Back to Journals » Hepatic Medicine: Evidence and Research » Volume 9
Economic impact of the use of rifaximin 550 mg twice daily for the treatment of overt hepatic encephalopathy in Italy
Authors Roggeri DP, Roggeri A
Received 14 July 2017
Accepted for publication 6 September 2017
Published 25 September 2017 Volume 2017:9 Pages 37—43
DOI https://doi.org/10.2147/HMER.S146438
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Daniela Paola Roggeri, Alessandro Roggeri
ProCure Solutions, Nembro, Bergamo, Italy
Purpose: Hepatic encephalopathy (HE) is associated with a reduced survival, an increased risk of hospitalization for recurrences, and a reduced health-related quality of life. The purpose of the present economic analysis was to evaluate the impact on the Italian National Health Service (INHS) expenditure of the treatment with rifaximin 550 mg twice daily (Tixteller®/Tixtar®) for the reduction of the recurrences of overt HE, with respect to the current treatment approach.
Patients and methods: Costs associated with patients treated with rifaximin 550 mg twice daily were estimated considering the reduction in hospitalizations for HE recurrences revealed by registrative clinical trial (−50%) applied to the hospitalization rate (42.5%) emerging from an Italian observational real-world study; costs associated with patients not treated with rifaximin were estimated based on the hospitalization rate, resulting from the same Italian observational study. Sensitivity analyses considering possible different discount levels to INHS structures for rifaximin were performed. The INHS perspective for a period of 3 years was considered.
Results: The treatment with rifaximin 550 mg twice daily, although increasing drug costs, is associated with a reduction in hospitalizations for HE recurrences that leads to an overall reduction of total costs charged to INHS, which could be estimated, based on the forecasted uptake of the treatment, at about €130,000 in the first year, reaching ~€260,000 in the third year. Considering a possible discount for rifaximin 550 mg to INHS structure of 20%, the total saving at the third year accounts for ~€3,000,000. Moreover, a relevant reduction in the number of hospitalizations and bed days is associated with rifaximin treatment.
Conclusion: The treatment with rifaximin 550 mg twice daily, even if associated with an increase in drug expenditure, results in a reduction in total health care costs charged to INHS due to a reduction in hospitalizations for HE recurrences.
Keywords: overt hepatic encephalopathy recurrences, health care expenditure, costs, hospitalizations, budget impact
Introduction
The term “hepatic encephalopathy” (HE) comprises a wide spectrum of neurological or psychiatric abnormalities in patients with liver disease commonly associated with hepatic cirrhosis.1 Symptoms range from mild neurological abnormalities (ie, mood swings and changes in reaction times during daily activities, such as driving) to serious neurological damage (ie, difficulty moving and communicating) and, in extreme cases, coma.1–4 Approximately 70% of patients with cirrhosis have subclinical or mild HE; 23%–40% of cases progress to more severe forms of the disease.5–7 The development of HE is a predictor of reduced survival in patients with cirrhosis8–10 and of an increased risk of hospitalization.11–13 Furthermore, a significant consequence of HE is a lower health-related quality of life both physically and mentally,14–17 and it also has a substantial impact on caregivers.14 A number of studies have demonstrated how the onset of liver diseases, in particular HE, is associated with a significant and increasing consumption of health care resources and an increase in relative costs.13,18–20
Thus far, no studies have been carried out with the specific aim of determining the prevalence of HE in patients with cirrhosis in Italy. Data published by the Italian Ministry of Health21 reveal that in Italy in 2001 the hospital discharge records for 8,089 patients indicated HE in the diagnosis. This figure is confirmed by the extrapolation, to a national level, of data collected during an Italian real-world evidence survey13 conducted by CINECA based on data provided by the ARNO Observatory. This estimated that, considering the incidence of hospital admissions for HE in 2011 (0.014%) in the observed population (in various Italian regions), a total of 8,550 patients were affected throughout Italy.
Currently, the only pharmacological treatment specifically authorized in Italy for the reduction of overt HE recurrences is rifaximin at a dosage of 550 mg twice daily (Tixteller®/Tixtar®; Alfa Wassermann SpA, Alanno, Italy).
