stimulation.18 Furthermore, a number of research have revealed that social isolation causes aberrant pain sensitivity.19 Short- and long-term social isolation dramatically reduced rodent pain sensitivity, resulting in a higher thermal and mechanical pain threshold in the animal paws.8,14 Meanwhile, social isolation increased morphine’s analgesic effect.6 Clinical studies, on the other hand, have indicated that those who are socially isolated are more prone to experience pain, such as muscular and joint pain.20,21 For the first time, we examined the impact of social isolation on visceral pain sensitivity. In contrast to the effect of social isolation on somatic pain, social isolation increased the sensitivity of rats to visceral pain. Our findings revealed that social isolation has a distinct influence on visceral and somatic pain.
Pain measurement includes external stimuli-induced pain response and spontaneous pain behaviors. In the present study, we found that acute social isolation increased visceral pain sensitivity in rats when pain was detected with CRD stimulation. Acute social isolation, on the other hand, had no effect on spontaneous pain in resting rats. The physiological stimulation in the resting state is insufficient to cause a difference in pain sensitivity between rats raised in isolation and those raised in groups. For future experiments, more powerful stimuli are required. In our unpublished study, we found that spontaneous pain behaviors induced by formalin and capsaicin differed significantly between isolated and grouped rats.
Isolation from others, particularly acute isolation, is a strong source of stress.22 Models of visceral pain are induced using the acute stress response, such as water avoidance and forced swimming stress.23 In rodents, these stressors cause significant visceral hypersensitivity. By counting the number of fecal bolus, we found that the stress level of acute social isolation rats was much higher than that of group rearing rats. Acute social isolation-induced visceral hypersensitivity is thought to be mediated by increased stress levels.24 This supports with our findings that acute social isolation increased visceral pain sensitivity rather than reducing somatic pain sensitivity. In addition to stress, acute social isolation causes an aberrant immunological response. Pro-inflammatory factors are produced more effectively in response to acute stress.25 One of the key mechanisms for visceral hypersensitivity is the inflammatory response of the digestive tract.26 At the level of the central nervous system, acute stress can cause brain alterations and defensive actions in response to the threat of an unknown environment.27 This results in a high level of awareness, which is one of the main causes for the HPA axis activation and stress reaction.
Why was somatic pain reduced but visceral pain enhanced after acute social isolation? Previous studies have reported that acute social isolation-induced decrease in somatic pain sensitivity is related to endogenous opioid and 5-HT systems. Administering naloxone or the serotonin receptor antagonist WAY100635 reduced the analgesic effect of acute social isolation on somatic pain.9,10 Although we are not well aware of the role of endogenous opioid systems in the different effect of acute social isolation on somatic and visceral pain, the serotonin system may contribute, in part, to these differences. Serotonin has different effects on somatic pain and visceral pain. The threshold of somatic pain was enhanced when serotonin levels were elevated.9,28 Increased serotonin levels, on the other hand, cause visceral hypersensitivity.29
In conclusion, our study found that acute short-term social isolation increases visceral pain sensitivity. Preclinical evidence of the impact of acute social isolation on visceral pain is presented.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests in this work.
References
1. Lee CR, Chen A, Tye KM. The neural circuitry of social homeostasis: consequences of acute versus chronic social isolation. Cell. 2021;184(6):1500–1516. doi:10.1016/j.cell.2021.02.028
2. Ma XC, Jiang D, Jiang WH, et al. Social isolation-induced aggression potentiates anxiety and depressive-like behavior in male mice subjected to unpredictable chronic mild stress. PLoS One. 2011;6(6):e20955. doi:10.1371/journal.pone.0020955
3. Paulus MP, Varty GB, Geyer MA. The genetic liability to stress and postweaning isolation have a competitive influence on behavioral organization in rats. Physiol Behav. 2000;68(3):389–394. doi:10.1016/s0031-9384(99)00193-6
4. Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110(15):5797–5801. doi:10.1073/pnas.1219686110
