Back to Journals » Journal of Pain Research » Volume 11
ED50 of intrathecal ropivacaine for cesarean delivery with and without epidural volume extension with normal saline: a randomized controlled study.
Received 15 May 2018
Accepted for publication 22 August 2018
Published 8 November 2018 Volume 2018:11 Pages 2791—2796
DOI https://doi.org/10.2147/JPR.S174176
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
M Lv,1 P Zhang,2 Z Wang3
1Department of Obestetrics and Gynecology, Affiliated Women and Children’s Hospital of Jiaxing University, Jiaxing, China; 2Department of Anesthesiology, Affiliated Women and Children’s Hospital of Jiaxing University, Jiaxing, China; 3Institute of Clinical Research, Jiaxing university, Jiaxing, China
Background: It was reported that epidural volume extension could decrease the ED50 of intrathecal plain bupivacaine. In this study, we investigated the ED50 of intrathecal hyperbaric ropivacaine followed by epidural normal saline bolus for cesarean section.
Methods: Sixty parturients were allocated into two groups in this prospective study. About 10 mL of epidural normal saline was given after the intrathecal dose of hyperbaric ropivacaine in the Group S (normal saline group), and no epidural injection of normal saline was given after the intrathecal ropivacainve injection in the Group C (control group). The dose of intrathecal ropivacaine for each parturient was decided by up-down allocation method. The initial dose was set as 10 mg. Effective anesthesia was defined as the level of T6 or above achieved within 10 minutes after intrathecal injection and no additional epidural drug to complete operation. The Massey formula was applied to calculate the ED50 of intrathecal ropivacaine.
Results: The ED50 of intrathecal ropivacaine for cesarean section determined by up-and-down method was 7.51 mg (95% CI, 7.09–7.93 mg) in the Group S and 8.29 mg (95% CI, 7.73–8.85 mg) in the Group C, and there was a significant difference in ED50 of ropivacaine between the two groups (P<0.05). Compared with the Group C, the ED50 of intrathecal ropivacaine decreased when followed by epidural normal saline bolus.
Conclusion: The ED50 of intrathecal hyperbaric ropivacaine for cesarean section is 8.29 mg, and it is reduced when followed by epidural normal saline bolus (www.chictr.org.cn, registration number: ChiCTR-ROC-17013382).
Keywords: ropivacaine, cesarean section, combined spinal–epidural anesthesia, median effective dose
Introduction
Neuraxial anesthesia has been used widely for cesarean section. Small dose of intrathecal local anesthetic was advocated, accompanied with low incidence of hypotension,1–3 but it possibly increased the failures of spinal anesthesia. It was reported that epidural volume extension could increase the extent of sensory blockade from spinal bupivacaine,4–6 but some documents reported that epidural volume extension had no effect on the profile of spinal anesthesia using hyperbaric bupivacaine.7–10 It is uncertain whether epidural volume extension decreased the dose requirement of spinal bupivacaine during combined spinal epidural anesthesia.11 Ropivacaine administrated intrathecally has a slower onset of sensory block and motor block compared to hyperbaric bupivacaine.12 Therefore, we hypothesized that the median effective dose (ED50) of intrathecal hyperbaric ropivacaine followed by epidural normal saline bolus for cesarean section would decrease. To verify our hypothesis, we investigated the ED50 of intrathecal hyperbaric ropivacaine followed immediately by epidural normal saline bolus in parturients undergoing elective cesarean section using an up-down sequential allocation method.
Methods
The study was approved by the Ethical Committee of Jiaxing University (Chairman Prof Yin) and written informed consent was provided by all parturients. From November 2017 to February 2018, 60 full-term parturients undergoing elective cesarean delivery were eligible for this randomized, double-blind, prospective study. Exclusion criteria were patients with scarred uterus, body weight >85 kg, height <150 or >170 cm, Amerian Society of Anesthesiogists > II, multiple pregnancies, and contraindication to combined spinal-epidural anesthesia. All parturients were randomly divided into the control group (Group C) and the normal saline group (Group S), based on a computer-generated random number code.
All parturients had no premedication. On arrival in the operating center, standard monitoring, including electrocardiogram, noninvasive blood pressure (BP), heart rate (HR), and pulse oximetry (SpO2), was applied and venous access was established. Ringer’s solution was infused at a rate of 10 mL kg−1 h−1 during the study period.
