Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Economic Impact of Low Adherence to COPD Management Guidelines in Spain
Authors Miravitlles M , Solé A, Aguilar H, Ampudia A, Costa-Samarra J , Mallén-Alberdi M , Nieves D
Received 8 June 2021
Accepted for publication 20 October 2021
Published 16 November 2021 Volume 2021:16 Pages 3131—3143
DOI https://doi.org/10.2147/COPD.S322793
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Marc Miravitlles,1 Alexandra Solé,2 Helena Aguilar,3 Ana Ampudia,2 Jaume Costa-Samarra,4 Maria Mallén-Alberdi,4 Diana Nieves4
1Pneumology Department, Vall d’Hebron University Hospital, Vall d’Hebron Research Institute (VHIR), Vall d’Hebron Barcelona Hospital Campus, CIBER for Respiratory Diseases (CIBERES), Barcelona, Spain; 2Market Access Department of Boehringer Ingelheim SA, Barcelona, Spain; 3Medical Department of Boehringer Ingelheim SA, Barcelona, Spain; 4Oblikue Consulting SL, Barcelona, Spain
Correspondence: Marc Miravitlles
Pneumology Department, Vall d’Hebron University Hospital, Vall d’Hebron Barcelona Hospital Campus, P. Vall d’Hebron 119-129, Barcelona, 08035, Spain
Email [email protected]
Objective: The objective of this study was to assess the non-adherence level of Spanish clinical practice to guideline recommendations for the treatment of chronic obstructive pulmonary disease (COPD) and to estimate the potential impact on pharmaceutical expenditure resulting from transitioning current treatment patterns according to guidelines.
Methods: A model was developed to compare current prescribing patterns with two alternative scenarios: the first aligned with the Global Initiative for Chronic Obstructive Lung Disease (GOLD 2020) recommendations, and the second with the Spanish Guidelines for COPD (GesEPOC 2017). Current treatment practice was obtained from publications that describe treatment patterns by pulmonology departments in Spain. The economic impact between patterns was calculated from the perspective of the Spanish National Health System (NHS), considering the annual pharmacological costs of COPD inhaled maintenance therapy. Two additional analyses were performed: one that included current prescribing patterns of patients managed by pulmonology and primary care centers in Spain (published aggregated data); and another that only considered the appropriate use of inhaled corticosteroids (ICS) treatment according to guidelines.
Results: It was estimated that 54% and 38% of patients were not treated in line with GOLD and GesEPOC recommendations, respectively, mainly due to a broader use of ICS-based therapies. Adapting treatment to recommendations could provide a potential annual cost-saving of € 17,792,022 (according to GOLD) and € 5,881,785 (according to GesEPOC). In scenario analysis 1, a 26% of non-adherence to GesEPOC guideline was observed with a potential annual pharmacological cost-saving of € 2,707,554. In scenario analysis 2, considering only inappropriate use of ICS treatment, an annual cost-saving of € 17,863,750 (according to GOLD) and € 9,904,409 (according to GesEPOC) was calculated.
Conclusion: More than a third of treatments for COPD patients in Spain are not prescribed in accordance with guideline recommendations. The adaptation of clinical practice to guideline recommendations could provide important cost-savings for the Spanish NHS.
