Back to Journals » Cancer Management and Research » Volume 9
Economic burden of the management of metastatic castrate-resistant prostate cancer in Italy: a cost of illness study
Authors Restelli U , Ceresoli GL, Croce D, Evangelista L, Maffioli LS , Gianoncelli L, Bombardieri E
Received 4 August 2017
Accepted for publication 17 October 2017
Published 7 December 2017 Volume 2017:9 Pages 789—800
DOI https://doi.org/10.2147/CMAR.S148323
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Umberto Restelli,1,2 Giovanni Luca Ceresoli,3 Davide Croce,1,2 Laura Evangelista,4 Lorenzo Stefano Maffioli,5 Letizia Gianoncelli,3 Emilio Bombardieri6
1Center for Health Economics, Social and Health Care Management, LIUC – Università Cattaneo, Castellanza, Italy; 2School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; 3Medical Oncology Department, Thoracic and Urologic Oncology Unit, Cliniche Humanitas Gavazzeni, Bergamo, 4Nuclear Medicine and Molecular Imaging Unit, Veneto Institute of Oncology IOV – IRCCS, Padova, 5Nuclear Medicine Department, Cancer Centre – ASST Ovest Milanese, Legnano, 6Nuclear Medicine Department, Cliniche Humanitas Gavazzeni, Bergamo, Italy
Background: Prostate cancer (PCa) accounts for 20% of all cancers in subjects over 50 years in Italy. The majority of patients with PCa present with localized disease at the time of diagnosis, but many patients develop recurrent metastatic disease after treatment with curative intent. Androgen deprivation therapy is the standard of care for metastatic PCa patients; unfortunately, most of them progress to castrate-resistant prostate cancer (CRPC) within 5 years. Metastatic CRPC (mCRPC) heavily affects patients in terms of quality of life, side effects, and survival, and greatly impacts economic costs. The approval of new effective agents in recent years, including cabazitaxel, abiraterone acetate, enzalutamide, and radium-223, has dramatically changed patient management.
Materials and methods: Here, we aimed to estimate the current costs of illness of mCRPC in Italy. All patients affected by mCRPC and treated with a single agent in an annual time horizon were considered. Therefore, the analysis was not focused on the management pathway of single patients through different lines of treatment. Direct medical costs referred to therapy, adverse event management, and skeletal-related event management were analyzed. A bottom-up approach was used to estimate the resource consumption: through national guidelines and expert opinions, the mean cost per patient was estimated and then multiplied by the total number of patients diagnosed with mCRPC.
Results: Direct medical costs ranged from €196.5 million to €228.0 million, representing ~0.2% of the financing of the Italian National Health Service in 2016. The main cost driver was the cost of treatment, which represented more than 77% of the overall economic burden.
Conclusion: Our analysis, reflective of real clinical practice, shows for the first time the high economic cost of mCRPC in Italy.
Keywords: metastatic castrate-resistant prostate cancer, cost analysis, resource consumption, Italy, skeletal related events, oncology
Corrigendum for this paper has been published.
Background
Prostate cancer epidemiology
Prostate cancer (PCa) is one of the most frequent cancers among the male population. Its incidence varies across geographical areas, depending more on different diagnostic strategies than on risk factors (eg, age, family history, genetics, ethnicity, androgen status, diet, and lifestyle).1 Approximately 900,000 PCa diagnoses per year are registered worldwide. PCa incidence varies widely among continents, being 104.2 cases per 100,000 person-years in Australia and New Zealand, 73.1 and 93.1 per 100,000 person-years in Northern and Western Europe, respectively, 85.6 cases per 100,000 person-years in North America, and 7.2 per 100,000 person-years in Asia, namely in India and China. Age-adjusted mortality for PCa is 12 per 100,000 person-years in Europe and 9.9 per 100,000 person-years in North America.2
In Italy, PCa is the most frequent cancer among the male population and accounts for 20% of all cancers diagnosed in subjects over 50 years.3 In 2015, 35,000 patients were diagnosed with PCa. A north-south gradient is observed in terms of incidence, with 99.8 cases per 100,000 person-years in northern regions, 79.8 cases in central regions, and 66.8 cases in southern regions. The mortality registered in 2012 was 7,282 deaths.4 However, it is difficult to differentiate cancer-specific mortality from the mortality caused by the other age-related diseases. Despite its highest incidence among cancer diagnosis, PCa is the third cause of cancer death in Italy (8% of the overall deaths for cancer) among males older than 70 years. However, death rates have declined in the past 20 years due to improved diagnostic detection (–2.3% per year). No consistent differences are observed in terms of mortality at a regional level, with ~17 cases per 100,000 person-years. In terms of prevalence, patients affected with PCa in Italy in 2015 were 398,708,4 representing 26% of males with any cancer diagnosis.