With the parturients in the left lateral position, an 18-G epidural needle was inserted into the epidural space at the third and fourth lumbar vertebral interspace and then a 27-G spinal needle (Sujia Medical Company, China) was inserted into the intrathecal space via the epidural needle. When cerebrospinal fluid (CSF) was aspirated, the premixed solution (total volume 2.5 mL of hyperbaric ropivacaine in all cases) was injected over 10 seconds with the needle orifice directed cephalad. After the spinal needle was withdrawn, 10 mL of epidural normal saline was given over 10 seconds through the epidural needle with the needle orifice directed cephalad immediately after intrathecal injection, but no epidural injection of normal saline was given after the intrathecal ropivacaine injection in the Group C, then epidural catheter was threaded 3–5 cm into the epidural space. The patient was then rapidly turned to supine position.
The ED50 of intrathecal ropivacaine was determined through the up-and-down allocation method. The initial dose of intrathecal ropivacaine was 10 mg for the first patient in every group according to clinical experiment. The dose of intrathecal ropivacaine for the next one was determined by anesthetic effect of the last patient in each group. If spinal anesthesia of the previous patient was effective, the dose of intrathecal ropivacaine for the next one was decreased by 1 mg gradient. If spinal anesthesia of the last patient was ineffective, the dose of intrathecal ropivacaine for the next one was increased by 1 mg. The operation was performed after T6 sensory block was achieved. 10U oxytocin was administered by intramuscular injection in uterus after delivery.
In the previous studies, an effective spinal anesthesia was defined as T6 sensory block level was achieved by pinprick in the middle of body within 15 minutes after the intrathecal injection according to previous studies without additional epidural drug.13–15 In our study, the effective spinal anesthesia was defined as a bilateral T6 sensory block level of the spinal anesthesia that was achieved by pinprick using 7-G needle within 10 minutes after inducing spinal anesthesia and no additional epidural drug or venous analgesic was administrated to complete operation. About 10–15 mL of 2% epidural lidocaine was administrated to complete the operation if a bilateral T6 sensory block level was not achieved or duration of anesthesia was not enough to complete the surgery. If the systolic BP was below 80 mmHg, a bolus of 50 µg phenylephrine was administered intravenously. If the HR was <60 beats/min, 0.3–0.5 mg atropine was administered intravenously.
Measurements
The primary outcome was the anesthetic effect of spinal anesthesia. The secondary outcomes were the side effects of spinal anesthesia. BP and HR were monitored at 5-minute intervals until the end of the surgery. The maximum of sensory block level, the onset time of sensory block or motor, and the duration of spinal anesthesia were recorded. The onset time of sensory block was defined as the time from intrathecal injection to T6 sensory block level achieved, and the duration of sensory block was defined as the time from intrathecal injection to the recovery of T12 sensory block level. The duration of spinal anesthesia was defined as the period from intrathecal injection to the recovery of T10 sensory block level or time of incision pain. Motor block was assessed using a Bromage score16 (0=no motor loss, 1=inability to flex hip, 2=inability to flex hip and knee, 3=inability to flex hip, knee, and ankle). The onset of motor block was defined as the time from intrathecal injection to Bromage 1 block. Motor block regression was defined as the Bromage score was 0.
Side effects of spinal anesthesia (hypotension, bradycardia, urinary retention, shivering, nausea, and vomiting) were recorded. Hypotension was defined as systolic BP was <80 mmHg and bradycardia was defined as HR was <60 beats/min. Urinary retention was defined as urine volumes >50 mL by ultrasound. The neonatal Apgar score at the first and fifth minute was assessed, and umbilical artery pH was measured immediately after delivery.
Statistical analysis
According to the analogous study13–15 and our clinical experience, 26 patients were demanded in every group in the present study, because sample sizes were regarded as adequate when six pairs of reversal of sequences were achieved. Demographic data were presented as number or mean ± SD. Means were analyzed using Student’s t-test and counts were analyzed by Fisher’s exact test. Statistical analysis was performed with SPASS 19.0 software. A P-value <0.05 was considered statistically significant.
Results
The consort diagram of the study is showed in Figure 1. Sixty-two patients were assessed for eligibility (two patients refused to participate), then 60 patients were enrolled in this study. There were no significant differences in the data of parturient women between the two groups (Table 1). The Apgar scores and umbilical artery pH immediately after delivery were similar between the two groups.
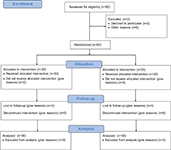  | Figure 1 Flow diagram of study. |
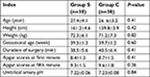  | Table 1 Data of the parturients Notes: Group S is the normal saline group, Group C is the control group. Data are presented as mean ± SD. |
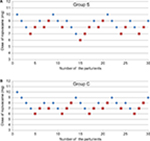
The ED50 of hyperbaric ropivacaine determined by up-and-down method was 7.51 mg (95% CI, 7.09–7.93 mg) in the Group S and 8.29 mg (95% CI, 7.73–8.85 mg) in the Group C. There was a significant difference in the ED50 of ropivacaine between the two groups (P<0.05). “” represents an effective spinal anesthesia and “” represents an ineffective spinal anesthesia (Figure 2).