Keywords: cost, chronic obstructive pulmonary disease, Spanish, treatment, recommendations
Introduction
Chronic obstructive pulmonary disease (COPD) affects 11.8% of the Spanish population aged 40 years or older.1 Although it has an irreversible component, an early diagnosis and appropriate guideline based treatment can modify the prognosis of its clinical evolution.2,3 However, it is an under-diagnosed disease with high morbidity and mortality and it constitutes a major public health problem.4
Recommendations for the management of patients with COPD suggest an individualized approach to treatment according to patient characteristics, based primarily on symptom burden and risk of exacerbations.5–7 The goal of pharmacological therapy for the management of COPD is to reduce the symptoms, frequency and severity of exacerbations, as well as to improve the prognosis of the disease.5,8
Similar to the ABCD groups of the classification according to the GOLD strategy,5 four phenotypes are defined in the GesEPOC 2017 guidelines: patients with ≤1 exacerbation/year without hospitalization belong to the non-exacerbator phenotype, in patients with ≥2 exacerbations/year or ≥1 hospitalizations a distinction is made between exacerbator with emphysema phenotype and exacerbator with chronic bronchitis phenotype and, finally, a fourth phenotype is defined, the asthma and COPD overlapping (ACO) phenotype.6
Bronchodilator therapy is the main initial and maintenance treatment for stable COPD in patients not at increased risk of exacerbations.5–7 According to several observational studies conducted in Spain, these patients represent around 60% of all COPD patients.9–11 Bronchodilator therapy may consist of a long-acting muscarinic antagonist bronchodilator (LAMA) or a long-acting β2 adrenergic agonist (LABA), in monotherapy or in combination. These therapies combined with inhaled corticosteroid (ICS) are recommended in patients with a history of previous exacerbations (2 or more moderate or 1 or more leading to hospitalization), especially if blood eosinophils are elevated – according to Global Initiative for Chronic Obstructive Lung Disease (GOLD)5,12 –, or triple therapy (LAMA/LABA/ICS) in uncontrolled high risk patients with exacerbations treated with LAMA/LABA – according to Spanish Guidelines for the diagnosis and treatment of COPD patients (GesEPOC) –, as well as in patients with overlapping asthma and COPD (asthma-COPD overlap).6,13,14
Several studies carried out in Spain have reported a lack of adherence of clinical practice to guideline recommendations, both in the primary care setting9,10,15,16 and in specialized care.9,10,17,18 The lack of adherence is mainly due inadequate evaluation and segmentation of COPD patients, a low use of spirometry and a high use of ICS in patients with mild-moderate COPD and without exacerbations.16,18,19 Different studies have found that more than 60% of patients with mild COPD receive ICS therapy, often at high doses.18,20
The correct evaluation of COPD patients and the optimization of pharmacological treatment in accordance with guideline recommendations could result in a clinical benefit for patients21–24 plus cost-savings for the Spanish National Health Service (NHS).25–28
This study aims to assess the degree of adherence of clinical practice to guideline recommendations for COPD in Spain, and estimate the potentials savings derived from the optimization of drug treatment to guideline recommendations.
Methods
Model
A model was developed to compare current prescribing patterns with two alternative patterns in line with the recommendations of: (1) international GOLD 2020 document,5 and (2) Spanish-level reference guide GesEPOC 2017.6
In the model, the natural history of COPD was not simulated, only the COPD population and associated pharmacological treatments. Costs have been estimated considering a time horizon of a year, 100% adherence to medications and no change in treatment over that period.
Two COPD studies conducted in Spain with a detailed description of COPD population and pharmacological treatment were identified during the literature review. These studies provide detailed information regarding the distribution of the COPD population according to the GOLD and/or GesEPOC classification criteria (ABCD GOLD groups and GesEPOC phenotypes), and the pharmacological treatment used in each group of patients:
- Multicenter observational study conducted in pulmonology departments with classification of patients according to GOLD groups and according to GesEPOC phenotypes,17 used in the base case analysis of this study.
- Multicenter observational epidemiological study (FENEPOC study) conducted in primary care and pulmonology services (aggregated data), with classification of patients according to GesEPOC phenotypes.9 This study was used in scenario analysis 1, considering an alternative source of current treatment patterns in Spain.
Base Case Analysis
Population and Classification
The model considered the Spanish population aged 40 years or older29 with diagnosed1 and treated COPD,30 with a total of 365,142 patients (Figure 1).
The classification of patients by group was based on the data from the study conducted in the field of pulmonology (Figure 2),17 with distribution based on: (1) GOLD classification according to four groups (ABCD, according to symptoms and history of exacerbations) and (2) GesEPOC classification according to four phenotypes of high-risk patients (non-exacerbator phenotype, exacerbator with emphysema, exacerbator with chronic bronchitis and ACO [asthma-COPD overlap]; according to clinical profile).
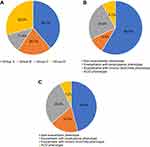 |
Figure 2 Distribution of patients considered in each GOLD group and GesEPOC phenotype in the base case analysis (A and B) and scenario analysis 1 (C), based on literature a. (A) Distribution of patients according to the GOLD strategy used in the base case analysis.17 (B). Distribution of patients according to the GesEPOC guide used in the base case analysis.17 (C). Distribution of patients to each GesEPOC phenotype used in scenario analysis 1.9 Abbreviations: ACO, asthma-COPD overlap; COPD, chronic obstructive pulmonary disease; GesEPOC, Spanish Guidelines for Chronic Obstructive Pulmonary Disease; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; ICS, inhaled corticosteroids; LABA, long-acting β2 adrenergic agonists; LAMA, long-acting muscarinic antagonists. Notes: aThe current treatment prescription patterns only take into account patients treated with the main treatments (LAMA, LABA, LAMA/LABA, LABA/ICS and LAMA/LABA/ICS). |
Based on the GOLD 2020 strategy,5 peripheral eosinophilia is a determining clinical parameter for selecting ICS treatment. According to recently published data, in Spain 28.4% of the patients have blood eosinophilia levels >300 cel/µL.31 It was assumed this proportion of patients in GOLD groups C and D had high blood eosinophils.