5. Almeida ILL, Rego JF, Teixeira ACG, Moreira MR. Social isolation and its impact on child and adolescent development: a systematic review. Rev Paul Pediatr. 2021;40:e2020385. doi:10.1590/1984-0462/2022/40/2020385
6. Adler MW, Mauron C, Samanin R, Valzelli L. Morphine analgesia in grouped and isolated rats. Psychopharmacologia. 1975;41(1):11–14. doi:10.1007/BF00421298
7. Kosten TA, Miserendino MJ, Bombace JC, Lee HJ, Kim JJ. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behav Brain Res. 2005;157(2):235–244. doi:10.1016/j.bbr.2004.07.001
8. Horiguchi N, Ago Y, Hasebe S, et al. Isolation rearing reduces mechanical allodynia in a mouse model of chronic inflammatory pain. Pharmacol Biochem Behav. 2013;113:46–52. doi:10.1016/j.pbb.2013.10.017
9. Horiguchi N, Ago Y, Asada K, et al. Involvement of spinal 5-HT1A receptors in isolation rearing-induced hypoalgesia in mice. Psychopharmacology. 2013;227(2):251–261. doi:10.1007/s00213-012-2959-7
10. Konecka AM, Sroczynska I. Stressors and pain sensitivity in CFW mice. Role of opioid peptides. Arch Int Physiol Biochim. 1990;98(5):245–252. doi:10.3109/13813459009113984
11. Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450(1–2):153–169. doi:10.1016/0006-8993(88)91555-7
12. Laird JMA, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92(3):335–342. doi:10.1016/S0304-3959(01)00275-5
13. Miampamba M, Chery-Croze S, Gorry F, Berger F, Chayvialle JA. Inflammation of the colonic wall induced by formalin as a model of acute visceral pain. Pain. 1994;57(3):327–334. doi:10.1016/0304-3959(94)90008-6
14. Han RT, Lee H, Lee J, et al. Brief isolation changes nociceptive behaviors and compromises drug tests in mice. Pain Pract. 2016;16(6):749–757. doi:10.1111/papr.12325
15. Bonaz B, Tache Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641(1):21–28. doi:10.1016/0006-8993(94)91810-4
16. Miyata K, Kamato T, Nishida A, et al. Role of the serotonin3 receptor in stress-induced defecation. J Pharmacol Exp Ther. 1992;261(1):297–303.
17. Gentsch C, Lichtsteiner M, Frischknecht HR, Feer H, Siegfried B. Isolation-induced locomotor hyperactivity and hypoalgesia in rats are prevented by handling and reversed by resocialization. Physiol Behav. 1988;43(1):13–16. doi:10.1016/0031-9384(88)90091-1
18. Varty GB, Powell SB, Lehmann-Masten V, Buell MR, Geyer MA. Isolation rearing of mice induces deficits in prepulse inhibition of the startle response. Behav Brain Res. 2006;169(1):162–167. doi:10.1016/j.bbr.2005.11.025
19. Ago Y, Takuma K, Matsuda T. Potential role of serotonin1A receptors in post-weaning social isolation-induced abnormal behaviors in rodents. J Pharmacol Sci. 2014;125(3):237–241. doi:10.1254/jphs.14r05cp
20. Smith TO, Dainty JR, Williamson E, Martin KR. Association between musculoskeletal pain with social isolation and loneliness: analysis of the English longitudinal study of ageing. Br J Pain. 2019;13(2):82–90. doi:10.1177/2049463718802868
21. Zheng C, He MH, Huang JR, He Y. Causal relationships between social isolation and osteoarthritis: a Mendelian randomization study in European population. Int J Gen Med. 2021;14:6777–6786. doi:10.2147/IJGM.S331864
22. Taylor JH, Mustoe AC, French JA. Behavioral responses to social separation stressor change across development and are dynamically related to HPA activity in marmosets. Am J Primatol. 2014;76(3):239–248. doi:10.1002/ajp.22228
23. Schwetz I, Bradesi S, McRoberts JA, et al. Delayed stress-induced colonic hypersensitivity in male Wistar rats: role of neurokinin-1 and corticotropin-releasing factor-1 receptors. Am J Physiol Gastrointest Liver Physiol. 2004;286(4):G683–91. doi:10.1152/ajpgi.00358.2003
24. Bradesi S, Eutamene H, Garcia-Villar R, Fioramonti J, Bueno L. Acute and chronic stress differently affect visceral sensitivity to rectal distension in female rats. Neurogastroenterol Motil. 2002;14(1):75–82. doi:10.1046/j.1365-2982.2002.00305.x
25. Cole SW, Gibson G. Human social genomics. PLoS Genet. 2014;10(8):e1004601. doi:10.1371/journal.pgen.1004601
26. Elsenbruch S. Abdominal pain in irritable bowel syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25(3):386–394. doi:10.1016/j.bbi.2010.11.010
27. Cacioppo S, Bangee M, Balogh S, Cardenas-Iniguez C, Qualter P, Cacioppo JT. Loneliness and implicit attention to social threat: a high-performance electrical neuroimaging study. Cogn Neurosci. 2016;7(1–4):138–159. doi:10.1080/17588928.2015.1070136
28. Liu QQ, Yao XX, Gao SH, et al. Role of 5-HT receptors in neuropathic pain: potential therapeutic implications. Pharmacol Res. 2020;159:104949. doi:10.1016/j.phrs.2020.104949
29. Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–486. doi:10.1038/nrgastro.2013.105
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


