Back to Journals » Drug Design, Development and Therapy » Volume 15
Effect of 2% Hyaluronic Acid on the Rate of Healing of Corneal Epithelial Defect After Pterygium Surgery: A Randomized Controlled Trial
Authors Chaidaroon W, Satayawut N, Tananuvat N
Received 31 August 2021
Accepted for publication 18 October 2021
Published 27 October 2021 Volume 2021:15 Pages 4435—4443
DOI https://doi.org/10.2147/DDDT.S336372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Winai Chaidaroon, Narudom Satayawut, Napaporn Tananuvat
Department of Ophthalmology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
Correspondence: Winai Chaidaroon Tel +66-53-935512
; +66-891415512
Fax +66-53-936121
Email [email protected]
Purpose: The study aimed to investigate the effects of 2% hyaluronic acid (HA) on corneal epithelial defect after pterygium surgery in comparison with the control group, measured in terms of the healing rate of corneal epithelial defect and pain score after surgery.
Methods: In this double-blind randomized clinical trial, fifty patients with primary pterygium were randomized into 2 groups: a control group or the group treated with a single topical application of 2% HA. Comprehensive ophthalmological examinations included measuring the area of corneal epithelium defect using ImageJ freeware and the pain score assessment after the operation.
Results: The mean and SD of the area of epithelial defect measured on postoperative Day 0, 1, and 2 were 10.89 ± 1.33 mm2, 5.04 ± 0.87mm2, and 2.44 ± 0.74 mm2 for the HA group, and 11.14 ± 1.11 mm2, 7.74 ± 1.17 mm2, and 5.31 ± 1.15 mm2 for the control group, respectively. While the initial area of the defect on Day 0 was essentially the same for both groups (p = 0.478), the area of the defect in the HA group was significantly smaller on both Day 1 and Day 2 (p < 0.001, p < 0.001), respectively. Similarly, the HA group exhibited a statistically significant higher rate of healing for the cornea epithelial defect over Day 0 and 1 compared to the control group (5.85 ± 0.89 mm2/day vs 3.14 ± 1.28 mm2/day, p < 0.001), respectively. The median (range) pain scores were evaluated at Day 0 was 7 (4– 10) in the HA group and 7 (3– 10) in the control group (p = 0.953). There was no statistically significant difference between two groups (p > 0.05) for Days 1, 2, and 3.
Conclusion: A single topical application of 2% HA tended to accelerate the healing process of corneal epithelium defect after pterygium surgery without any observable adverse effects during short-term follow-up.
Keywords: 2% hyaluronic acid, corneal epithelial defect, pterygium surgery
Introduction
Pterygium is an ocular surface disease characterized by a triangular-shaped fibrovascular neo-formation arising from the conjunctiva onto the cornea.1 A major risk factor for progression of pterygium is prolonged sunlight exposure, which damages the limbal stem cell barrier with subsequent conjunctivalization of the cornea.2,3 Pterygium may cause several ocular problems, including dryness, irritation, discomfort, foreign body sensation, ocular movement limitation, and decreased vision.4,5 Pterygium excision with superior conjunctival autografting is a widely accepted procedure as a gold standard treatment for pterygium.6 It is not only associated with a low recurrence rate but also without the risk of potentially serious complications associated with adjunctive therapy.7 However, postoperative corneal epithelial defects may cause pain, irritation, light sensitivity, and tearing. Rapid epithelial wound healing after conjunctival autograft transplantation with pterygium removal could reduce postoperative pain and the risk of corneal infection.
Hyaluronic acid (HA) is a linear glycosaminoglycan which consists of disaccharide units of glucuronic acid and N-acetyl-glucosamine.8 HA plays a role in cell proliferation and anti-inflammation, and exhibits antioxidant properties.8,9 HA has accelerated the wound healing by adhering to specific cell surface receptors. Cluster of differentiation 44 (CD 44), the receptor for hyaluronan-mediated motility, lymphatic vessel endothelial hyaluronan receptor 1, and the liver endothelial cell receptor are the main receptors in the repairing wound process.9 The ophthalmic applications of HA are established in diverse pharmaceutical preparations, such as artificial tears, eye drops, in situ forming hydrogels, modified nanoparticles, and intravitreal injections.10 HA eye drops hasten the corneal epithelialization in many clinical situations.11,12 Previous study showed that rate of corneal wound healing related to the concentration of HA.13 This study aims to investigate the data on the healing rate of corneal epithelial defect after pterygium operation receiving a single topical of 2% HA.