Management of metastatic castration-resistant prostate cancer (mCRPC)
PCa tumor growth is driven by androgen receptor (AR) that, when bound by androgens, acts as a transcription factor5 and activates non-genomic cytoplasmic signaling cascades. Most patients with PCa have localized disease at diagnosis and are managed with curative intent by radical prostatectomy or external beam radiation. However, many patients develop recurrent metastatic disease. For these patients, the standard treatment is based on androgen deprivation therapy (ADT) that reduces AR signaling through medical or surgical castration. Although this therapeutic approach is initially effective in almost all patients, most of them progress to castration-resistant prostate cancer (CRPC) within 5 years, and 84% of them have metastatic disease at the time of CRPC diagnosis. The definition of CRPC status includes a conventionally defined testosterone level of <50 ng/dL or <1.7 nmol/L and a rising prostate-specific antigen (PSA) level with 50% increments over the nadir in two consecutive determinations (with a PSA >2 ng/mL), despite ADT.6 The term “castration resistant” replaced previously used nomenclature such as “androgen independent” and “hormone refractory,” because during the progression to the castrate status, PCa cells remain dependent on androgens and on the AR signaling pathway.7
The treatment options for mCRPC have increased significantly over the past decade, and its management has changed dramatically.1 Before 2010, docetaxel was the only approved treatment to have shown a survival benefit in this setting. More recently, several large randomized Phase III trials have led to the approval of multiple new agents, including cabazitaxel, abiraterone acetate, enzalutamide, and radium-223.8 With the availability of these new drugs, the choice of a proper therapeutic strategy for mCRPC patients has become increasingly challenging. The clinical approach should be patient specific and aim at improving cancer control while minimizing side effects. Treatment characteristics (eg, mechanism of action, dosing, and toxicity profile) and aspects relating to the individual (eg, performance status, comorbidity, prior therapies, symptom burden, and sites of metastases) should be carefully taken into account when choosing the right treatment at the right time for a specific patient. Nonetheless, despite the numerous treatment options, there are limited data on appropriate sequencing of available drugs.9
Treatment options for mCRPC
Chemotherapy
Docetaxel is a chemotherapeutic agent that induces apoptosis through inhibition of microtubule assembly and mitotic arrest. It was approved for clinical use in the mCRPC setting in 2004, on the basis of two landmark Phase III trials: the TAX-327 and the SWOG 9916. In the TAX-327 study,10 patients receiving docetaxel every 3 weeks had a significantly longer median overall survival (mOS) compared with those randomized to receive mitoxantrone (18.9 months vs 16.5 months), with a hazard ratio (HR) of 0.76 (95% confidence interval [CI]: 0.62–0.94; P=0.009). Similarly, in the SWOG 9916 trial,11 which compared docetaxel plus estramustine with mitoxantrone plus prednisone, mOS was longer in the group receiving docetaxel and estramustine (17.5 months vs 15.6 months), with a HR of 0.8 (95% CI: 0.67–0.97; P=0.02). Docetaxel is, therefore, recommended in patients with asymptomatic/minimally symptomatic mCRPC, as well as in those with symptomatic mCRPC and good performance status.
Cabazitaxel is a next-generation taxane, approved by regulatory authorities as a second-line therapy for mCRPC based on an open-label Phase III multicenter trial (the TROPIC study).12 In this study, 775 men with mCRPC that had progressed during or after docetaxel-based chemotherapy were randomized to receive intravenous cabazitaxel versus mitoxantrone every 3 weeks for up to 10 cycles, each in combination with daily prednisone. mOS in the cabazitaxel group was 15.1 months (95% CI: 14.1–16.3), compared with 12.7 months (95% CI: 11.6–13.7) in the mitoxantrone group, with a HR of 0.70 (95% CI: 0.59–0.83; P<0.0001).
An updated analysis of the TROPIC trial with longer follow-up confirmed a sustained OS benefit for treatment with cabazitaxel.13 The most common clinically significant grade 3 or higher adverse event observed with cabazitaxel was neutropenia, which was observed in 82% of patients. The standard use of prophylactic granulocyte colony-stimulating factors has significantly reduced this risk, as reported in several retrospective reports of patients treated in real-world setting.14 Furthermore, in the PROSELICA trial, the standard dose of cabazitaxel 25 mg/m2 every 3 weeks was compared to a lesser dose of 20 mg/m2, which demonstrated non-inferiority for OS and an improved safety profile compared with the higher dosage.15 Finally, a Phase III trial comparing cabazitaxel at two different dose levels (20 or 25 mg/m2 every 3 weeks) with docetaxel at standard doses as first-line therapy (FIRSTANA trial) failed to demonstrate superiority for cabazitaxel, showing that docetaxel remains the standard first-line chemotherapy in patient with mCRPC.16
AR targeting agents
Abiraterone acetate is a small-molecule inhibitor of the CYP17A1 enzyme inhibiting the synthesis of steroid precursors that can be transformed into testosterone.17 This drug was approved for the treatment of mCRPC following the results of the COU-AA-301 study, a Phase III study comparing abiraterone acetate versus placebo in patients progressing after docetaxel, in both cases in combination with prednisone.18,19 The mOS was 14.8 months in the abiraterone group compared to 10.9 months in the placebo group (HR 0.64; 95% CI: 0.54–0.77; P<0.0001). This trial demonstrated that abiraterone was highly effective in bone metastases that were present in the great majority of patients.20 The COU-AA-302 study evaluated the efficacy of abiraterone in a population of chemotherapy-naive, asymptomatic or minimally symptomatic patients.21 Abiraterone and prednisone resulted in improved OS over prednisone alone (34.7 months vs 30 months, HR 0.81; P<0.001). In both studies, abiraterone was well tolerated, with the main adverse events consisting of manageable hypokalemia, hypertension, and fluid overload.