Characteristics of effective spinal anesthesia were presented in Table 2. There were significant differences in the maximum sensory block level and onset of sensory block between the two groups (6.7±1.2 vs 7.6±1.4, P<0.05). The duration of spinal anesthesia was also similar between the two groups (58.2±13 vs 55.3±14, P>0.05). There were significant differences in the onset of motor block between the two groups (P>0.05).
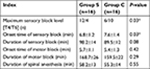  | Table 2 Characteristic of effective spinal anesthesia Notes: Group S is the normal saline group, Group C is the control group. Data are presented as mean ± SD or median. *P<0.05. |
The side effects of spinal anesthesia, such as hypotension, shivering, nausea, and vomiting were shown in Table 3. There were no significant differences in the side effects between the two groups (P>0.05).
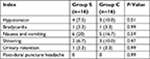  | Table 3 Side effects of effective anesthesia Notes: Group S is the normal saline group, Group C is the control group. Data are presented as number (%). |
Discussion
We found that the ED50 of intrathecal hyperbaric ropivacaine for cesarean section with or without following epidural normal saline bolus were 7.51 mg (95% CI, 7.09–7.93 mg) and 8.29 mg (95% CI, 7.73–8.85 mg), respectively. Our study also indicated that the ED50 of intrathecal hyperbaric ropivacaine decreased by 11% when followed by epidural normal saline bolos in cesarean section.
Our study verified that the ED50 of intrathecal ropivacaine decreased by 11% under epidural normal saline bolus using up-and-down allocated method. Up-and-down sequential method is a common method to decide the ED50 of drugs and saves ample size in trials.17 The studies of the ED50 of intrathecal ropivacaine followed by epidural volume extension were few in obstetric patients. So we investigated the ED50 of intrathecal ropivacaine for cesarean section followed by epidural volume extension.
Our study found that the onset of sensory block was shorter and that the maximum level of sensory block was greater when epidural normal saline bolus was administrated in the Group S than that in the Group C. The reason could be as following: epidural normal saline bolus brought about an increase of vertebral epidural cavity pressure. Changes of epidural cavity pressure would result in the redistribution of CSF between the cranial cavity and vertebral canal. Therefore, CSF transferred from the vertebral canal to the cranial direction with epidural normal saline bolus. At the same time, a greater amount of ropivacaine may shift in the cranial direction, and it improves the uppermost extent of sensory block. Our results were similar with many studies on the onset and maximum level of sensory block when epidural normal saline was administrated. It was reported that epidural volume extension led to an increase in the extent of sensory block,4,5 which was in agreement with our study. Epidural normal saline bolus brought about a decrease in ED50 of intrathecal ropivacaine or hastened the spread of intrathecal ropivacaine in our study. However, another study reported that epidural volume extension did not decrease the dose of intrathecal hyperbaric bupivacaine or raise the level of sensory block when hyperbaric bupivacaine is used for spinal anesthesia.18 The reason was probably as following: first, spinal anesthesia was performed in the left lateral position in the present study, not in the sitting position, as lumbar epidural normal saline flows into sacral canal during spinal anesthesia in the sitting position because of gravity. Second, the block level for surgery was different. The block level of T6 was achieved in obstetric patients, but the block level of T10 was achieved in lower limb orthopedic surgery. Lastly, the intrathecal anesthetic in our study was different from their studies. Epidural volume extension could not hasten the onset of motor block in cesarean section in the present study. Lew et al19 reported that combined spinal–epidural anesthesia using epidural volume extension hastened motor recovery after elective cesarean delivery, but the onset of motor block was not hastened in our study, it was in relation to the extent of intrathecal hyperbaric ropivacaine under epidural volume extension. As there were some difficulties in the assessment of motor recovery, we did not observe the accurate time of the motor recovery in the present study.
Limitations
Many factors may play a role on the results of experiment, for example, height, abdominal perimeter, vertebral length, and operative time. Moreover, the ED50 of spinal ropivacaine was calculated by a formula.
Conclusion
The ED50 of intrathecal hyperbaric ropivacaine for cesarean section is 8.29 mg, and it is reduced further by epidural normal saline bolus.
