Back to Journals » International Journal of General Medicine » Volume 15
Effect of a Dexamethasone Implant on Rheological Blood Parameters in Patients Treated for Retinal Vein Thrombosis
Authors Śpiewak D, Heinke A , Michalska-Małecka K
Received 9 November 2021
Accepted for publication 17 August 2022
Published 30 August 2022 Volume 2022:15 Pages 6931—6944
DOI https://doi.org/10.2147/IJGM.S348512
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Dorota Śpiewak,1 Anna Heinke,2,3 Katarzyna Michalska-Małecka4,5
1University Clinical Center, University Hospital Medical University of Silesia, Katowice, Poland; 2University of California San Diego Department of Ophthalmology at the Shiley Eye Institute, La Jolla, CA, USA; 3Joan and Irwin Jacobs Retina Center, La Jolla, CA, USA; 4Department of Ophthalmology, Medical University of Gdańsk, Gdańsk, Poland; 5University Clinical Center, University Hospital Medical University of Gdańsk, Gdańsk, Poland
Correspondence: Katarzyna Michalska-Małecka, Department of Ophthalmology, University Hospital Medical University of Gdańsk, Mariana Smoluchowskiego 17, Gdańsk, 80-214, Poland, Tel +48 600 064 150, Email [email protected]
Purpose: To assess the effect of the prolonged-release dexamethasone implant (Ozurdex) in patients with retinal venous thrombosis on blood rheological parameters and evaluate the impact on morphological and functional parameters.
Methods: Prospective cross-sectional study involved 35 subjects treated with central retinal vein and vein branch occlusion with intravitreal dexamethasone implant and 35 healthy subjects in control group. Hematocrit, blood and plasma viscosity, erythrocyte aggregation amplitude (EAA), index (EAI), aggregation half-life time (EAT1/2), and erythrocyte deformability (ED) were evaluated. The best-corrected visual acuity (BCVA), central retinal thickness (CRT) and visual field (VF) were examined. All the parameters were examined at the baseline, 7 and 30 days after the treatment. The results were analyzed with correlation and regression and compared to the baseline values.
Results: In the studied group, three hemorheological parameters improved as early as on the 7th day after the drug administration, and this improvement remained at a similar level on the 30th day. The EAA increased by 21.26% on day 7 and by 19.78% on day 30, the EAI decreased by 10.55% on day 7 and by 9.14% on day 30 and the EAT1/2 time increased by 38.50% on day 7 and by 31.02% on day 30. On day 7, the BCVA was improved by 1 row, CRT was reduced by 170.77 μm (24.49%), and the VF was improved by 9.8 dB (49.92%) compared to baseline parameters. On day 30, the BCVA was improved by three rows, CRT reduced by 244.68 μm (35.09%), and VF improved by 12.09 dB (61.59%).
Conclusion: The intravitreal dexamethasone implant used in treating macular oedema in patients with RVO has a statistically significant effect on some rheological parameters of blood, reduction of CRT and clinical improvement in BCVA and VF.
Keywords: retinal venous vein thrombosis, dexamethasone, hemorheology
Introduction
Retinal vein occlusion is the second most common retinal vascular disease after diabetic retinopathy, leading to reduced visual acuity or even blindness. The RVO manifests as a sudden, painless, unilateral decrease in visual acuity, which depends on the degree of closure of the vessel, the size and location of the occupied retinal area and the size of the macular area affected by the clot. Metamorphopsia, relative loss of visual field, appears,1 and the fundus of the eye shows retinal oedema, “cotton-wool spots”, blurred optic disc margins, intraretinal haemorrhages and dilated, tortuous veins. Patients with peripheral thrombosis may not have any symptoms. A complication of RVO may be neovascularization, which usually develops within 6–12 months. Neovascularization outside the optic disc (NVE – neovascularization elsewhere) develops more often than the form with vessels on the disc (NVD – neovascularization on the disc) and is usually formed at the border of perfusion-free areas drained by a closed vein. Proliferating vessels may cause recurrent haemorrhages to the vitreous, tractional retinal detachment, and development of secondary glaucoma.