Enzalutamide is a second-generation non-steroidal AR inhibitor with higher receptor affinity than conventional anti-androgens, which impairs AR nuclear localization and transcriptional activity even under conditions of AR overexpression.22 This drug was approved for clinical use in docetaxel-pretreated patients with mCRPC on the basis of the AFFIRM trial, as well as in chemo-naïve patients on the basis of the PREVAIL trial. The AFFIRM trial23 demonstrated the superiority of enzalutamide compared to placebo in 1,199 patients, achieving an mOS of 18.4 months versus 13.6 months, with an HR of 0.63 (95% CI: 0.52–0.75; P<0.0001). In the PREVAIL study,24 82% of patients in the enzalutamide group and 73% of those in the placebo group were alive at 18 months; the estimated mOS was not reached in the enzalutamide group and was 31.0 months in the placebo group, with an HR of 0.73 (95% CI: 0.63–0.85; P<0.001). The more common side effects recorded in these two trials included fatigue, hot flashes, diarrhea, musculoskeletal pain, headache, and hypertension.
Radium-223
The alpha emitter radium-223 chloride is a calciomimetic agent that accumulates in osteoblastic areas and deposits a very high energy of irradiation in a short penetration range. This mechanism causes irreparable DNA damage resulting in cell death.25 Radium-223 is the first alpha-emitting radiopharmaceutical agent approved for the treatment of mCRPC with bone metastases, based on an advantage in mOS reported in the ALSYMPCA trial.26,27 Radium-223 treatment showed a 30% reduction in the risk of death versus placebo and better control of skeletal related events (SREs) both in post-docetaxel and chemo-naïve patients;28 furthermore, bone pain control and quality of life were improved.29
On the basis of these results, radium-223 was approved worldwide for clinical use in patients with mCRPC and at least two symptomatic bone metastases and no visceral disease. Although definitive protocols for combined therapy are still lacking, since the introduction of radium-223, there has been a clear trend toward the use of radionuclide metabolic therapy in the early stages of bone metastatic disease with curative intent, rather than in late stages only for pain palliation and after the failure of other therapeutic measures.30
Other bone-targeting agents
Bone-modifying agents are able to affect the biological activity of osteoclasts, osteoblasts, and osteocytes. Several bisphosphonates (eg, zoledronic acid) and an inhibitor of the RANK/RANKL pathway, denosumab, were approved for the treatment of bone metastases in solid tumors with the goal to reduce SREs.31 Clinical studies have demonstrated that denosumab has superior activity over zoledronic acid in delaying and preventing SREs in patients with skeletal metastases from mCRPC.32 However, none of these agents have demonstrated an improvement in OS.
Response assessment to systemic treatments
Based on the main clinical trials,10,12,18,21,23,24 national and international guidelines,33–35 and a consensus conference,6 radiographic imaging techniques, such as computed tomography (CT) and bone scan (BS), are recommended before and after the start of systemic treatments in patients with mCRPC. CT imaging is able to detect the presence of lymph nodal, bone, and visceral metastasis. Moreover, by using Response Evaluation Criteria in Solid Tumors (RECIST), CT represents a useful instrument for evaluating the response to treatments, mainly at lymph node and visceral sites. On the other hand, BS, which is able to detect the presence of bone metastasis in a simple and economical way, is often employed for monitoring response or progression at the bone level. Lastly, new imaging modalities (eg, 18F/11C-choline or 68Ga-PSMA positron emission tomography/CT) have been included in the work-up of recurrent PCa, showing better performance than CT and BS. However, few data are available on their usefulness in the monitoring of patients affected with mCRPC.36 Therefore, despite their intrinsic limitations, CT and BS still represent standard imaging in detecting metastases, as well as in monitoring response to treatment.
The cost of mCRPC
To assess the costs associated with mCRPC, a literature review was performed on the PubMed database. Keywords used in the search were as follows: prostate cancer AND castration resistant AND (metastasis OR metastatic) AND ([cost OR burden] AND [illness OR disease]). Only articles in English and those published from January 1, 2006, to February 2017 were taken into consideration. Eighty articles were identified; 79 were excluded, as they did not consider costs or had a target population not affected by mCRPC.