Acknowledgment
We thank all the colleagues in the Department of Anesthesiology in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Kang FC, Tsai YC, Chang PJ, Chen TY. Subarachnoid fentanyl with diluted small-dose bupivacaine for cesarean section delivery. Acta Anaesthesiol Sin. 1998;36(4):207–214. | ||
Ben-David B, Miller G, Gavriel R, Gurevitch A. Low-dose bupivacaine-fentanyl spinal anesthesia for cesarean delivery. Reg Anesth Pain Med. 2000;25(3):235–239. | ||
Sarvela J, Halonen P, Soikkeli A, Korttila K. A double-blinded, randomized comparison of intrathecal and epidural morphine for elective cesarean delivery. Anesth Analg. 2002;95(2):436–440. | ||
Salman C, Kayacan N, Ertuğrul F, Bıgat Z, Karslı B. Combined spinal-epidural anesthesia with epidural volume extension causes a higher level of block than single-shot spinal anesthesia. Braz J Anesthesiol. 2013;63(3):267–272. | ||
Takiguchi T, Okano T, Egawa H, Okubo Y, Saito K, Kitajima T. The effect of epidural saline injection on analgesic level during combined spinal and epidural anesthesia assessed clinically and myelographically. Anesth Analg. 1997;85(5):1097–1100. | ||
Mcnaught AF, Stocks GM. Epidural volume extension and low-dose sequential combined spinal-epidural blockade: two ways to reduce spinal dose requirement for caesarean section. Int J Obstet Anesth. 2007;16(4):346–353. | ||
Tyagi A, Girotra G, Kumar A, Kumar S, Sethi AK, Mohta M. Single-shot spinal anaesthesia, combined spinal-epidural and epidural volume extension for elective caesarean section: a randomized comparison. Int J Obstet Anesth. 2009;18(3):231–236. | ||
Kucukguclu S, Unlugenc H, Gunenc F, et al. The influence of epidural volume extension on spinal block with hyperbaric or plain bupivacaine for Caesarean delivery. Eur J Anaesthesiol. 2008;25(4):307–313. | ||
Loubert C, O’Brien PJ, Fernando R, et al. Epidural volume extension in combined spinal epidural anaesthesia for elective caesarean section: a randomised controlled trial. Anaesthesia. 2011;66(5):341–347. | ||
Beale N, Evans B, Plaat F, Columb MO, Lyons G, Stocks GM. Effect of epidural volume extension on dose requirement of intrathecal hyperbaric bupivacaine at Caesarean section. Br J Anaesth. 2005;95(4):500–503. | ||
Heesen M, Weibel S, Klimek M, Rossaint R, Arends LR, Kranke P. Effects of epidural volume extension by saline injection on the efficacy and safety of intrathecal local anaesthetics: systematic review with meta-analysis, meta-regression and trial sequential analysis. Anaesthesia. 2017;72(11):1398–1411. | ||
Dar FA, Mushtaq MB, Khan UM. Hyperbaric spinal ropivacaine in lower limb and hip surgery: A comparison with hyperbaric bupivacaine. J Anaesthesiol Clin Pharmacol. 2015;31(4):466–470. | ||
Carvalho B, Durbin M, Drover DR, Cohen SE, Ginosar Y, Riley ET. The ED50 and ED95 of intrathecal isobaric bupivacaine with opioids for cesarean delivery. Anesthesiology. 2005;103(3):606–612. | ||
Ginosar Y, Mirikatani E, Drover DR, Cohen SE, Riley ET. ED50 and ED95 of intrathecal hyperbaric bupivacaine coadministered with opioids for cesarean delivery. Anesthesiology. 2004;100(3):676–682. | ||
Tyagi A, Kakkar A, Kumar S, Sethi AK, Salhotra R. ED50 of hyperbaric bupivacaine with fentanyl for cesarean delivery under combined spinal epidural in normotensive and preeclamptic patients. Reg Anesth Pain Med. 2012;37(1):40–44. | ||
Xu H, Li H, Zuo Y, et al. A multicenter study of the analgesic effects of epidural chloroprocaine after lower limb orthopedic surgery. J Clin Anesth. 2016;35:313–320. | ||
Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15(1):47–50. | ||
Tyagi A, Kumar A, Sethi AK, Mohta M. Epidural volume extension and intrathecal dose requirement: plain versus hyperbaric bupivacaine. Anesth Analg. 2008;107(1):333–338. | ||
Lew E, Yeo SW, Thomas E. Combined spinal-epidural anesthesia using epidural volume extension leads to faster motor recovery after elective cesarean delivery: a prospective, randomized, double-blind study. Anesth Analg. 2004;98(3):810–814. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.