The factors predisposing to clotting can be divided into systemic and local. The first group includes factors that increase blood viscosity: Waldenström’s macroglobulinemia, proliferative diseases of the hematopoietic system, sickle cell anemia, thrombophilia, diabetes mellitus,2 hypertension,3 hyperlipidemia, cardiovascular diseases,4 obesity, oral contraceptives, smoking, dehydration, and hot climate. The second group includes glaucoma,5,6 hyperopia, inflammatory conditions of the eye, bends and compression of eye veins and many venous and arterial vessel crossings.7,8 In the development of retinal vein occlusion, three factors that create the so-called Virchow’s Triad play a fundamental role: of destruction to the blood vessel with impaired function of the vascular endothelium, excessive blood clotting and blood flow disorders, such as its slowdown or vortex flow. As a result of their interaction, hypoxia, and ischemia results in increased hydrostatic pressure in venous vessels and capillaries, development and progression of inflammatory processes, dysfunction of vascular endothelial cells and increased VEGF concentration in the vitreous.9,10 Depending on the thrombus localization, we distinguish between central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO).11 The presence or absence of abnormalities in blood flow through retinal capillaries in CRVO enables to divide CRVO into ischemic forms if there are nonperfusion zones and non-ischemic forms if the capillary flow is maintained.
In venous thromboembolism, plasma viscosity, erythrocyte aggregation and deformability are increased. Increased viscosity of blood creates the risk of CRVO and hemostasis abnormalities promote the development of neovascularization.12,13
In retinal venous thrombosis, corticosteroids reduce macular oedema, inhibit expression of endothelial growth factor (VEGF),14,15 reduce the formation of mediators of cystic macular oedema,16,17 stabilize the blood-retinal barrier and improve hemorheological parameters in retinal vessels. The administration of corticosteroid directly to the vitreous chamber allows to achieve the optimal therapeutic concentration in the eye tissues. The use of biodegradable implants with dexamethasone, which have prolonged action time, enables their use less frequently.14,18,19
Materials and Methods
The study was conducted at the Prof. K. Gibiński University Clinical Center in Katowice. The consent of the Bioethics Committee operating at the Medical University of Silesia was obtained for the study: Resolution No. KNW/022/KB1/99/I/13.
Thirty-five Caucasian subjects with central retinal vein thrombosis (CRVO) and central retinal vein branch thrombosis (BRVO) aged between 28 and 77 years were included in the study. In the study, central retinal vein thrombosis was not divided into ischemic and non-ischemic types due to the design of the study, which aimed to evaluate the effect of intravitreal dexamethasone on hemorheological parameters, regardless of the type of thrombus. All the study patients were diagnosed with acute macular edema due to RVO and were all treatment naive. The treatment with intravitreal dexamethasone implant was started within the first month of symptom onset and diagnosis of RVO in all the subjects. The patient baseline demographics and clinical characteristics are presented in Table 1. Patients, who used the medication for hypertension, diabetes and hypercholesterolemia before the study, continued the treatment throughout the duration of the study. None of the study patients used anticoagulation. The control group consisted of 35 healthy patients. The control group baseline characteristics are shown in Table 2. None of the healthy control subjects used anticoagulation or other medication.
 |
Table 1 Baseline Demographics and Clinical Characteristics of Study Group |
 |
Table 2 Baseline Demographics and Clinical Characteristics of Healthy Control Group |
Numerous publications support a 6-month duration of action for the intravitreal dexamethasone implant. Our study lasted only one month because its purpose was to show the effect of intravitreal dexamethasone on hemorheological parameters and not to evaluate the duration of its action. Intravitreal dexamethasone in RVO affects hemorrhagic parameters because it penetrates the systemic circulation through the damaged blood-retinal barrier. The train of thought was based on the available literature on an animal model of drug permeation into the systemic circulation through a damaged internal blood-retinal barrier (iBRB), in the course of central retinal vein thrombosis.20
The hemorheological parameters of peripheral blood were analyzed: hematocrit, blood viscosity, plasma viscosity, erythrocyte aggregation amplitude, erythrocyte aggregation index, erythrocyte half-life and erythrocyte deformability. In addition, the best corrected visual acuity (BCVA, Snellen charts), central retinal thickness (CRT, Optical Coherence Tomography – OCT) and visual field (static examination, mean loss of retinal sensitivity, MD – mean defect, Octopus 300) were examined. Multiple studies demonstrate the validity of microperimetry in the diagnosis and treatment of retinal diseases, and it is likely that microperimetry would have been a better choice in our study. Nevertheless, the main aim of the study was to assess blood rheological parameters, and visual field testing was only an additional clinical and functional parameter.