Drug Treatment Patterns
The model used a treatment-mapping matrix to hypothetically redistribute patients with COPD undergoing long-acting inhaler therapy from their current treatment pathway (actual prescribing patterns) to one of two alternative treatment pathways, which represents treatment optimization with prescribing in accordance with GOLD strategy and GesEPOC guideline (Figure 3).
 |
Figure 3 Base case analysis: current treatment patterns and proposed treatment according to GOLD (A) and GesEPOC (B) recommendations. (A) Current treatment patterns in pulmonology according to GOLD groups17 and proposed treatment patterns based on the GOLD strategy.5 (B) Current treatment patterns in pulmonology according to GesEPOC phenotypes17 and proposed treatment patterns based on the recommendations of the GesEPOC guidelines.6 Abbreviations: ACO, asthma-COPD overlap; COPD, chronic obstructive pulmonary disease; GesEPOC, Spanish Guidelines for Chronic Obstructive Pulmonary Disease; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; ICS, inhaled corticosteroids; LABA, long-acting β2 adrenergic agonists; LAMA, long-acting muscarinic antagonists; Treat., treatment. Notes: aIt was considered that 28.4% of the patients in GOLD groups C and D had eosinophilia >300 cells/µL31 assuming that these patients were treated with ICS therapies. It was considered to maintain the treatment to patients with high blood eosinophils and to treat with LAMA/LABA the rest of the patients currently treated with ICS therapies. bPatients with inappropriate therapy who can be treated with more than one therapeutic option, have been distributed according to the current treatment shares of the phenotype considered. |
Current Treatment
The current drug treatment patterns used for each of the GOLD groups and GesEPOC phenotypes were obtained from the same observational study in pulmonology17 (Figure 3). The therapies considered were the inhaled therapies LAMA, LABA, LAMA/LABA, LABA/ICS and LAMA/LABA/ICS.
Treatment Proposal According to Guideline Recommendations
Two patterns were mapped for the analysis of adherence to guidelines: (1) the recommendations of the GOLD 2020 strategy5 and (2) the recommendations of the Spanish GesEPOC 2017 guidelines6 (Figure 3).
Costs
The impact on pharmaceutical expenditure as a result of changing the current treatments that did not follow guideline recommendations to the appropriate therapies according to guidelines was estimated. The impact was conducted from the perspective of the Spanish NHS in 2020.
The annual pharmacological costs by therapeutic group (LAMA, LABA, LAMA/LABA, LABA/ICS and LAMA/LABA/ICS) were considered based on the public price including (PPI) Value Added Tax (VAT) (PPI VAT),32 applying the deductions of Royal Decree Law (RDL) 8/2010,33 the recommended dosage of each drug according to the summary of product characteristics34 and weighting the cost of each pharmacological option according to its consumption in Spain35 (Table 1). All patients treated with dual or triple therapies were assumed to use fixed-dose combination inhalers. Likewise, when calculating the annual pharmacological cost of LAMA and LAMA/LABA, specifically for tiotropium and tiotropium/olodaterol Respimat® reusable, due to the fact that the same inhaler can be used with up to 6 cartridges, a maximum use of refills was assumed (annual prescription of 2 packs of the reusable inhaler plus cartridge and 10 refill cartridges).
 |
Table 1 Annual Pharmacological Cost Considered by Each Therapeutic Group |
Analysis in Autonomous Communities
The distribution of the economic impact for the 17 individual budget holding Autonomous Communities of Spain were estimated, as they are individually responsible for providing healthcare services. To this end, the population ≥40 years of age29 with diagnosed COPD1 and treated30 was estimated in each autonomous community. The same classification of patients and distribution of treatments by group/phenotype were applied as in the total Spanish population,17 since no published data were identified at the Autonomous Community level.
The annual pharmacological costs per therapeutic group were weighted based on the pharmacological consumption of each region.35
Scenario Analyses
Two scenario analyses were performed.