Materials and Methods
Study Design
A prospective, randomized, parallel group interventional study was performed among patients diagnosed with primary pterygium at the Ophthalmology Clinic, Chiang Mai University Hospital between May 2020 and December 2020. The study protocol was conducted in accordance with the tenets of the Declaration of Helsinki and approved by the Ethics committee of Faculty of Medicine, Chiang Mai University (Study code: OPT-2560-04526), and Thai Clinical Trial Registry Committee (Study ID 20180822002, https://www.thaiclinicaltrials.org/). All participants provided written informed consent prior to the study. The treatment outcome of a single intraoperative topical application of 2.0% HA (TRB CHEMEDICA AG, Visiol®, München, Germany) was investigated in terms of healing rate of corneal epithelial defect after pterygium surgery, in comparison with the control group (without HA). The study was divided into 4 visits for assessments, the first visit was immediately after operation, Day 0. Three follow-up visits were scheduled on Days 1, 2 and 3. Simultaneously, the level of postoperative pain was measured using the Wong-Baker FACES Pain Rating Scale.14
Study Population and Treatment
Patients aged between 20 years and 60 years diagnosed with unilateral primary pterygium were included in this study. Patients with recurrent pterygium, corneal abnormalities, limbal stem cell deficiency, corneal decompensation, ocular malignancy, ocular infections, glaucoma, concurrent ophthalmic drug use, and systemic diseases that interfere with corneal wound healing were excluded.
Participants were divided into 2 groups (the HA group and the control group), stratified by age (<50 or ≥50 years old) using permuted block of 4 randomization within strata methods as aging is a significant confounding factor for corneal wound healing. Then, the patients were randomized into either the HA or the control group using 1:1 block randomization methods.
The surgical technique of pterygium excision with superior conjunctival autografting involved transferring a free graft of superior bulbar conjunctiva to cover the sclera exposed by pterygium excision. All surgeries were performed by one surgeon (W.C.), with local anesthesia and an operating microscope.15 Neither retrobulbar nor eyelid block was used.
Surgical Procedure
- Two percent Lidocaine jelly (AstraZeneca, Xylocaine Jelly 2%®, Södertälje, Sweden) was applied to induce anesthesia of the conjunctiva and cornea 10 minutes before operation.
- A lid speculum was used to provide maximal exposure.
- The pterygium was injected with 1% lidocaine/1:100,000 adrenaline mixture (AstraZeneca, Xylocaine 1%®, Södertälje, Sweden).
- A disposable surgical blade was used to superficially excise involved cornea to the limbus at the head of pterygium. Westcott scissors were used to excise pterygium from surrounding conjunctiva. All fibrovascular pterygium was removed at the scleral bed and limbus. The remaining subtenon tissue was totally also removed.
- Minimal cautery was used to control bleeding.
- The patient was asked to look down in order to expose the superior conjunctiva. One percent lidocaine without adrenaline was injected subconjunctivally to separate the conjunctiva and tenon’s capsule adjacent to the limbus in the 6 o’clock position.
- Westcott scissors were used to excise a free conjunctival graft in the extract size of the scleral bed.
- The free graft was placed in the corrected orientation onto the scleral bed and was sutured with 8 to 9 interrupted 8–0 polyglactin.
- At the end of surgery, all epithelial defects caused by pterygium excision were stained by fluorescein paper strip (Chona Surgical Co., Fluorescein Sodium – Test Strip®, Delhi, India) in two groups.
In both groups, on postoperative Day 1, the patch was removed. A topical 0.5% levofloxacin (Santen, Cravit®, Osaka, Japan) eye drops and 1% prednisolone acetate (Allergan, Pred-Forte®, Westport, Ireland) eye drops were prescribed 4 times daily for 4 weeks. Sutures were allowed to absorb spontaneously without removal.