The erythrocyte aggregation parameters were measured using the LORCA (Laser-assisted Optical Rotational Cell Analyzer) by Mechatronics (LORCA), obtaining the curves of dependence of scattered light intensity on time and shear rate, the so-called sylektograms.21,22
The measurements of erythrocyte deformability (elongation), after choosing the appropriate software option, were also performed with the use of LORCA analyzer to obtain a diffraction image from which the elongation index (EI) is calculated by computer. The value of EI depends on the size of the diffractive image. The larger the diffraction image is, the greater the deformability of red blood cells, ie, the greater is EI.21–23
Hematocrit was determined by traditional micromethod. Blood and plasma viscosity were determined using Brookfield DV-II+ viscometer in cone-plate system (Wells-Brookfield Engineering Laboratories) at constant temperature 37°C. Blood viscosity measurements were taken at shear rates of 150 s-1, 300 s-1 and 450 s-1 and plasma viscosity at shear rate of 900 s-1. Due to the influence of hematocrit on blood viscosity, viscosity corrected for hematocrit 45% was calculated.21,24
Inclusion and Exclusion Criteria for the Study
Inclusion Criteria
- Retinal vein thrombus on ophthalmoscopic examination
- Retinal vein thrombus confirmed by fluorescein angiography
- Cystoid macular edema in the course of retinal vein thrombosis confirmed by OCT (retinal thickness >300 μm)
- Best corrected distance visual acuity (BCVA) 5/10 (0.5) according to Snellen chart
- Intraocular pressure up to </= 21 mmHg controlled by a maximum of one topical hypotensive medication
- Written informed consent for drug administration
- Written informed consent for venous blood sampling
- Duration of disease – maximum 18 months.
Exclusion Criteria
- Lack of patient’s consent for administration of drug
- Lack of patient’s consent to venous blood sampling for research
- Hypersensitivity to the drug
- Advanced glaucoma not amenable to medical treatment
- Infection of the eye or its surroundings
- Aphakia with rupture of posterior capsule of the lens
- Anterior chamber implantation of artificial lens with the interruption of posterior capsule of the lens
- Post-pars plana vitrectomy condition
- Status after trabeculectomy with surgical iridectomy.
Statistical Analysis
All obtained measurement results were subjected to statistical analysis. At the beginning of the analysis, their statistical description was made considering the minimum, maximum, mean, median, standard deviation, skewness, standard error of skewness, kurtosis and standard error of kurtosis. For skewness and kurtosis, a range of values ±0.7 was adopted for symmetrical distribution (skewness) and moderately flattened (kurtosis), ie, close to normal or normal (skewness: 0, kurtosis: 0). The obtained results were statistically analyzed: Shapiro–Wilk test (W statistic) was performed to check the normality of distribution, Mauchly’s test was used to assess sphericity (homogeneity of covariance) and correlation between variables was assessed by determining Pearson’s correlation coefficient and determination coefficient.
The aim of the statistical analysis was to verify the alternative hypothesis that the values of individual variables changed during the month (7th and 30th day) after the administration of Ozurdex. The null hypothesis assumed that the measurement values did not change within one month after the administration of Ozurdex. The significance level was assumed to be α <0.05. The Wilcoxon test (labelled rank test, Wilcoxon pair order test) was used as a non-parametric alternative to the Student’s t-test, the analysis of variance (ANOVA) by Friedman (Friedman test, chi-quadrate statistics) and the Kendall compliance factor (W Kendall) was determined.
Results
The best corrected visual acuity (BCVA) was assessed before the administration of Ozurdex, 7 days after the administration of Ozurdex and 30 days after the administration of Ozurdex, respectively: 0.196 ±0.174, 0.337 ±0.242, 0.470 ±0.255.
The results of statistical analysis allowed rejecting the null hypothesis with no differences between the variables and adopting an alternative hypothesis, which indicates that:
- The average visual acuity before the administration of Ozurdex (VIS0) and after 7 (VIS7) and after 30 (VIS30) days from its administration differ and the difference is statistically significant with high strength (W = 0.87).
- Mean visual acuity after intravitreal administration of Ozurdex, both after 7 (VIS7) and after 30 (VIS30) days of administration, improves after administration of the drug (VIS0), and this difference is statistically significant with high strength (ε = 0.78, 0.85).
- Moreover, it was shown that visual acuity after 30 (VIS30) days from administration of the drug is better than after 7 (VIS7) days and the difference is also statistically significant with high strength (ε=0.79).