Dragomir et al in 2014 performed an analysis on the cost of drugs for the management of mCRPC in Canada.37 The authors structured a Markov microsimulation model based on clinical practice derived from national guidelines, considering the evolution of the pathology and costs related to the primary drug treatment, medical castration to maintain castration testosterone levels, and bone-targeted therapies. A total per capita cost (in 2013 Canadian dollars) between $41,182 and $51,106 and between $95,227 and $114,245 was estimated in two different scenarios. The total cost of mCRPC in Canada was estimated to be $193,604,000 and $416,284,000, respectively, in the two scenarios considered.
Due to the high prevalence of PCa and due to the lack of published data with regard to the Italian context, we aimed to estimate the cost of illness (COI) of mCRPC in Italy.
Materials and methods
Cost of illness
From a methodological point of view, to estimate the COI of mCRPC, we referred to the methodological considerations of the cost of PCa studies published by Molinier et al.38 Concerning the design of the analysis, referring to the approaches identified by Akobundu et al, we adopted the “Sum_Diagnosis Specific” method.39 This method considers only the costs directly related to the pathology of interest. The target population of the analysis was composed of the subjects affected by mCRPC in Italy. The approach adopted was that of prevalence with an annual time horizon, year 2016, adopting the perspective of the Italian National Health Service (NHS). Therefore, we considered all patients affected by mCRPC in an annual time horizon, both being treated with first- and second-line treatments.
The Italian NHS is publicly oriented both in terms of financing and providers, with a universalistic approach. All patients have the right to be treated without any co-payment for oncologic drugs, hospitalizations, and outpatient activities. The costs considered were direct medical costs related to ADT, drugs used for mCRPC as described earlier, adverse events management, management of SREs, monitoring activities in terms of drugs, hospitalizations, and outpatient activities. A bottom-up approach was used to estimate the resource consumption: through national guidelines and expert opinions, the mean cost per patient was estimated and then multiplied by the total number of patients diagnosed with mCRPC. This approach is consistent with previously published COI studies in Italy, for the estimation of direct medical costs, as in Marcellusi et al.40
Due to the 1-year time horizon considered, it was not necessary to identify a discount rate. The characteristics of the analysis are summarized in Table 1.
  | Table 1 Characteristics of the analysis Abbreviation: mCRPC, metastatic castrate-resistant prostate cancer. |
Target population
There is currently a lack of publications concerning the number of patients affected by mCRPC in Italy. The estimation of such a population has to take into consideration the difficulties related to the definition of “castrate-resistant pathology”, which implies a biochemical and radiologic progression during ADT. Carteni and Pappagallo3 estimated that 4,902 patients affected by mCRPC in 2012 would receive a first-line treatment and 2,417 patients would be eligible for second-line treatment after a first-line chemotherapy with docetaxel, based on data from the IMS Onco3 database.
To estimate the number of patients affected by mCRPC in Italy in 2016, we considered data relating to patients affected by mCRPC receiving first-line treatment between April 2015 and March 2016, collected from a 2016 report from the IMS Oncoview database (http://www.imshealth.com/it_IT/). The number of patients receiving first-line treatment was 6,497. To estimate the number of patients affected by mCRPC receiving second-line treatment, we applied the same ratio between first- and second-line treatments as observed by Carteni and Pappagallo.3 The estimated total number of patients affected by mCRPC was 9,700: 6,497 receiving first-line treatment and 3,203 receiving second-line treatment.
Patient management and clinical inputs
To identify the activities related to the management of patients affected by mCRPC, partially presented in the previous sections, we considered expert opinions based on real clinical practice and guidelines.
In detail, for metastatic disease, we considered ADT to be performed in all patients, external beam radiotherapy for patients with symptomatic bone metastases in 10% of patients, denosumab in 20% of patients, and zoledronic acid in 80% of patients. The treatments considered in first-line and second-line are reported in Table 2.
  | Table 2 Market shares per line of treatment Note: *Estimates from Verzoni et al.46 |
Adverse events of grade 3 or higher were considered for each treatment. The incidence of adverse events and SREs is reported in Table 3, based on published data. When incidence data were unavailable, different data were taken into consideration: the mean incidence, the minimum incidence, and the maximum incidence among other treatments for which data were available.
  | Table 3 Incidence of adverse events and skeletal-related events per treatment Notes: The rate of events was taken from randomized trials that compared the efficacy of the experimental drug with that of placebo.27 Incidence values considered when N/A: for anemia a mean value of 8.77%, a minimum value of 5.00%, a maximum value of 12.67%; for thrombocytopenia a mean value of 2.86%, a minimum value of 1.00%, a maximum value of 6.50%; for neutropenia a mean value of 23.22%, a minimum value of 0.13%, a maximum value of 81.67%; for febrile neutropenia a mean value of 2.12%, a minimum value of 0.17%, a maximum value of 7.55%; for nausea a mean value of 1.57%, a minimum value of 0.00%, a maximum value of 2.15%; for vomiting a mean value of 1.77%, a minimum value of 0.00%, a maximum value of 2.65%; for pathological fracture a mean value of 4.50%, a minimum value of 3.00%, a maximum value of 6.00%; for radiation to bone a mean value of 23.00%, a minimum value of 22.00%, a maximum value of 24.00%; for spinal cord compression a mean value of 5.80%, a minimum value of 2.10%, a maximum value of 8.00%; for surgery to bone a mean value of 1.35%, a minimum value of 1.00%, a maximum value of 1.70%. *Due to lack of second-line evidence, the same value as first-line was considered. Abbreviation: N/A, not available. |
Patients were also considered to have received abdominal CT and total body bone scintigraphy twice a year. Furthermore, the monitoring activity of patients considered was as follows: PSA and testosterone levels every 3 months and renal and liver function tests, blood tests, and diagnosis of lactate dehydrogenase and alkaline phosphatase each month.