Study Material
Visiol® (2.0% HA, molecular weight 1.8 × 106 Da; TRB CHEMEDICA AG, München, Germany) was used to this study. It was a high concentrated isotonic viscoelastic gel indicated for use as a surgical aid in ocular surgery, such as cataract surgery and intraocular lens implantation. The compositions of this viscoelastic gel are 2% HA, disodium phosphate, sodium dihydrogen phosphate, mannitol, and water for injection.
The Evaluations of Corneal Epithelial Healing
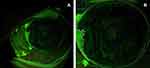
Immediately after the surgery, Day 0, the photos of fluorescein-stained epithelial defect with the cobalt blue exciter filter were taken using digital camera equipped with SL-D701 slit-lamp (Topcon Corporation, Topcon®, Tokyo, Japan). Then, a thin coat16 of 2% HA was applied topically to the stained-positive epithelial defect in the HA group but not in the control group by 1 investigator (N.S.). The eye was patched for 24 hours postoperatively.
All photos were saved as red green blue uncompressed Joint Photographic Experts Group (JPEG) files (2576 × 1934 pixels) using the IMAGEnet®6, version 3.0.1. They were analyzed by 1 investigator (N.T.) to determine the size of epithelial defects using the ImageJ freeware 1.53k (Figure 1).17 Patients were asked to visit for ophthalmic examination and daily photograph at a fixed time for three days. Postoperative treatments remained the same in two groups. The pain score using “Wong-Baker FACES Pain Rating Scale”,14 ranged from 0 to 10, were recorded daily for 4 days.
Statistical Analysis
According to the study of effect of 2% HA on the healing rate of corneal epithelial defect after pterygium surgery has not been reported, sample size of 50 eyes was hypothesized to provide sufficient power to demonstrate a difference between the HA group and the control group. All data had been analyzed with the SPSS 21.0 statistical software (SPSS Inc., Chicago, IL, USA). Mean (standard deviation (SD)), median (range), t test, Chi-square test, and Mann–Whitney U-test were conducted on the demographic and clinical characteristics of participants at baseline. Mann–Whitney U-test and t test were utilized to compare the pain score and the area of corneal epithelial defect between the two groups, respectively. A p-value of <0.05 was considered as a significant difference.
Result
The CONSORT flow diagram is shown in Figure 2. The study comprised 58 patients. Four patients declined to participate the protocol, three patients had diabetes, and one had a history of cataract extraction in the affected eye. A total of 50 patients were randomized to one of the two groups, yielding the HA group of 25 patients in total, and 25 patients in the control group.
 |
Figure 2 CONSORT flow diagram of a randomized controlled trial of the HA group versus the control group for patients who undergone pterygium excision. |
Baseline Characteristics
Baseline characteristics are summarized in Table 1. There were no significant differences between the baselines of age, sex, initial corneal epithelium defect, and pain score after operation (p > 0.05). Fifty patients, with 25 females and 25 males, were enrolled in the study.
 |
Table 1 Demographic and Clinical Characteristics of Patients |
Corneal Epithelium Healing
The corneal epithelium healing, in terms of area of corneal epithelial defect, was significantly different between two groups at Day 1 (P < 0.001) and Day 2 (P < 0.001). The HA group, area of epithelial defect by mean ± SD were 5.04 ± 0.87mm2, 2.44 ± 0.74 mm2, and 1.10 mm2 (according to 24 eyes were completely healed); and were 7.74 ± 1.17 mm2, 5.31 ± 1.15 mm2 and 1.80 ± 0.50 mm2 (according to 23 eyes were completely healed) for the control group at postoperative Days 1, 2 and, 3, respectively (Figure 3).
 |
Figure 3 Area of corneal epithelial defect at Days 0, 1, 2, and 3 between the HA and the control groups. Notes: □ = HA, ∆ = control. |
Figure 4 illustrates the healing rate of corneal epithelial defect. The mean ± SD of healing rate of corneal epithelial defect from Day 0 to Day 1 was statistically greater in the HA group compared to the control group (5.85 ± 0.89 mm2/day versus 3.14 ± 1.28 mm2/day, p < 0.001). But the healing rate of corneal epithelial defect from Day 1 to Day 2 showed no significant difference between the two groups (p < 0.394). On postoperative Day 3, 4% (1/25) of the patients in the HA group had small epithelial defect, and 8% (2/25) in the control group. All corneal epithelial defects in both groups had completely healed by Day 4.