- An alternative hypothesis for the significance level ɑ = 0.05 was assumed, but it is also true for the level ɑ = 0.01 and ɑ = 0.001. The results are presented in Figure 1.
Retinal thickness (μm) was assessed prior to administration of Ozurdex, 7 days after administration of Ozurdex, and 30 days after administration of Ozurdex, with results: 697.314 ± 239.054, 526.542 ± 253.403, 452.628 ± 253.387 μm, respectively.
The results of statistical analysis allowed to reject the zero hypothesis about the lack of differences between the variables and to adopt an alternative hypothesis, which indicates that:
- The average thickness of the retina before the intravitreal administration of Ozurdex (RET0) and after 7 (RET7) and 30 (RET30) days after its administration differs, and this difference is statistically significant with high strength (W = 0.80).
- The mean thickness of the retina after the administration of Ozurdex, both after 7 (RET7) and after 30 (RET30) days of administration, is less than before administration of the drug (RET0), and this difference is statistically significant with high strength (ε=0.84, 0.85).
- Moreover, it has been shown that the average retinal thickness after 30 (RET30) days is less than after 7 (RET7) days of administration, and this difference is also statistically significant with moderate strength (ε=0.77).
- An alternative hypothesis for the significance level ɑ=0.05 was assumed, but it is also true for the level ɑ=0.01 and ɑ=0.001. The results are presented in Figure 2.
Mean loss of retinal sensitivity (MD – mean defect) (db) was assessed before Ozurdex, 7 days after Ozurdex administration and 30 days after Ozurdex administration, respectively: 19.625 ± 6.303, 9.828 ± 3.032, 7.540 ± 2.663 dB.
The results of statistical analysis allowed to reject the zero hypothesis with no differences between the variables and to adopt an alternative hypothesis, which indicates that:
- The average mean loss of retinal sensitivity (MD) prior to the administration of Ozurdex (TIM0) and after 7 (TIM7) and 30 (TIM30) days after its administration differs, and the difference is statistically significant with moderate strength (W = 0.76).
- The average mean loss of retinal sensitivity (MD) after the administration of Ozurdex, both after 7 (MD7) and after 30 (MD30) days of administration, is smaller than before the administration of the drug (MD0), and this difference is statistically significant with high strength (ε=0.84, 0.86).
- Moreover, it has been shown that the average loss of retinal sensitivity (MD) after 30 (MD7) is lower than after 7 (MD30) days of administration of the drug, and this difference is also statistically significant with high strength (ε=0.86).
- An alternative hypothesis for the significance level ɑ=0.05 was assumed, but it is also true for levels ɑ=0.01 and ɑ=0.001. The results are presented in Figure 3.
The erythrocyte (au) aggregation amplitude was studied before Ozurdex, 7 days after Ozurdex administration and 30 days after Ozurdex administration, respectively: 20.987 ± 2.982, 25.444 ± 2.845, 25.132 ± 3.484 au.
The results of statistical analysis allowed to reject the null hypothesis about the lack of differences between variables and to adopt an alternative hypothesis, which indicates that
- The average amplitudes of erythrocyte aggregation before the administration of Ozurdex (AMP0) and after 7 (AMP7) and after 30 (AMP30) days from its administration differ, and the difference is statistically significant of moderate strength (W = 0.44).
- The average erythrocyte aggregation amplitude after the administration of Ozurdex, both after 7 (AMP7) and after 30 (AMP30) days of administration, is greater than before administration (AMP0), and the difference is statistically significant with high (AMP7) or moderate (AMP30) strength (ε=0.82, 0.67).
- The zero hypothesis for the pair of AMP7 and AMP30 measurements was not rejected, which means that the erythrocyte aggregation amplitudes after 7 (AMP7) and 30 (AMP30) days after administration of the drug do not differ.
- An alternative hypothesis for the significance level ɑ=0.05 was assumed, but it is also true for the level ɑ=0.01 and ɑ=0.001. The results are presented in Figure 4.
The erythrocyte aggregation index (%) was determined prior to the administration of Ozurdex, 7 days after the administration of Ozurdex and 30 days after the administration of Ozurdex, respectively: 64.431 ± 7.310, 57.625 ± 7.591, 58.542 ± 8.146%.