The purpose of health sector financial assessments is to satisfy the need to rationalize the allocation and use of the financial resources available, providing the decision-maker with objectifiable evaluation criteria (depending on the information available and its reliability), which can justify the therapeutic choices made; in particular, the budget impact analysis (BIA) is aimed at estimating, in the short term (1–3 years), the financial consequences of adopting and distributing a new health-related technology (ie, a drug) in a specific territorial area (using, as far as possible, outcome and cost data related to and collected in that area).
The aim of this economic model (developed with Microsoft® Excel for Mac, 2011) was to evaluate the impact of the costs associated with using rifaximin 550 mg twice daily to reduce the recurrences of episodes of overt HE with respect to the current treatment approach on the Italian National Health Service (INHS) expense budget.
Materials and methods
In order to evaluate the impact of the reimbursement cost of rifaximin 550 mg twice daily on the INHS expense budget, the current costs of outpatient treatment (tests and medical consultations), drugs, and hospitalizations for patients with overt HE were compared with a possible future scenario whereby a proportion of the patients previously hospitalized for HE are treated with rifaximin 550 mg twice daily.
Assumption of the BIA
The estimated reduction in the number of hospitalizations due to HE was taken from the rifaximin 550 mg registrative study12 and applied to this group. The present economic evaluation was conducted from an INHS perspective and covered a time horizon of 3 years.
The distinction between the two hypothetical scenarios considered relates to the possibility, through the use of rifaximin 550 mg twice daily, of reducing the probability of HE recurrences and, in particular, of reducing recurrences that require hospitalization.12
Sources of clinical efficacy of rifaximin 550 mg
In a 6-month, randomized, double-blind, placebo-controlled study, rifaximin 550 mg twice daily was the only treatment that was shown to bring about a reduction in HE recurrences and hospitalizations versus placebo.12 In particular, rifaximin 550 mg twice daily, in combination with lactulose, significantly reduced (p<0.001) the risk of overt HE recurrences (−58%; hazard ratio [HR] =0.42) and (p=0.01) the risk of HE hospitalizations (−50%; HR =0.50) in comparison with placebo + lactulose over a 6-month period. The incidence of adverse and severe adverse occurrences during the study was comparable between the group undergoing active treatment and the placebo group. Furthermore, it was demonstrated that long-term treatment with rifaximin 550 mg twice daily (over 24 months)22 sustains the results in terms of continuous protection from HE recurrences and maintains the rate of reduction in hospitalizations due to HE recurrences without any compromise in patient safety. Conservatively, only the differences in hospitalizations due to HE recurrences (HR =0.50) were assessed in financial terms during this analysis and not the reduction in HE recurrences not requiring hospitalization.12
Source of Italian data on hospitalizations due to HE recurrences and related costs
In order to evaluate the impact of the reduction in episodes of HE recurrences resulting from rifaximin 550 mg treatment, the frequency of hospitalizations due to HE recurrences in Italy and the related costs were taken from the study of Roggeri et al.13 This retrospective, observational study analyzed the data of ~3,000,000 INHS patients nationally, whose records are held on the ARNO Observatory database (http://arno.cineca.it/portal/). From this sample, 381 patients who were hospitalized due to HE during the period from January 1, 2011, to December 31, 2011, were selected. These patients were observed during the year following the index event to evaluate the consumption of health service resources and INHS costs (ie, drugs, outpatient diagnostic procedures and specialized outpatient services, and hospitalizations). The percentage of patients who died during the index hospitalization was 21.5% (n=82), 5.8% (n=22) of patients died during the follow-up period, and the survival rate at the end of the observation period was 72.7% (n=277). Of those patients surviving during the index hospitalization (n=299), 42.5% had at least one episode of HE recurrence which led to hospitalization during the follow-up; of those patients with at least one recurrence, the mean number of hospitalizations was 2.13 hospitalizations/year. The percentage of patients who did not suffer any recurrences leading to hospitalization during the follow-up period was 57.5%. The mean hospitalization duration due to HE recurrences observed by this study was 9.67 days.