Costs
The costs considered in the analysis were direct medical costs, related to the management of the pathology (follow-up activities and monitoring), therapy, and management of treatment-related adverse events and SREs. The costs of mCRPC treatments were taken from published documents of the Technical Commission of Veneto Region, approved in 2015 and 2016, in which details on the total cost of different therapies were reported, inclusive of mandatory discounts and cost-sharing agreements between pharmaceutical companies and the Italian Medicines Agency. Treatments and costs are reported in Table 4.
For each treatment, the costs related to adverse events management and management of SREs were considered. Management of adverse events was quantified based on actual clinical practice, through agreement of three clinicians (a director of a hospital oncology department, a director of a nuclear medicine department, and a nuclear medicine clinician). The costs related to SREs (pathologic fracture, spinal cord compression, radiation to bone, surgical intervention) were derived from the available literature41 and inflated at their 2016 level, considering the Italian national annual average inflation as reported by the International Monetary Fund.42
The costs of management of AEs and SREs are reported in Table 5. The costs of monitoring activities, derived from the Italian nomenclature tariffs, are reported in Table 6.
  | Table 5 Costs of adverse events and skeletal-related events Abbreviation: DRGs, diagnosis related groups. |
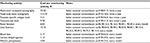  | Table 6 Monitoring activity costs |
Uncertainty of the parameters considered in the analysis was dealt with by considering net and gross values observed for treatment costs, the incidence of adverse events and SREs in the case of missing values, and the cost of ADT, as described. Furthermore, we implemented a univariate sensitivity analysis varying the following variables: percentage of each treatment market share (±10%) (varying the market shares of other treatments maintaining their proportion among each other); number of patients treated with first- and second-line treatments (±10%); percentage of patients receiving external beam radiotherapy (+100%); percentage of patients receiving denosumab (+100%); percentage of patients receiving zoledronic acid (±10), and percentage of patients receiving ADT (−10%).
Results
The yearly cost to treat mCRPC patients in Italy was estimated to be between €196.5 million and €228.0 million, as presented in Table 7. The costs related to patients receiving first-line treatment are between €136.9 million and €160.3 million and the costs related to patients receiving second-line treatment are between €59.7 million and €67.8 million.
Concerning single costs components, the costs related to PCa treatment (drug cost plus administration cost) represent 77.7% of the total cost (considering net treatment cost) and 79.5% (considering gross treatment cost). Considering the net treatment cost, management of SREs represents 5.3% of the total cost, management of adverse events 1.2%, and other costs (external beam radiotherapy, denosumab, zoledronic acid, monitoring of patients, ADT) 15.7%. When considering the gross treatment cost, management of SREs represents 4.9% of the total cost, management of adverse events 1.1%, and other costs 14.5%.
The total annual per capita cost is between €31,011 and €38,842 for patients treated with abiraterone as first-line treatment; €23,161 and €27,622 for patients treated with abiraterone as second-line treatment; €20,567 and €22,199 for patients treated with cabazitaxel; €7,453 and €9,143 for patients treated with docetaxel; €32,388 and €34,214 for patients treated with enzalutamide as first-line treatment; €23,634 and €25,461 for patients treated with enzalutamide as second-line treatment; and €20,833 and €22,079 for patients treated with radium-223.
The sensitivity analysis results (presented as Tables S1 and S2) showed a total costs lower value of €182,838.2 (decreasing by 10% the number of patients treated with first-line treatments) and a higher value of €244,054.6 (increasing by 10% the number of patients treated with first-line treatments).
Discussion and conclusion
According to the literature review performed (presented earlier), the only published study that reported costs related to mCRPC was that of Dragomir et al.37 The mean per capita cost of medications (referenced to 2013) was estimated to be €35,390 (equal to Canadian $48,428, converted into euros using the yearly average exchange rate of 2013, as reported by EUROSTAT).43 The cost presented by Dragomir et al was calculated considering a period of 28.1 months. The same data related to a 12-month period would be equal to €15,113, comparable with the mean annual per capita costs here estimated for the Italian context. The higher cost observed in Italy might be partially explained by the fact that we considered further costs, such as those related to monitoring activities.