The results of statistical analysis allowed to reject the zero hypothesis about the absence of differences between variables and adopt an alternative hypothesis, which indicates that:
- The average erythrocyte aggregation index before the administration of Ozurdex (INA0) and after 7 (INA7) and after 30 (INA30) days from its administration differ, and the difference is statistically significant with moderate strength (W = 0.57).
- The average erythrocyte aggregation index after the administration of Ozurdex, both after 7 (INA7) and after 30 (INA30) days of administration, is lower than before administration (INA0), and this difference is statistically significant with high (INA7) or moderate (INA30) strength (ε=0.85, 0.75).
- The zero hypothesis for the pair of INA7 and INA30 measurements was not rejected, which means that the erythrocyte aggregation index after 7 (INA7) and 30 (INA30) days after drug administration does not differ.
- An alternative hypothesis for the significance level ɑ=0.05 was assumed, but it is also true for levels ɑ=0.01 and ɑ=0.001. The results are presented in Figure 5.
The half-time of erythrocyte aggregation recovery (T1/2) (s) was assessed prior to the administration of Ozurdex, 7 days after the administration of Ozurdex and 30 days after the administration of Ozurdex, respectively: 1.869 ± 0.676, 2.592 ± 0.808, 2.450 ± 0.791 s.
The results of statistical analysis allowed to reject the zero hypothesis with no differences between the variables and to adopt an alternative hypothesis, which indicates that:
- The average half-life of the aggregation recovery (T1/2) before the administration of Ozurdex (TIM0) and after 7 (TIM7) and 30 (TIM30) days after its administration differs, and the difference is statistically significant of moderate strength (W = 0.48).
- The average half-life of the aggregation recovery (T1/2) after the administration of Ozurdex, both after 7 (TIM7) and 30 (TIM30) days after administration, is greater than before administration (TIM0), and the difference is statistically significant with high (TIM7) or moderate (TIM30) strength (ε=0.86, 0.68).
- However, the zero hypothesis for the pair of TIM7 and TIM30 measurements was not rejected, which means that the half-time aggregation (T1/2) after 7 (TIM7) and 30 (TIM30) days after drug administration does not differ.
- An alternative hypothesis for the significance level ɑ=0.05 was assumed, but it is also true for the level ɑ=0.01 and ɑ=0.001. The results are presented in Figure 6.
After testing and assessing the statistical significance of changes in the values of individual variables, at three successive time points (before the administration of Ozurdex and after 7 and 30 days from its administration), the relationships between the variables were examined (correlation), the shape of this relationship (regression) and the linear regression parameters were calculated, and regression lines were determined. The results are presented in the form of regression lines and a graph of the scatter of the rest.
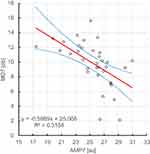
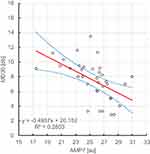
It was shown that there is a statistically significant (ɑ=0.05), negative, linear relationship between the erythrocyte aggregation amplitude at day 7 after Ozurdex administration and the visual field at day 7 and day 30 after Ozurdex administration, r2, respectively: 0.316, 0.280. Figures 7 and 8.
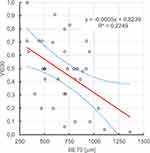
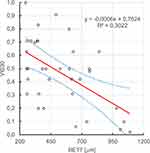
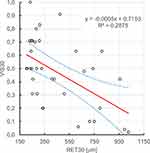
It has been shown that there is a statistically significant (ɑ=0.05), negative, linear relationship between retinal thickness before Ozurdex administration, retinal thickness on the 7th and 30th day after Ozurdex administration and visual acuity on the 30th day after Ozurdex administration, r2 respectively: 0.225, 0.302, 0.288. Figures 9-11.
Discussion
The corticosteroids used in the treatment of cystic oedema in the course of RVO reduce the permeability of retinal capillaries and inhibit gene expression and metabolic pathways of VEGF (vascular endothelial growth factor).25,26 SCORE showed structural improvement in retinal morphology and functional improvement in RVO patients, ie, improvement of visual acuity after triamcinolone, but the effect was short term.27 There is also a greater increase in intraocular pressure. The technological progress made it possible to develop a biodegradable implant slowly releasing dexamethasone (Ozurdex) and a system for its single administration.