- Scenario analysis 1
This scenario included prescribing patterns of COPD patients who attended pulmonology and primary care centers in Spain (aggregated data).9
For the calculation, the distribution of patients and the detail of current prescription patterns was considered according to the four phenotypes of the GesEPOC guidelines based on the FENEPOC study (Figure 2; Annex 1).9
The treatment changes used in this analysis were those recommended by the GesEPOC 2017 guideline,6 shown in Annex 1.
- Scenario analysis 2
Here the focus was limited to the economic impact as a result of aligning ICS treatment with guideline recommendations. The population distribution and current COPD treatment used were those used in the base case analysis.
The estimated recommended treatment was based on the appropriate use of ICS treatment where its use is not recommended in certain populations according to GOLD 20205 and GesEPOC 2017,6 without modifying the rest of the non-recommended therapeutic options that did not include ICS.
Results
Base Case Analysis
Analysis According to GOLD Recommendations
It was observed that 54% of patients managed in pulmonology departments were not treated in line with GOLD recommendations for their GOLD category (Table 2).
 |
Table 2 COPD Patients Not Adherent to GOLD Recommendations and GesEPOC Guidelines in Pulmonology Units (Base Case Analysis) |
The lack of adherence to guideline recommendations in current practice was mainly due to a broader use of ICS-based therapies. Ninety-nine percent (195,051 of the 196,164 patients who were not treated in accordance with recommendations) of patients were inappropriately treated with LABA/ICS or LAMA/LABA/ICS, and the remaining 1% of patients treated with LABA not in accordance with guidelines (Table 2).
In analyzing the results according to patient type, 46% and 65% of patients in population GOLD groups A and B (low risk of exacerbations), respectively, were not treated in line with the recommended pharmacological treatment. Additionally, groups C and D (high exacerbation risk) amounted to 49% and 57% of patients, respectively (Table 2; Figure 3).
If patients undergoing long-acting inhaler therapy from their current treatment pathway are hypothetically redistributed to treatment aligned with GOLD recommendation, it could result in potential cost-savings on the NHS of €17,792,022 (Figure 4).
Analysis According to GesEPOC Guideline
This analysis showed that the patterns of treatment from 38% of patients managed in the pulmonology setting were not prescribed in line with recommendations of GesEPOC guidelines for their specific phenotype (Table 2).
Lack of adherence to guideline recommendations in current practice was mainly due to a broader use of ICS-based therapies. Ninety-four percent of patients (128,929 of the 137,464) who were not treated in accordance with the Spanish guideline were treated with ICS-based therapies, as opposed to the remaining 6% who were treated with bronchodilator therapy (Table 2).
Regarding the different phenotypes, it was observed that half of the patients with the non-exacerbator phenotype were not treated according to GesEPOC recommendations because they were treated with ICS (Table 2; Figure 3). In 25% of patients with exacerbator phenotype with emphysema and in 13% of patients with exacerbator phenotype with chronic bronchitis, the change to dual-bronchodilator treatment should be considered (Table 2; Figure 3). Patients with ACO phenotype should all be treated with ICS-based therapies (Figure 3); however, 32% of ACO patient therapies were not prescribed in line with guideline recommendations (Table 2).
The model showed that if patients are hypothetically redistributed to treatment in accordance with GesEPOC guideline recommendations, it could provide potential cost-savings of €5,881,785 on the NHS (Figure 4).
Analysis of Savings by Autonomous Communities
Additionally, the potential savings were calculated by autonomous community. The factors with the greatest impact on savings were the population size and the prevalence of diagnosed and treated COPD in each region. The Community of Madrid, Catalonia and Andalusia were the autonomous communities with the greatest potential savings, as a result of re-aligning current treatment to GOLD and GesEPOC guidelines (Figure 5).
 |
Figure 5 Base case analysis: annual savings in pharmaceutical expenditure in patients treated in pulmonology services distributed by Autonomous Community according to the GOLD (A) and GesEPOC (B) guidelinesa. (A) Following GOLD strategy recommendations. (B) Following GesEPOC guideline. Abbreviations: GesEPOC, Spanish Guidelines for Chronic Obstructive Pulmonary Disease; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease. Notes: aThe sum of the costs of the regions does not correspond to the total Spanish cost (Figure 4) as the pharmacological costs have been weighted according to their sales in each region. |
Scenario Analyses
In scenario analysis 1, it was observed that 26% of patients were not treated in line with GesEPOC recommendations for their specific phenotype (Table 3). The lack of adherence was most prominent in the inappropriate use of ICS in most COPD phenotypes; 94% of the total number of patients not treated in accordance with recommendations were treated with an ICS therapy, compared with 6% that were inappropriately treated with bronchodilators (Table 3; Annex 1).