 |
Figure 4 The healing rate of corneal epithelial defect at postoperative period between the HA and the control groups. Notes: □ = HA, ∆ = control. |
Pain Score
The median (range) pain scores in the HA and the control group at Days 0, 1, 2, and 3 are demonstrated in Table 2. However, there was no statistically significant difference between the two groups (p > 0.05).
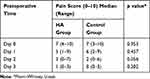 |
Table 2 Pain Scores Immediately After Operation (Day 0), Days 1, 2, and 3 Between the HA and the Control Groups |
Safety Profiles
No observed adverse effect was found in either the HA or the control groups.
Discussion
Corneal epithelium defect is a common lesion following the pterygium surgery. Treatments to promote wound healing could reduce the risk of developing complications, such as infective keratitis, corneal perforation, or corneal scar. Moreover, pain is one of the most common symptoms occurred after pterygium surgery. Other comorbidities such as photophobia, eye irritation, and tearing have also been observed in these patients. A variety of modalities for fostering re-epithelialization were proposed including mechanical treatment (eg, bandage contact lens, firm patching), pharmacological intervention (eg, growth factors, cytokines, HA), biological substances containing HA (eg, amniotic membrane, autologous serum).18
HA has been used to speed up re-epithelialization in many clinical and experimental situations. Turley et al indicated that HA could promote corneal epithelial wound healing in vitro, possibly via the CD44 receptor binding in the extracellular domain, resulting in the stimulation of aggregation, proliferation and migration of corneal epitheliums.19 In vivo models demonstrated the role of HA in promoting corneal wound healing through different mechanisms, including the generation of the provisional fibronectin-hyaluronate complexes,12,13 the suppression of stromal neutrophils, the enhancement of keratocyte repopulation, and the production of fibronectin.20,21 One clinical study showed that 0.3% HA in eye drops promoted human corneal epithelium wound healing caused by mechanical damage.11 Therefore, both in vitro and in vivo studies have given evidence of HA that accelerates corneal epithelial healing.
The previous study done by Camillieri et al showed that 0.2% and 0.4% HA were not different for the time needed to complete wound healing in rabbit eye model.21 Meanwhile, Nakamura et al found that the effects of HA on wound healing depended on HA concentrations in both diabetic and non-diabetic rats which was 0.1% and 0.3%, respectively. They concluded that HA accelerated the healing rate in a dose-dependent manner.13 However, these 2 studies revealed only low concentration of HA (less than 1%). One study that related to high dose of HA, Reed et al evaluated corneal epithelial healing after penetrating keratoplasty using between topical 1% HA (Kabi Pharmacia, Healon®, Uppsala, Sweden) and the balanced salt solution. They concluded that the group treated with 1% HA demonstrated a high correlation to more complete graft healing one week after operation.16 In this study, we topically applied high concentrated HA (2.0% HA), which had a double concentration compared to the previous study, once immediately after operation. This study also provided the first data of the healing rate of corneal epithelial defect using 2% HA after pterygium surgery. The corneal epithelium defect area was significantly smaller in the 2% HA group on Day 1 and Day 2. The healing rate of corneal epithelial defect from Day 0 to Day 1 also showed a statistically significant greater in the 2% HA group compared to the control group. Besides the HA itself, eye patching after operation might be one of cofactors to promote the corneal epithelial healing rate. These results supported Nakamura’s study. In addition, ultraviolet (UV) light is a widely accepted causative factor for pterygium. UV radiation may induce the production of reactive oxidative species (ROS).22 Mannitol contained in 2.0% HA that used in this study acts as a free radical scavenger, may decrease the degradation of HA long chains by ROS which may prolong the contact time between the HA and the eye.23
HA has a mucoadhesive effect, therefore it has a coatability effect on the cornea. Increasing the residual time in wound region depends on its concentration.24 However, high concentration of HA may increase patient discomfort because of lash crusting or vision decreasing25 but this adverse effect was not found in this study according to a single topical application and eye patching. However, this novel clinical application must be considered in any cost-effectiveness analysis. Pain scores exhibited no statistically significant difference between the two groups. Thus, the application of high concentration of HA did not cause any additional uncomfortable feeling in the eye.