In the course of iBRB damage, elevated concentrations of endothelial growth factor VEGF, cytokines, inflammatory factors, and glycation end products, among others, are found. Transport across the retinal vascular wall extracellular, but also intercellular, is increased by the opening of endothelial intercellular junctions and by qualitative and quantitative changes in intercellular transport. Retinal hypoxia leads to the collapse of the iBRB, which is associated with increased vascular permeability, causing angioedema. Damage to the iBRB is associated with increased production of vascular endothelial growth factor (VEGF), nitric oxide (NO), oxidative stress and inflammation. Understanding the complex cellular and molecular processes involved in iBRB leakage has increased rapidly in recent years. These insights have provided tools for the rational design of drugs aimed at restoring blood–retinal barrier function of the iBRB.
Ozurdex effectively reduces macular oedema and improves visual acuity in patients with CRVO and BRVO, as demonstrated in the multicenter GENEVA study. On the 180th day after injection, a statistically significant difference in visual acuity was found between the group that received Ozurdex and the placebo group, but without a significant difference in retinal thickness, which suggests the involvement of additional mechanisms of action of the drug on visual acuity, in addition to the reduction of retinal thickness in patients with RVO and macular oedema. Similar conclusions were drawn after the analysis of the SCORE study, in which triamcinolone was administered by the retina. The correlation between retinal thickness and improvement of visual acuity was poor.28 In our study, after the use of prolonged-action dexamethasone (Ozurdex), this correlation was stronger (about 7%) and statistically significant, in contrast to the effect of triamcinolone administration. Our study, compared to the SCORE and GENEVA studies, was enriched with an analysis of mean loss of retinal sensitivity (MD). The results were analyzed at the same time points as visual acuity.
Improvement of visual acuity, reduction of central retinal thickness and reduction of mean loss of retinal sensitivity were statistically significant at all time points studied, ie, after 7 and 30 days and between 7 and 30 days after Ozurdex administration.
To identify other factors explaining the influence of Ozurdex on clinical improvement (in terms of visual acuity) in patients with retinal vein occlusion, its influence on some hemorheological parameters was analyzed. One of the studied parameters was erythrocyte aggregation, which is an important factor determining blood flow parameters and is responsible for increasing its viscosity under slow flow conditions (low shear rate). The size of erythrocyte aggregates is a result of repulsive forces between negatively charged erythrocytes, erythrocyte adhesion induced by plasma proteins and shear forces generated by blood flow. Under physiological conditions, blood flow is sufficient to break down the forming aggregates (disaggregation), which ensures proper tissue perfusion. Under slow flow conditions and other pathological states related to increased erythrocyte aggregation, microcirculation and closure of venous capillaries may be disturbed.29 Venous microcirculation in the retina is characterized by slow flow and increased vascular resistance, which significantly limits its adaptive abilities in response to increased blood viscosity.30 In the available literature, hemorheological abnormalities are described not only in RVO but also in other ophthalmologic diseases, eg, age-related macular degeneration (AMD), which suggests the relationship between angiogenesis factors (VEGF), hematological factors, hemostasis, endothelial dysfunction, and AMD. The interaction between abnormal angiogenesis and Virchow’s triad components and thrombogenesis may partially contribute to AMD development.31 An interesting observation is hemorheological changes in patients with diabetic retinopathy compared to the control group. Statistically significantly higher values of hematocrit, fibrinogen, increased blood and plasma viscosity and increased erythrocyte aggregation were found. There was no significant difference in hemorheological variables in different stages of retinopathy (non-proliferative, pre-proliferative and proliferative), but the participation of these factors in the development of diabetic microangiopathy was indicated.32
The hemodynamic characteristics of venous circulation in the retina make it particularly dependent on rheological parameters of blood, and the shape deformability of erythrocyte aggregates begins to be crucial for flow. In our examined group of patients, three out of several evaluated hemorheological parameters changed significantly after administration of Ozurdex: erythrocyte aggregation amplitude (Amp), erythrocyte aggregation index (AI) and erythrocyte aggregation recovery half-life (T1/2), which suggests that dexamethasone in the form of a long-acting, biodegradable implant, administered intravitreally (Ozurdex), used in the treatment of macular oedema in patients with RVO, directly affects the RVO pathomechanism, which may be the key mechanism of its action in the treatment of RVO. Clinical improvement in visual acuity and visual field in patients with RVO treated with dexamethasone administered intravitreally can be associated with the reduction of central retinal oedema. Clinical improvement is also influenced by the change in microcirculation flow associated with shortened erythrocyte disaggregation time. Increase in erythrocyte aggregation amplitude in patients with RVO treated with dexamethasone administered intravenously correlates negatively with MD visual field parameter, and reduction of retinal oedema correlates negatively with improvement of visual acuity during observation (30 days) and may be a predictor of improvement of visual acuity. However, this hypothesis needs to be confirmed in a large prospective study. The improvement of hemorheological profile (Amp – increase, AI – decrease, T1/2 – elongation), which reflects the erythrocyte disaggregation process induced by dexamethasone, shown in our study, was stable, constant, and sustained until the end of the study.