Considering direct medical costs to treat PCa, in 2010, Fourcade et al44 estimated a mean cost for the first year after the diagnosis of €5,226 (referenced to 2006) in Italy. The difference in terms of annual cost compared to the analysis performed is due to the earlier stage of disease in the Fourcade et al’s analysis (while only patients with mCRPC are considered in the analysis presented) and the lack of the most recent therapeutic options (eg, cabazitaxel, enzalutamide, and radium-223), which are more expensive compared with older treatments, such as docetaxel. The higher cost of most recent treatments, however, might be partly compensated by the decrease in hospitalizations related to SREs as shown for the use of radium-223 compared with docetaxel.45
To the best of our knowledge, the analysis presented here is the first economic analysis conducted on mCRPC in Italy. Two articles have been published at a national level, both analyzing radium-223 in terms of the financial sustainability of its use in Italy and of cost utility, compared with abiraterone, cabazitaxel, docetaxel, and enzalutamide.46,47
The main limitation of our analysis is the lack of real-world data collected within the Italian context. However, the data considered for the analysis are based on a thorough literature review and expert opinion of clinicians, and this reflects the real-life clinical practice of Italian hospitals. A further limitation of the analysis is related to the lack of costs referred to the adverse events of the drugs considered for ADT, leading to a possible underestimation of the cost of illness.
The direct medical costs related to the management of mCRPC, identified as between €196.5 million and €228.0 million, represented in 2016 ~0.2% of the financing of the Italian National Health Service. This is less than half of the one estimated by Gerace et al for urothelial bladder cancer.60 However, the latter results are calculated on a number of patients higher than 30,000 and all stages of the disease are considered, while in the analysis presented, only metastatic patients are considered.
The main cost driver is the cost of treatment (drugs and administration), which represents more than 77%, followed by the cost of ADT (considered within the “other costs” category in the analysis, representing more than 50% of such cost category in the mean scenario).
The results of the analysis presented could support decision makers, providing estimates of the total cost of the last stage of PCa disease, in particular considering the costs associated with different drugs for first- and second-line treatments. The results of the analysis presented, therefore, will allow future benchmarking activities.
Disclosure
The authors report no conflicts of interest in this work.
References
Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet. 2016; 387(10013):70–82. | ||
Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61(6):1079–1092. | ||
Carteni G, Pappagallo G. Carcinoma della prostata resistente alla castrazione in Italia: un unmet medical need in via di risoluzione. [Castrate resistant prostate cancer in Italy: an unmet medical need about to be solved]. Ital J Public Health. 2011;8(Suppl 4):S3–S8. Italian. | ||
AIOM. CCM. AIRTUM. I numeri del cancro in Italia 2015. [The numbers of cancer in Italy 2015]. Available http://www.registri-tumori.it/PDF/AIOM2015/I_numeri_del_cancro_2015.pdf. Accessed November 10, 2017. | ||
Zarif JC, Miranti CK. The importance of non-nuclear AR signaling in prostate cancer progression and therapeutic resistance. Cell Signal. 2016;28(5):348–356. | ||
Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. Epub 2017 June 24. | ||
Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67(10):5033–5041. | ||
Ritch CR, Cookson MS. Advances in the management of castration resistant prostate cancer. BMJ. 2016;355:i4405. | ||
Lorente D, Fizazi K, Sweeney C, de Bono JS. Optimal treatment sequence for metastatic castration-resistant prostate cancer. Eur Urol Focus. 2016;2(5):488–498. | ||
Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. | ||
Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. | ||
de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. | ||
Bahl A, Oudard S, Tombal B, et al. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann Oncol. 2013;24(9):2402–2408. | ||
Heidenreich A, Scholz HJ, Rogenhofer S, et al. Cabazitaxel plus prednisone for metastatic castration-resistant prostate cancer progressing after docetaxel: results from the German compassionate-use programme. Eur Urol. 2013;63(6):977–982. | ||
de Bono J, Hardy-Bessard A-C, Kim C-S, et al. Phase III non-inferiority study of cabazitaxel (C) 20 mg/m2 (C20) versus 25 mg/m2 (C25) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) previously treated with docetaxel (D) [Abstract]. J Clin Oncol. 2016;34(15 Suppl):5008. | ||
Oudard S, Fizazi K, Sengelov L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J Clin Oncol. 2017. doi: JCO2016721068. | ||