 |
Table 3 COPD Patients Treated Without Following GesEPOC Guidelines in Pulmonology and Primary Care Units (Scenario Analysis 1) |
Forty-four percent of the patients with a non-exacerbator phenotype were treated with ICS, while they should not have received ICS according to GesEPOC guidelines (Table 3; Annex 1). Among exacerbating patients, 12% of patients with emphysema and 7% with chronic bronchitis were treated with another option than recommended according to GesEPOC guidelines (Table 3; Annex 1). Patients with ACO phenotype should all be treated with ICS-based therapies; however, 28% of these patients were not prescribed in line with guideline recommendations (Table 3; Annex 1).
The model also showed a potential reduction in annual pharmaceutical expenditure resulting from alignment of treatment to the GesEPOC guidelines of €2,707,554 (Figure 4), equivalent to 0.9% of the 2020 pharmaceutical expenditure on COPD in Spain.
In scenario analysis 2, it was observed that adapting clinical practice regarding the management of ICS-based therapies to guideline recommendations would lead to savings in pharmaceutical expenditure of €17,863,750 and €9,904,409 according to the GOLD and GesEPOC recommendations for the Spanish NHS, respectively.
Discussion
This study illustrates the low adherence of Spanish clinical practice in the management of stable COPD to GOLD strategy and GesEPOC guidelines.
According to guidelines, the population who can benefit from ICS, in addition to long-term bronchodilator therapy, are those with a history of exacerbations and elevated blood eosinophil levels, as well as the population with a history of asthma. However, the present analysis demonstrates a broader inappropriate use of ICS being the main reason for the low adherence to guidelines.
This trend in the use of ICS has also been observed in other Spanish studies. In particular, in the field of pulmonology, the EPOCONSUL clinical audit revealed that more than 60% of patients with a phenotype other than ACO were treated with ICS.18 Likewise, the results of the COACH clinical audit, carried out in primary care centers, showed up to 55% of COPD patients without a history of exacerbations followed treatment regimens that included ICS.15 More recently, a big data analysis has been published with data from patients from Castile La Mancha, in which 68% of COPD patients were receiving ICS, mostly in the form of triple therapy.10
Given the high use of ICS, the GOLD strategy recommends a periodic review of treatment and proposes an assessment of ICS withdrawal in patients who experience an ICS-related adverse effect (such as pneumonia), inappropriate original indication, and/or lack of response to ICS. Although the current analysis did not follow individual patients over time, –so any de-escalation of treatment was taken into account–, it has been observed in the literature that the gradual withdrawal of ICS in patients treated with LAMA/LABA/ICS does not increase the risk of exacerbation, and may reduce the risk of adverse events, such as pneumonia.36 In this respect, and based on all the available evidence, the European Respiratory Society (ERS) has recently issued a conditional recommendation of ICS withdrawal in patients with blood eosinophils <300 cel/µL and no history of frequent exacerbations.37
In contrast, the present study shows that 13% of patients with an ACO phenotype are not treated with ICS, despite GesEPOC recommendations. These results were similar to EPOCONSUL, where a quarter part of ACO patients were not receiving ICS.18
From an economic perspective, the model showed that treating patients in accordance with guideline recommendations could lead to a 6.3% (according to GOLD) and 2.1% (according to GesEPOC) reduction in pharmaceutical spending on COPD in Spain in 2020 (total cost of €282 million [Figure 4]).
Overall, the degree of adherence to recommendations was higher for the GesEPOC guideline compared with the GOLD strategy. Consequently, regarding the economic implications, the appropriateness of clinical practice to the GOLD recommendations would entail an additional pharmacological saving of €11,910,237, compared with the potential savings generated by aligning with GesEPOC guidelines. The main reason for these differences observed from GOLD in comparison with GesEPOC, is associated with the more restrictive recommendations regarding the use of ICS in the GOLD strategy. Likewise, the decrease in the use of triple therapy in line with the recommendations also contributed to savings over GesEPOC. On the other hand, the GesEPOC guideline recommends dual bronchodilator therapy as the main treatment in high-risk patients, reserving the LABA/ICS combination as initial therapy only for patients with ACO phenotype. This implies scaling up the treatment of patients treated with less costly therapies (such as bronchodilator monotherapy or LABA/ICS) to more expensive ones (such as dual bronchodilation), adding an additional pharmacological cost. These additional costs, however, are offset by a reduction of the use of triple therapy (a more costly therapy), generating overall savings for the NHS.