In considering safety issues, there were no adverse effects related to the HA application. There were three eyes that needed 4 days to heal completely. Two cases in the control group had histories of severe eye rubbing. One case in the HA group was 54 years old and was the highest age among the patients studied. Age may cause a delayed wound healing. All patients had completely healed by Day 4. There were no complications, such as, recurrence, epithelial defect, or subconjunctival hemorrhage, seen in either group at the 3-month follow-up.
Instead of using subjective scale to measure the size of corneal epithelial defect, ImageJ freeware was introduced to determine the area of corneal epithelial defect after pterygium surgery. This provided precise measurements of the extent of the defect and minimized evaluator bias. In addition, a single topical application of 2% HA was more convenient for the patients compared to the daily use of lower concentrations of HA.
Study Limitations
First, this study had a limitation in the number of patients and had a short-time follow-up. A large-scale and longer follow-up may help to confirm the results. Second, the 2% HA containing mannitol may potentially effect on the speed of corneal epithelial healing. Third, this study may have a bias owing to the misinterpretation of pain score which may not represent the true eye pain. Fourth, the pressure related to eye patching at Day 0 may be different from patient to patient. The difference of pressure in the eye patching may effect eye blinking which interferes wound healing.
Conclusion
A single topical application of 2% HA tended to accelerate the healing process of corneal epithelial defect after pterygium surgery. No adverse effects were observed during short-term follow-up. High concentrated form did not cause any additional discomfort to the eye. However, the clinical applications of 2% HA in corneal epithelial defect after pterygium surgery required further study. In addition to the outcome of this study, cost-effectiveness analysis and patient compliance should also be considered before clinical use.
Data Sharing Statement
Datasets collected, used, and analyzed for the study can be obtained from the corresponding author on a reasonable request. Besides the study, no specific data is intended to share. No other study documents will be available.
Ethics Approval and Informed Consent
The study protocol was conducted in accordance with the tenets of the Declaration of Helsinki and approved by the Ethics committee of Faculty of Medicine, Chiang Mai University (Study code: OPT-2560-04526), and Thai Clinical Trial Registry Committee (Study ID 20180822002, https://www.thaiclinicaltrials.org/). Written informed consent was obtained from all participants.
Consent for Publication
All participants provided written informed consent prior to the study.
Acknowledgments
The authors thank associate professor Janejit Choovuthayakorn, M.D., PhD., and Miss Kittika Kanjanarattanakorn for kind assistance in statistical analysis and Dr. Robert Paul Batzinger, PhD., for English manuscript revision.
Funding
This study was supported by the Faculty of Medicine Endowment Fund, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (Grant Number 014/2561).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Sánchez-Thorin JC, Rocha G, Yelin JB. Meta-analysis on the recurrence rates after bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygium. Br J Ophthalmol. 1998;82(6):661–665. doi:10.1136/bjo.82.6.661
2. Dushku N, Reid TW. Immunohistochemical evidence that human pterygia originate from an invasion of vimentin-expressing altered limbal epithelial basal cells. Curr Eye Res. 1994;13(7):473–481. doi:10.3109/02713689408999878
3. Kwok LS, Coroneo MT. A model for pterygium formation. Cornea. 1994;13(3):219–224. doi:10.1097/00003226-199405000-00005
4. Ang M, Li X, Wong W, et al. Prevalence of and racial differences in pterygium: a multiethnic population study in Asians. Ophthalmology. 2012;119(8):1509–1515. doi:10.1016/j.ophtha.2012.02.009
5. Li L, Zhong H, Tian E, et al. Five-year incidence and predictors for pterygium in a rural community in China: the Yunnan minority eye study. Cornea. 2015;34(12):1564–1568. doi:10.1097/ICO.0000000000000637
6. Janson BJ, Sikder S. Surgical management of pterygium. Ocul Surf. 2014;12(2):112–119. doi:10.1016/j.jtos.2014.01.001
7. Kenyon KR, Wagoner MD, Hettinger ME. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 1985;92(11):1461–1470. doi:10.1016/s0161-6420(85)33831-9
8. Wu H, Zhang H, Wang C, et al. Genoprotective effect of hyaluronic acid against benzalkonium chloride-induced DNA damage in human corneal epithelial cells. Mol Vis. 2011;17:3364–3370.