During the observation period (30 days), long-acting, biodegradable dexamethasone, used in the form of intravitreal implant in patients with RVO in the treatment of retinal post-thrombotic changes, statistically significantly increases the erythrocyte aggregation amplitude, reduces the erythrocyte aggregation index and extends the erythrocyte half-aggregation time, which can be associated with its effectiveness in the treatment of RVO.
The predictor of improving visual acuity in RVO can be both hemorheological and morphological parameters of the retina, ie, reduction of erythrocyte aggregation amplitude in the 7th day after the intravitreal administration of a long-acting, biodegradable dexamethasone implant, which correlates negatively with visual field parameters in the 7th and 30th day and reduction of retinal thickness in the central retina on the 7th and 30th day after intravitreal administration of a long-term biodegradable dexamethasone implant, which correlates with improved visual acuity. The limitation of our study is a relatively small group of 35 patients and short duration of observation (30 days) although the drug has been shown to last up to 6 months once released into the vitreous. Nevertheless, we have shown that significant changes in hemorheological profile are observed in the first 30 days after intravitreal application of dexamethasone implant. Longer observation on a larger group is needed to confirm whether the effect of dexamethasone implant on the hemorheological parameters of the blood is sustained for a longer period of time, and how these parameters change when the effect of the drug subsides over time.
The beneficial clinical effect of dexamethasone in the form of a long-acting, biodegradable, intravitreal implant can be associated with a reduction in the thickness of the central retina and an improvement in retinal microcirculation flow associated with a reduction in erythrocyte disaggregation time.
Conclusions
The results of the study and their statistical analysis suggest that dexamethasone in the form of a long-acting, biodegradable intravitreal implant (Ozurdex) is effective and safe in the treatment of post-thrombotic lesions in RVO patients and significantly affects the amplitude of aggregation of erythrocytes, erythrocyte aggregation index and half-time reconstitution of aggregation of erythrocytes, directly interferes with RVO pathomechanism, which may be a key mechanism of action in RVO therapy. Clinical improvement (visual acuity, visual field) in RVO patients treated with the intravitreal implant of dexamethasone can be associated with a reduction in central retinal thickness and improvement in microcirculation (erythrocyte disaggregation).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Funding
This research received no external funding.
Disclosure
The authors declare that there is no conflict of interest.
References
1. Manabe K, Tsujikawa A, Osaka R., et al. Metamorphopsia associated with branch retinal vein occlusion. PLoS One. 2016;11(4):e0153817.
2. Diaw M, Pialoux V, Martin C, et al. Sickle cell trait worsens oxidative stress, abnormal blood rheology, and vascular dysfunction in type 2 diabetes. Diabetes Care. 2015;38(11):2120–2127.
3. Łapuciak S. Zakrzepy I Zatory. Warszawa: PZWL; 2002.
4. Pabisiak A, Bromboszcz J, Kmiec S, et al. Changes in the complete blood count and blood rheology in patients after myocardial infarction participating in the rehabilitation programme. Clin Hemorheol Microcirc. 2015;61(3):541–547.
5. Michalska-Malecka K, Slowinska-Lozynska L, Romaniuk W. Influence of rheological factors on the development of primary open angle glaucoma. Klin Oczna. 2012;114(2):135–137.
6. Michalska-Malecka K, Slowinska-Lozynska L. Aggregation and deformability of erythrocytes in primary open-angle glaucoma (POAG); the assessment of arterial hypertension. Clin Hemorheol Microcirc. 2012;51(4):277–285.
7. Kubicka-Trząska A, Romanowska-Dixon B. Diagnosis and management of retinal venous and arterial occlusive disease. Przew Lek. 2010;2010(2):159–164.