In scenario analysis 1, a higher adherence of treatment patterns to GesEPOC guidelines was observed than in the base case analysis. The low adherence was mainly due to the broader use of ICS-based therapies, including patients with a non-exacerbator phenotype.
In scenario analysis 2, it was observed that the pharmacological savings resulting from limiting re-alignment to ICS-based therapies alone, were higher than observed in the base case analysis. The savings derived from switching from more expensive therapies (such as triple therapy) to less costly treatments (such as monotherapy or double bronchodilation) offset the additional pharmacological costs derived from switching away from less costly therapies (such as LABA/ICS) to LAMA/LABA. This suggests that a correct evaluation of ICS treatment use could be an effective cost-reducing strategy.
These data are consistent with those of a Spanish study which analyzed 10,711 patients with COPD in the primary care setting and showed that 34.5% received ICS and in 18.2% of them the use was inappropriate according to the GOLD strategy.25 Patients who received ICS treatment not aligned with the recommendations had a poorer quality of life and generated higher direct costs (€1,590/patient per year if the use of ICS was inappropriate vs. €1,157 if they followed the recommendations, p <0.05). In addition, a recent study performed in primary care in Spain showed that from more than 34,000 patients initiating triple therapy, 70% were GOLD A or B and up to 54% were non exacerbators, showing again a broader use of ICS, not aligned with current guidelines.38
A number of limitations in the current analysis should be noted. First, the use of fixed-dose combination inhalers was assumed in all patients treated with double or triple therapies. This simplified approach led to an underestimation of the costs of the combinations, since the pharmacological costs of the fixed-dose combinations are lower than the sum of the cost of the single components taken separately.
Another limitation was that this study only considers the costs associated with the pharmacological treatment of stable COPD. Thus, the potential variations in other direct or indirect costs in patients derived from low adherence to the recommendations have not been quantified, and the clinical side effects produced by the drugs have not been included.
On the other hand, the prescribing patterns of clinical practice in the scenario of patients classified according to GOLD groups of the base case analysis have been obtained from a study that was based on the version of the GOLD strategy from 2013,39 where obstruction level was considered in the classification of patients and, instead, the proposed treatment has been determined based on the recommendations of the 2020 version. This may lead to some patients not belonging to the same ABCD group in both versions of the guideline, specifically some patients from D group according to 2013 version may be classified as B group in the updated version.
In addition, another limitation is that the assumption of the number of patients with elevated eosinophils used for the estimations comes from a different study.31
Finally, in the autonomous communities analyses it was not possible to use regional data on patient distribution by typology and drug prescription, as there was no data published, so model projections are simplifications of reality.
The current analysis demonstrates the potential savings to the Spanish NHS as a result of aligning treatment with international and national guidance. However, any change in treatment should be evaluated by the clinician and agreed with the patient, in particular when the disease is not well controlled.8,40 In line with guidelines recommendations, a review of the diagnosis, management of comorbidities, history of symptoms and exacerbations, inhalation technique and adherence to medication should be included. However, it has been identified the potential economic benefits for the Health System to consider when assessing the risks and benefits of therapies, particularly those containing ICS for their COPD patients.
Conclusion
The results of this analysis show that more than a third of the patients with COPD in Spain are not treated in line with national and international clinical recommendations.
The adherence of clinical practice to COPD treatment recommendations, and the reduction in the use of ICS-based therapies in patients in whom their use is not recommended, could provide significant cost-savings for the Spanish NHS.
These findings encourage the need for establishing strategies for adapting the management of stable COPD in Spanish clinical practice to guideline recommendations, thus optimizing health outcomes for patients and reducing the pharmaceutical expenditure of the Spanish NHS.
Funding
This analysis has been funded by Boehringer Ingelheim Spain. Partial results were presented at the XXVII SEMG Congress (Sociedad Española de Médicos Generales y de Familia) in June 2021.
Disclosure
Dr Marc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Gebro Pharma, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, Spin Therapeutics, Palobiofarma SL, pH Pharma, Novartis, Sanofi and Grifols, and research grants from Grifols. Alexandra Solé, Helena Aguilar and Ana Ampudia are employees of Boehringer Ingelheim, S.A. Jaume Costa-Samarra, Maria Mallén-Alberdi and Diana Nieves work for an independent research entity, Oblikue Consulting, and have received remuneration for their contribution to the development of the draft. The authors affirm that the results of the research described in this manuscript, as well as the analysis and interpretation thereof, were the result of the free expression of opinions and the agreement of the publication’s co-authors, and that there were no conflicts, either to obtain or disclose said results.