9. Kim DJ, Jung MY, Pak HJ, et al. Development of a novel hyaluronic acid membrane for the treatment of ocular surface diseases. Sci Rep. 2021;11(1):2351. doi:10.1038/s41598-021-81983-1
10. Chang WH, Liu PY, Lin MH, et al. Applications of hyaluronic acid in ophthalmology and contact lenses. Molecules. 2021;26(9):2485. doi:10.3390/molecules26092485
11. Lin T, Gong L. Sodium hyaluronate eye drops treatment for superficial corneal abrasion caused by mechanical damage: a randomized clinical trial in the People’s Republic of China. Drug Des Devel Ther. 2015;9:687–694. doi:10.2147/DDDT.S77270
12. Ho WT, Chiang TH, Chang SW, Chen YH, Hu FR, Wang IJ. Enhanced corneal wound healing with hyaluronic acid and high-potassium artificial tears. Clin Exp Optom. 2013;96(6):536–541. doi:10.1111/cxo.12073
13. Nakamura M, Sato N, Chikama TI, Hasegawa Y, Nishida T. Hyaluronan facilitates corneal epithelial wound healing in diabetic rats. Exp Eye Res. 1997;64(6):1043–1050. doi:10.1006/exer.1997.0302
14. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker faces pain rating scale in pediatric emergency department patients. Acad Emerg Med. 2010;17(1):50–54. doi:10.1111/cxo.12073
15. Chaidaroon W, Wattananikorn S. Conjunctival autograft transplantation for primary pterygium. J Med Assoc Thai. 2003;86(2):111–115.
16. Reed DB, Mannis MJ, Hills JF, Johnson CA. Corneal epithelial healing after penetrating keratoplasty using topical Healon versus balanced salt solution. Ophthalmic Surg. 1987;18(7):525–528.
17. ImageJ. Image processing and analysis in Java. Available from: https://imagej.nih.gov/ij/.
18. Jullienne R, Garcin T, Crouzet E, et al. Evaluation of corneal epithelial wound healing after penetrating keratoplasty in patients receiving a new matrix therapy agent (regenerating agent). Eur J Ophthalmol. 2020;30(1):119–124. doi:10.1177/1120672118808971
19. Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277(7):4589–4592. doi:10.1074/jbc.R100038200
20. Yang G, Espandar L, Mamalis N, Prestwich GD. A cross-linked hyaluronan gel accelerates healing of corneal epithelial abrasion and alkali burn injuries in rabbits. Vet Ophthalmol. 2010;13(3):144–150. doi:10.1111/j.1463-5224.2010.00771.x
21. Camillieri G, Bucolo C, Rossi S, Drago F. Hyaluronan-induced stimulation of corneal wound healing is a pure pharmacological effect. J Ocul Pharmacol Ther. 2004;20(6):548–553. doi:10.1089/jop.2004.20.548
22. Kau HC, Tsai CC, Lee CF, et al. Increased oxidative DNA damage, 8-hydroxydeoxy- guanosine, in human pterygium. Eye (Lond). 2006;20(7):826–831. doi:10.1038/sj.eye.6702064
23. Mendoza G, Alvarez AI, Pulido MM, et al. Inhibitory effects of different antioxidants on hyaluronan depolymerization. Carbohydr Res. 2007;342(1):96–102. doi:10.1016/j.carres.2006.10.027
24. Saettone MF, Monti D, Torracca MT, Chetoni P. Mucoadhesive ophthalmic vehicles: evaluation of polymeric low-viscosity formulations. J Ocul Pharmacol. 1994;10(1):83–92. doi:10.1089/jop.1994.10.83
25. Ridder WH
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.