8. Humid S, Mirza A, Shokh I. Branch retinal vein occlusion. J Ayub Med College Abbotabad. 2008;20(2):128–132.
9. Noma H, Funatsu H, Mimura T, et al. Inflammatory factors in major and macular branch retinal vein occlusion. Ophthalmologica J Int D’ophtalmologie Int J Ophthalmol Zeitschrift Fur Augenheilkunde. 2012;227(3):146–152.
10. Noma H, Funatsu H, Harino S, et al. Vitreous inflammatory factors in macular edema with central retinal vein occlusion. Jpn J Ophthalmol. 2011;55(3):248–255.
11. Merkoudis N, Granstam E. Treatment of macular edema associated with retinal vein occlusion using sustained-release dexamethasone implants in a clinical setting. Eur J Ophthalmol. 2013;23(4):558–563.
12. Williamson TH, Rumley A, Lowe GD. Blood viscosity, coagulation, and activated protein C resistance in central retinal vein occlusion: a population controlled study. Br J Ophthalmol. 1996;80(3):203–208.
13. Michalska-Malecka K, Spiewak D, Slowinska-Lozynska L, et al. Influence of hemorheological factors on the development of retinal vein occlusion. Clin Hemorheol Microcirc. 2016;63(1):69–76.
14. Kubicka-Trząska A, Romanowska-Dixon B. Intravitreal dexamethasone implant (Ozurdex) in the treatment of chronic macular edema in patients with retinal vein occlusion. Kwartalnik Medyczny Okulistyka. 2012;2012:16–18.
15. Haller JA, Bandello F, Belfort R, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118(12):2453–2460.
16. Damasceno EF, Neto AM, Damasceno NA, et al. Branch retinal vein occlusion and anabolic steroids abuse in young bodybuilders. Acta Ophthalmol. 2009;87(5):580–581.
17. Kaiser PK. Steroids for branch retinal vein occlusion. Am J Ophthalmol. 2005;139(6):1095–1096.
18. Proenca Pina J, Turki K, Labreuche J, et al. Efficacy and safety in retinal vein occlusion treated with at least three consecutive intravitreal dexamethasone implants. J Ophthalmol. 2016;2016:6016491.
19. Kim M. Usefulness of anti-vascular endothelial growth factor combined with dexamethasone implant for retinal vein occlusion. Clin Interv Aging. 2016;11:1451–1453.
20. Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48.
21. Słowińska L, Monkos K. Kliniczne zastosowanie laserowo-optycznego rotacyjnego analizatora krwinek czerwonych LORCA. AnnAcadMedSiles. 2010;64(2):41–47.
22. Dobbe JG, Hardeman MR. Red blood cell aggregation as measured with the LORCA. Int j Artificial Organs. 2006;29(6):641–642.
23. Hardeman MR, Dobbe JG, Ince C. The laser-assisted optical rotational cell analyzer (LORCA) as red blood cell aggregometer. Clin Hemorheol Microcirc. 2001;25(1):1–11.
24. Antonova N. Methods in hemorheology and their clinical applications1. Clin Hemorheol Microcirc. 2016;64(4):509–515.
25. Tong JP, Lam DS, Chan WM, et al. Effects of triamcinolone on the expression of VEGF and PEDF in human retinal pigment epithelial and human umbilical vein endothelial cells. Mol Vis. 2006;12:1490–1495.
26. Ebrahem Q, Minamoto A, Hoppe G, et al. Triamcinolone acetonide inhibits IL-6- and VEGF-induced angiogenesis downstream of the IL-6 and VEGF receptors. Invest Ophthalmol Vis Sci. 2006;47(11):4935–4941.
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127(9):1101–1114.
28. Scott IU, VanVeldhuisen PC, Oden NL, et al. SCORE Study report 1: baseline associations between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology. 2009;116(3):504–512.
29. Chien S. Rheology in the microcirculation in normal and low flow states. Adv Shock Res. 1982;8:71–80.
30. Chabanel A, Glacet-Bernard A, Lelong F, et al. Increased red blood cell aggregation in retinal vein occlusion. Br J Haematol. 1990;75(1):127–131.
31. Lip PL, Blann AD, Hope-Ross M, et al. Age-related macular degeneration is associated with increased vascular endothelial growth factor, hemorheology and endothelial dysfunction. Ophthalmology. 2001;108(4):705–710.
32. Vekasi J, Marton ZS, Kesmarky G, et al. Hemorheological alterations in patients with diabetic retinopathy. Clin Hemorheol Microcirc. 2001;24(1):59–64.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.