References
1. Soriano JB, Alfageme I, Miravitlles M, et al. Prevalence and determinants of COPD in Spain: EPISCAN II. Arch Bronconeumol. 2021;57(1):61–69. doi:10.1016/j.arbres.2020.07.024
2. Decramer M, Miravitlles M, Price D, et al. New horizons in early stage COPD – improving knowledge, detection and treatment. Respir Med. 2011;105(11):1576–1587. doi:10.1016/j.rmed.2010.12.015
3. Duarte-de-Araújo A, Fonte P, Teixeira P, Hespanhol V, Correia-de-Sousa J. Is an early diagnosis of COPD clinically useful? Arch Bronconeumol. 2020;56(6):409–410. doi:10.1016/j.arbres.2019.11.018
4. Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi:10.1016/S0140-6736(16)31678-6
5. Vogelmeier C, Agusti A, Anzueto A, et al. Global initiative for chronic obstructive lung disease; 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
6. Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53(6):324–335. doi:10.1016/j.arbres.2017.03.018
7. Montes de Oca M, López Varela MV, Acuña A, et al. Incorporating new evidence on inhaled medications in COPD. The Latin American Chest Association (ALAT) 2019. Arch Bronconeumol. 2020;56(2):106–113. doi:10.1016/j.arbres.2019.09.023
8. Miravitlles M, Calle M, Soler-Cataluña JJ. GesEPOC 2021: one more step towards personalized treatment of COPD. Arch Bronconeumol. 2021;57(1):9–10. doi:10.1016/j.arbres.2020.08.002
9. Calle Rubio M, Casamor R, Miravitlles M. Identification and distribution of COPD phenotypes in clinical practice according to Spanish COPD Guidelines: the FENEPOC study. Int J Chron Obstruct Pulmon Dis. 2017;12:2373–2383. doi:10.2147/COPD.S137872
10. Izquierdo JL, Morena D, González Y, et al. Clinical management of COPD in a real-world setting. A big data analysis. Arch Bronconeumol. 2021;57(2):94–100. doi:10.1016/j.arbres.2019.12.025
11. Miravitlles M, Barrecheguren M, Romaín-Rodríguez M. Frequency and characteristics of different clinical phenotypes of chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2015;19(8):992–998. doi:10.5588/ijtld.15.0021
12. Golpe R, Dacal D, Sanjuán-López P, Martín-Robles I, Pérez-de-Llano LA. Plasma eosinophil count and patient-centered events in chronic obstructive pulmonary disease in real-life clinical practice. Arch Bronconeumol. 2020;56(2):129–130. doi:10.1016/j.arbr.2019.09.001
13. Nuñez A, Sarasate M, Loeb E, Esquinas C, Miravitlles M, Barrecheguren M. Practical guide to the identification and diagnosis of asthma-COPD overlap (ACO). COPD J Chronic Obstr Pulm Dis. 2019;16(1):1–7. doi:10.1080/15412555.2019.1575802
14. Soler-Cataluña JJ, Novella L, Soler C, et al. Clinical characteristics and risk of exacerbations associated with different diagnostic criteria of asthma-COPD overlap. Arch Bronconeumol. 2020;56(5):282–290. doi:10.1016/j.arbres.2019.08.023
15. Abad-Arranz M, Moran-Rodríguez A, Mascarós Balaguer E, et al. Community Assessment of COPD Health Care (COACH) study: a clinical audit on primary care performance variability in COPD care. BMC Med Res Methodol. 2018;18(1):1–13. doi:10.1186/s12874-018-0528-4
16. Barrecheguren M, Monteagudo M, Ferrer J, et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med. 2016;111:47–53. doi:10.1016/j.rmed.2015.12.004
17. Izquierdo JL, Miravitlles M, Esquinas C, et al. Characteristics of COPD patients managed in respiratory medicine departments in Spain, according to GOLD groups and GesEPOC clinical phenotypes. Arch Bronconeumol. 2018;54(11):559–567. doi:10.1016/j.arbres.2018.03.021
18. Calle Rubio M, Rodríguez Hermosa JL, Soler-Cataluña JJ, et al. Medical care according to risk level and adaptation to Spanish COPD Guidelines (GesEPOC): The Epoconsul study. Arch Bronconeumol. 2018;54(5):270–279. doi:10.1016/j.arbres.2017.11.015
19. Monteagudo M, Rodriguez-Blanco T, Parcet J, et al. Variability in the performing of spirometry and its consequences in the treatment of COPD in primary care. Arch Bronconeumol. 2011;47(5):226–233. doi:10.1016/S1579-2129(11)70057-X