Yap TA, Carden CP, Attard G, de Bono JS. Targeting CYP17: established and novel approaches in prostate cancer. Curr Opin Pharmacol. 2008;8(4):449–457. | ||
de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. | ||
Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983–992. | ||
Logothetis CJ, Basch E, Molina A, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13(12):1210–1217. | ||
Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. | ||
Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. | ||
Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. | ||
Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. | ||
Henriksen G, Breistol K, Bruland OS, Fodstad O, Larsen RH. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res. 2002;62(11):3120–3125. | ||
Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. | ||
Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15(7):738–746. | ||
Hoskin P, Sartor O, O’Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15(12):1397–1406. | ||
Nilsson S, Cislo P, Sartor O, et al. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann Oncol. 2016;27(5):868–874. | ||
Saad F, Carles J, Gillessen S, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17(9):1306–1316. | ||
Body JJ, Casimiro S, Costa L. Targeting bone metastases in prostate cancer: improving clinical outcome. Nat Rev Urol. 2015;12(6):340–356. | ||
Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. | ||
AIOM – Associazione Italiana di Oncologia Medica. APP Linee Guida 2014: Carcinoma della prostata. 2015. [AIOM - Italian Association of Medical Oncology. APP Guidelines 2014: Prostate cancer. 2015]. Available from: http://www.aiom.it/. Accessed November 10, 2017. | ||
Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479. | ||
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Prostate Cancer. National Comprehensive Cancer Network (NCCN); 2015. | ||
Evangelista L, Bertoldo F, Boccardo F, et al. Diagnostic imaging to detect and evaluate response to therapy in bone metastases from prostate cancer: current modalities and new horizons. Eur J Nucl Med Mol Imaging. 2016;43(8):1546–1562. | ||
Dragomir A, Dinea D, Vanhuyse M, Cury FL, Aprikian AG. Drug costs in the management of metastatic castration-resistant prostate cancer in Canada. BMC Health Serv Res. 2014;14:252. | ||
Molinier L, Bauvin E, Combescure C, et al. Methodological considerations in cost of prostate cancer studies: a systematic review. Value Health. 2008;11(5):878–885. | ||
Akobundu E, Ju J, Blatt L, Mullins CD. Cost-of-illness studies: a review of current methods. Pharmacoeconomics. 2006;24(9):869–890. | ||
Marcellusi A, Viti R, Mecozzi A, Mennini FS. The direct and indirect cost of diabetes in Italy: a prevalence probabilistic approach. Eur J Health Econ. 2016;17(2):139–147. | ||
Hechmati G, Cure S, Gouépo A, et al. Cost of skeletal-related events in European patients with solid tumours and bone metastases: data from a prospective multinational observational study. J Med Econ. 2013;16(5):691–700. | ||
International Monetary Fund. World Economic Outlook Database – April 2017. Available from: http://www.imf.org/external/pubs/ft/weo/2017/01/weodata/index.aspx. Accessed November 10, 2017. | ||
EUROSTAT. Euro/ECU Exchange Rates – Annual Data. Available from: http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=ert_bil_eur_a&lang=en. 2017. Accessed November 10, 2017. | ||
Fourcade RO, Benedict A, Black LK, Stokes ME, Alcaraz A, Castro R. Treatment costs of prostate cancer in the first year after diagnosis: a short-term cost of illness study for France, Germany, Italy, Spain and the UK. BJU Int. 2010;105(1):49–56. | ||
Parker C, Zhan L, Cislo P, Reuning-Scherer J. Effect of radium-233 dichloride (Ra-223) on hospitalization and its economic implications in ALSYMPCA trial [Abstract 1230]. Eur J Cancer. 2015;51(Suppl):S183. | ||
Verzoni E, Tucci M, Caruggi M, Colombo GL. Radio-223 dicloruro nel trattamento del carcinoma metastatico della prostata resistente alla castrazione: un’analisi di Budget Impact per il Servizio Sanitario Nazionale. [Radium-223 dichloride for the treatment of metastatic Castrate Resistant Prostate Cancer: a budget impact analysis for the Italian National Health Service]. ClinicoEconomics. 2016;11:29–44. Italian. | ||
Restelli U, Tucci M, Croce D. Impatto economico di Radio-223 nel trattamento del tumore della prostata metastatico resistente alla castrazione (mCRPC), 2016. In “Radio-223 nel trattamento del carcinoma della prostata metastatico resistente alla castrazione (mCRPC): risultati di una valutazione di Health Technology Assessment”. [Economic impact of Radium-223 for the treatment of metastatic castrate resistant prostate cancer (mCRPC), 2016. In “Radium-223 for the treatment of metastatic castrate resistant prostate cancer (mCRPC): Health Technology Assessment results]. Available from: http://www.ijph.it/pdf/2016-v5-n7.pdf. Accessed November 10, 2017. | ||
Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66(5):815–825. | ||