From a cost standpoint, the annual costs associated with patients not requiring hospitalization due to HE recurrences during the observation period were significantly lower (€12,098/patient/year) in comparison with patients who were hospitalized due to an HE recurrence (€21,272/patient/year).13
Pharmacological costs in the two scenarios and expected number of patients treated with rifaximin 550 mg
From a pharmacological cost standpoint, considering that in Italy there are no other pharmacological treatments approved for the reduction of HE recurrences and that, in the rifaximin 550 mg twice daily registrative study,12 the majority of patients were treated with rifaximin in combination with lactulose (~91% of patients also took lactulose in both arms of treatment), in the current scenario, no additional pharmacological costs were considered, whereas in the scenario with progressive rifaximin uptake, the additional rifaximin cost was considered. This approach also derived from the fact that the costs associated with the pharmacological treatment of patients with HE are taken from a real-world evidence study in which lactulose was already part of the current pattern of treatment in Italy in the majority of patients13 and is also recommended for the management of HE and indicated as standard of care by Italian Guidelines.21
In order to evaluate the pharmacological costs resulting from rifaximin 550 mg twice daily treatment, the ex-factory reimbursement price charged to INHS, net of price reductions stipulated by law and of selective reductions for rifaximin, was considered; furthermore, sensitivity analyses assuming different price levels were conducted to reflect the possible discounts negotiated and reserved to INHS structures reported in the Gazzetta Ufficiale (Official Gazette of the Italian Republic).23
The price considered for rifaximin 550 mg twice daily, which is, as mentioned previously, the ex-factory price net of provisional reductions stipulated by law and of selective reductions for rifaximin (ie, the cost actually borne by the INHS), was €5.23/day.
Therefore, to evaluate the impact of the cost of rifaximin 550 mg twice daily on the INHS, the 50% reduction in the number of hospitalizations due to HE recurrences as a result of the pharmacological treatment12 was applied to the actual rate of hospitalizations due to HE recurrences observed in the abovementioned Italian observational study on the untreated portion of the population (47.5%)13 and to the relative costs associated with patients with or without hospitalization due to HE recurrences, as well as to the drug costs.
It is expected that during the first year 3,500 patients will be treated with the new medicine, and this number will increase to 5,000 in the second year and to 7,000 in the third.
Results
Overall, considering the rifaximin 550 mg twice daily uptake in terms of patients per year, its use could lead to a national reduction in hospitalizations due to HE recurrences of 743 in the first year, 1,062 in the second, and 1,487 in the third, as well as a reduction in the number of hospitalization days required (Table 1).
On the basis of the hospitalization rate due to HE recurrences and the observed costs for patients with and without recurrences,13 the current mean annual cost borne by the INHS (the current scenario) weighted by the percentage of patients with and without recurrences is €15,995 (including hospitalizations, diagnostic procedures and visits, and drugs); similarly, considering a 50% reduction in hospitalizations due to HE recurrences (Bass et al12), the weighted mean annual cost per patient treated with rifaximin 550 mg twice daily would be €14,046 (including hospitalizations, diagnostic procedures and visits, and drugs excluding rifaximin), to which, for the purpose of this analysis, the cost of the rifaximin 550 mg twice daily treatment must be added, which amounts to €1,910/patient/year, resulting in a total cost of €15,957 (Table 2).
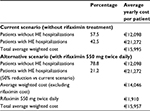  | Table 2 Current scenario versus alternative scenario: average yearly cost per patient weighted by the percentage of patients with and without recurrences Abbreviation: HE, hepatic encephalopathy. |
As Table 3 clearly shows, an increase in the cost associated with pharmacological treatment with rifaximin, thanks to the reduction due to rifaximin 550 mg twice daily on the number of hospitalizations due to HE recurrences, results in a progressive decrease in the total cost borne by the INHS in line with the increase in the number of patients treated with rifaximin, progressing from a saving of ~€133,000 in the first year to ~€267,000 in the third year.