20. Garrastazu R, García-Rivero JL, Ruiz-Nuñez M, et al. Reliability of a chronic obstructive pulmonary disease diagnosis register in primary care. Semergen. 2017;43(5):364–374. doi:10.1016/j.semerg.2016.06.001
21. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
22. Oba Y, Keeney E, Ghatehorde N, Dias S. Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2018;12:CD012620. doi:10.1002/14651858.CD012620.pub2
23. Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60. doi:10.1016/S2213-2600(12)70052-8
24. Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J COPD. 2015;10:1015–1026. doi:10.2147/COPD.S84436
25. de Miguel-Díez J, Carrasco-Garrido P, Rejas-Gutierrez J, et al. Inappropriate overuse of inhaled corticosteroids for COPD patients: impact on health costs and health status. Lung. 2011;189(3):199–206. doi:10.1007/s00408-011-9289-0
26. Miravitlles M, Brosa M, Velasco M, et al. An economic analysis of pharmacological treatment of COPD in Spain. Respir Med. 2009;103(5):714–721. doi:10.1016/j.rmed.2008.11.019
27. Miravitlles M, Sicras A, Crespo C, et al. Costs of chronic obstructive pulmonary disease in relation to compliance with guidelines: a study in the primary care setting. Ther Adv Respir Dis. 2013;7(3):139–150. doi:10.1177/1753465813484080
28. Fens T, van der Pol S, Kocks JWH, Postma MJ, van Boven JFM. Economic impact of reducing inappropriate inhaled corticosteroids use in patients with chronic obstructive pulmonary disease: ISPOR’s guidance on budget impact in practice. Value Heal. 2019;22(10):1092–1101. doi:10.1016/j.jval.2019.05.006
29. INE [National Statistics Institute]. Population and demographic phenomena by autonomous communities and cities: year 2020. Population residing in Spain on January 1, by sex, age and year; 2020. Available from: https://www.ine.es/.
30. Soriano JB, Miravitlles M, Borderías L, et al. Geographical variations in the prevalence of COPD in Spain: relationship to smoking, death rates and other determining factors. Arch Bronconeumol. 2010;46(10):522–530. doi:10.1016/j.arbres.2010.06.008
31. Miravitlles M, Monteagudo M, Solntseva I, Alcázar B. Blood eosinophil counts and their variability and risk of exacerbations in COPD: a Population-Based Study. Arch Bronconeumol. 2021;57(1):13–20. doi:10.1016/j.arbres.2019.12.015
32. CGCOF. Consejo general de colegios oficiales de farmacéuticos [Spanish General Council of Official Colleges of Pharmacy]. BotPlus: database of medicinal products; 2020. Available from: https://botplusweb.portalfarma.com/botplus.aspx.
33. MSCBS. List of medicines affected by the deductions of the Royal Decree-Law 8/2010 May 2020; 2020. Available from: https://www.mscbs.gob.es/profesionales/farmacia/pdf/DeduccionesMayo2020.pdf.
34. AEMPS (Spanish Agency for Medicines and Medical Devices). AEMPS online medicinal product information centre (CIMA); 2020. Available from: https://cima.aemps.es/cima/publico/home.html.
35. IQVIA. Sales data -sell in-. 2019.
36. Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi:10.1056/NEJMoa1407154
37. Chalmers JD, Laska IF, Franssen FME, et al. Withdrawal of inhaled corticosteroids in COPD: a European Respiratory Society guideline. Eur Respir J. 2020;55(6):2000351. doi:10.1183/13993003.00351-2020
38. Monteagudo M, Nuñez A, Solntseva I, et al. Treatment pathways before and after triple therapy in COPD: a population-based study in primary care in Spain. Arch Bronconeumol. 2021;57(3):205–213. doi:10.1016/j.arbres.2020.07.032
39. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
40. Miravitlles M, Sliwinski P, Rhee CK, et al. Changes in control status of COPD over time and their consequences: a Prospective International Study. Arch Bronconeumol. 2021;57(2):122–129. doi:10.1016/j.arbres.2020.06.003
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