Horgan AM, Seruga B, Pond GR, et al. Tolerability and efficacy of docetaxel in older men with metastatic castrate-resistant prostate cancer (mCRPC) in the TAX 327 trial. J Geriatr Oncol. 2014;5(2):119–126. | ||
Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15(10):1147–1156. | ||
Parker C, Zhan L, Cislo P, et al. Effect of radium-223 dichloride (Ra-223) on hospitalisation: an analysis from the phase 3 randomised Alpharadin in Symptomatic Prostate Cancer Patients (ALSYMPCA) trial. Eur J Cancer. 2017;71:1–6. | ||
Regione del Veneto, Giunta Regionale. Allegato A al Decreto n. 125 del 14 novembre 2016. [Veneto Region, Regional Commettee, Annex A to the Decree n. 125 - 14 November 2016]. Available from: http://www.regione.veneto.it/c/document_library/get_file?uuid=8cbaf3b9-6a7f-405b-8a9f-392327563208&groupId=10793. Accessed November 10, 2017. | ||
Regione del Veneto, Giunta Regionale. Allegato B al Decreto n. 291 del 7 ottobre 2015. [Veneto Region, Regional Commettee, Annex B to the Decree n. 291 - 7 October 2015]. Available from: bur.regione.veneto.it/BurvServices/Pubblica/Download.aspx?name=291_Allegato_B_DDR_291_07-10-2015_308781.pdf&type=7&storico=False. Accessed November 10, 2017. | ||
Agenzia Italiana del Farmaco. Determinazione 2 agosto 2013. Rinegoziazione del medicinale per uso umano «Prolia», ai sensi dell’art. 8, comma 10, della legge 24 dicembre 1993, n. 537. (Determina n. 732/2013) (Gazzetta n. 194 del 20 agosto 2013). [Italian Medicines Agency. Resolution 2 August 2013. Renegotiation of the human medicine “Prolia”, in accordance with article 8, comma 10, of the law of 24 December 1993, n. 537. (Resolution n. 732/2013) (Gazette n. 194 - 20 August 2013)]. Available from: http://www.gazzettaufficiale.it/eli/id/2013/08/20/13A06863/sg. Accessed November 10, 2017. | ||
Agenzia Italiana del Farmaco. Determinazione 7 marzo 2016. Autorizzazione all’immissione in commercio del medicinale per uso umano «Acido Zoledronico Sun Pharma» (Determina n. 732/2016) (Gazzetta n. 70 del 24 marzo 2016). [Italian Medicines Agency. Resolution 7 March 2016. Marketing authorisation of the human medicine “Acido Zoledronico Sun Pharma” (Resolution n. 732/2016) (Gazette n. 70 - 24 March 2016)]. Available from: http://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario;jsessionid=WMHMtgUOYHLyIvdYvhNMnA__.ntc-as3-guri2a?atto.dataPubblicazioneGazzetta=2016-03-24&atto.codiceRedazionale=16A02346&elenco30giorni=false. Accessed November 10, 2017. | ||
Agenzia Italiana del Farmaco. Determinazione 31 luglio 2012. Procedure di payback – anno 2012 (Gazzetta n. 183 del 17 agosto 2012). [Italian Medicines Agency. Resolution 31 July 2012. Payback procedures - year 2012 (Gazette n. 283 - 17 August 2012)]. Available from: www.gazzettaufficiale.it/eli/gu/2012/08/07/183/sg/pdf. Accessed November 10, 2017. | ||
Agenzia Italiana del Farmaco. Determinazione 1 agosto 2013. Classificazione del medicinale per uso umano «Enantone», ai sensi dell’art. 8, comma 10, della legge 24 dicembre 1993, n. 537. (Determina n. 715/2013) (Gazzetta n. 194 del 20 agosto 2013). [Italian Medicines Agency. Resolution 1 August 2013. Classification of the human medicine “Enantone”, in accordance with article 8, comma 10, of the law of 24 December 1993, n. 537. (Resolution n. 715/2013) (Gazette n. 194 - 20 August 2013)]. Available from: http://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2013-08-20&atto.codiceRedazionale=13A06866&elenco30giorni=false. Accessed November 10, 2017. | ||
Agenzia Italiana del Farmaco. Comunicato. Determinazioni di rimborsabilità e prezzo di vendita, autorizzazioni, modificazioni e rettifiche all’immissione in commercio di vari medicinali (Gazzetta n. 271 del 21 novembre 2007 suppl. ordinario n. 239). [Italian Medicines Agency. Pubblic announcement. Resolution on reimbursement and selling price, authorizations, modifications and amendments to the marketing authorization of various medicines (Gazette n. 271 - 21 November 2007 ordinary supplement n. 239)]. Available from: http://www.gazzettaufficiale.it/eli/id/2007/11/21/07A09472/sg. Accessed November 10, 2017. | ||
Agenzia Italiana del Farmaco. Determinazione 11 aprile 2011. Regime di rimborsabilità e prezzo di vendita del medicinale «Firmagon» (degarelix). (Determinazione/C n. 2168/2011) (Gazzetta n. 92 del 21 aprile 2011). [Italian Medicines Agency. Resolution 11 April 2011. Reimbursement regime and selling price of the medicine “Firmagon” (degarelix). (Resolution/C n. 2168/2011) (Gazette n. 92 - 21 April 2011)]. Available from: http://www.gazzettaufficiale.it/eli/id/2011/04/21/11A04989/sg. Accessed November 10, 2017. | ||
Gerace C, Montorsi F, Tambaro R, et al. Cost of illness of urothelial bladder cancer in Italy. Clinicoecon Outcomes Res. 2017;9:433–442. |
Supplementary materials
  | Table S1 Per capita cost Abbreviations: min, minimum; max, maximum. |
  | Table S2 Sensitivity analysis results Abbreviations: min, minimum; max, maximum; ADT, androgen deprivation therapy. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