  | Table 3 Budget impact analysis results Abbreviation: HE, hepatic encephalopathy. |
Since the Gazzetta Ufficiale indicates that INHS structures receive a discount for rifaximin 550 mg, in order to better assess the true impact of the cost of this drug on the INHS expense budget, two sensitivity analyses, considering a 10% and a 20% discount, respectively, were carried out. As Table 4 shows, if the discount for INHS hospitals was ~20% of the market price, the saving for the INHS would increase to ~€1.5 million in the first year and to ~€3 million in the third year.
  | Table 4 Sensitivity analysis with 10% and 20% price reductions for rifaximin Abbreviation: HE, hepatic encephalopathy. |
Discussion
The key strength of this economic evaluation, which outlines a financial saving associated with rifaximin 550 mg twice daily treatment due to the reduction in HE recurrences leading to hospitalization (which constitutes the main cost component for this category of patient),13,18–20 is the fact that it is based on Italian real-world evidence data13 relating to an unselected wide population covering different regions of the country (ie, epidemiology, frequency of HE recurrences, current health care costs associated with patients with and without HE recurrences borne by the INHS relating to drugs, ordinary hospitalizations and one-day hospitalizations, diagnostic procedures, and outpatient medical consultations), with the exception of the data on the effectiveness of the drug in reducing hospitalizations due to HE recurrences, which was taken from the registrative study.12 These 6-month effectiveness data were also confirmed for long treatment periods through the extension of the registrative study, using the open-label approach, for >24 months.22
Thus far, no studies have been carried out to evaluate the impact of the reimbursement cost of rifaximin 550 mg twice daily on the INHS expense budget, and any comparisons to foreign data in this kind of study would be inappropriate due to different market prices for the medicine and different reimbursement systems. However, in a European context, a study did underline the positive impact of treatment with rifaximin on the consumption of hospital resources for seven UK hospitals.20 This study showed that rifaximin 550 mg twice daily treatment leads to a reduction in hospitalizations due to HE recurrences and in the length of stay, which comfortably cover the cost of the drug, resulting in a total saving of between £1,480 and £3,228 per patient per year. Moreover, at the European level (ie, France, Sweden, UK, and Belgium), several cost effectiveness analyses have been carried out, which support the favorable cost-effectiveness ratio of rifaximin 550 mg twice daily used in combination with lactulose.24–27
The conservativeness of this analysis should be taken into account: HE recurrences not requiring hospitalization, but only pharmacological treatment, were not taken into financial consideration. Indeed, rifaximin leads to a significant reduction in the rate of these episodes when compared to the standard pattern of treatment (58% relative reduction in the risk of a breakthrough episode).12
Conclusion
This evaluation of the impact of the use of rifaximin 550 mg twice daily, which, in addition to pharmacological costs, also considers costs related to hospitalizations, diagnostic procedures, and medical consultations taken from Italian sourced data, on the INHS expense budget shows how, despite the increase in drug costs, rifaximin leads to significant clinical benefits in terms of reducing hospitalizations due to HE recurrences and related costs, resulting in an overall cost reduction for the INHS; this saving increases proportionally in line with the number of patients treated.
Acknowledgments
The authors are grateful to Maria Elena Calicchia and David Dickens for the English language editing. This economic evaluation was supported by an unrestricted grant from Alfa Wassermann.
Author contributions
Both the authors devised and performed the analysis, wrote the paper, contributed to drafting and revising the paper, and agreed to be accountable for all aspects of the work.
Disclosure
AR and DPR are consultants of Alfa Wassermann. The authors report no other conflicts of interest in this work.
References
Wijdicks EF. Hepatic encephalopathy. N Engl J Med. 2016;375(17):1660–1670. | ||
Mullen KD. Review of the final report of the 1998 Working Party on definition, nomenclature and diagnosis of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25(Suppl 1):11–16. | ||
Morgan M. Chapter 8: Hepatic encephalopathy in patients with cirrhosis. In: Dooley JS, Lok A, Burroughs A, Heathcote J, editors. Sherlock’s Diseases of the Liver and Biliary System. 12th ed. Hoboken, NJ, USA: Blackwell Publishing; 2011:121–151. | ||
Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50:2014–2021. | ||
Gilberstadt SJ, Gilberstadt H, Zieve L, Buegel B, Collier RO Jr, McClain CJ. Psychomotor performance defects in cirrhotic patients without overt encephalopathy. Arch Intern Med. 1980;140(4):519–521. | ||
Gitlin N, Lewis DC, Hinkley L. The diagnosis and prevalence of subclinical hepatic encephalopathy in apparently healthy, ambulant, non-shunted patients with cirrhosis. J Hepatol. 1986;3(1):75–82. | ||
Das A, Dhiman RK, Saraswat VA, Verma M, Naik SR. Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol. 2001;16(5):531–535. | ||
D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. | ||
Alvarez MA, Cirera I, Sola R, Bargallo A, Morillas RM, Planas R. Long-term clinical course of decompensated alcoholic cirrhosis: a prospective study of 165 patients. J Clin Gastroenterol. 2011;45(10):906–911. | ||
Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30(5):890–895. | ||
Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25(Suppl 1):3–9. | ||
Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081. | ||
Roggeri DP, Roggeri A, Rossi E, et al. Overt hepatic encephalopathy in Italy: clinical outcomes and health care costs. Hepat Med. 2015;7:37–42. | ||
Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106(9):1646–1653. | ||
Younossi ZM, Boparai N, McCormick M, Price LL, Guyatt G. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterol. 2001;96(2):579–583. | ||
Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci. 2003;48(8):1622–1626. | ||
Groeneweg M, Quero JC, De Bruijn I, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28(1):45–49. | ||
Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10(9):1034.e1–1041.e1. | ||
American Gastroenterological Association. The Burden of Gastrointestinal Diseases. Bethesda, MD: American Gastroenterological Association; 2001:41–42. | ||
Orr JG, Currie CJ, Berni E, et al. The impact on hospital resource utilisation of treatment of hepatic encephalopathy with rifaximin-α. Liver Int. 2016;36(9):1295–1303. | ||
Associazione Italiana Per Lo Studio Del Fegato. “Encefalopatia Epatica” dell’Associazione Italiana per lo Studio del Fegato. [Italian Association for the Study of the Liver. Hepatic Encephalopathy]. Available from: http://www.webaisf.org/media/8045/encefalopatia_epatica.pdf. Accessed June 30, 2017. Italian. | ||
Mullen DE, Sanyal AJ, Bass NM, et al. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2014;12(8):1390.e2–1397.e2. | ||
Determina 14 settembre 2016 Riclassificazione del medicinale per uso umano «Tixteller», ai sensi dell’articolo 8, comma 10, della legge 24 dicembre 1993, n. 537. (Determina n. 1211/2016). (16A07024) (GU Serie Generale n.230 del 01-10-2016). [Determination 14 September 2016. Reclassification of the medicinal product for use in human “Tixteller” within the meaning of Article 8, paragraph 10 of Law 24 December 1993, n. 537. (Determination No. 1211/2016). (16A07024) (General Series No.230 of 01-10-2016)]. Available from: http://www.gazzettaufficiale.it/eli/id/2016/10/01/16A07024/sg;jsessionid=ss8SvRMtGRI4TzilOUkjAA__.ntc-as5-guri2a. Accessed June 30, 2017. Italian. | ||
Kabeshova A, Launois R. Cost-effectiveness analysis of rifaximin-α administration for the reduction of the overt hepatic encephalopathy episodes in recurrence in France. Value Health. 2016;19(7):A513. | ||
Poole CD, Berni E, Conway P, Radwan A, Currie CJ. Evaluation of the cost effectiveness of rifaximin-α 550 mg in the reduction of recurrence of overt hepatic encephalopathy in Sweden. Value Health. 2015;18(7):A626. | ||
Berni E, Poole CD, Conway P, Radwan A, Currie CJ. Cost effectiveness of rifaximin-α 550 mg in the reduction of recurrence of overt hepatic encephalopathy in United Kingdom. Value Health. 2015;18(7): A626. | ||
Berni E, Connolly M, Conway P, Radwan A, Currie CJ. Evaluation of the cost effectiveness of rifaximin-α in the reduction of recurrence of overt hepatic encephalopathy in Belgium. Value Health. 2015;18(7): A628. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

